* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Future Challenges - Thrombosis Research Institute
Polysubstance dependence wikipedia , lookup
Drug design wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Drug discovery wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmacognosy wikipedia , lookup
Prescription costs wikipedia , lookup
Drug interaction wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Theralizumab wikipedia , lookup
Discovery and development of direct Xa inhibitors wikipedia , lookup
Dydrogesterone wikipedia , lookup
Discovery and development of direct thrombin inhibitors wikipedia , lookup
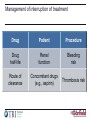
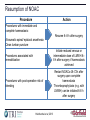
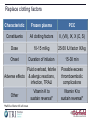
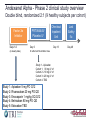
Future Challenges Alexander G G Turpie Professor Emeritus of Medicine McMaster University Hamilton ON Canada The GARFIELD Registry is funded by an unrestricted research grant from Bayer Pharma AG www.tri-london.ac.uk Disclosures for Dr A.G.G. Turpie Research Support None Employee None Consultant and/or Honoraria Bayer HealthCare, Boehringer-Ingelheim, Bristol-Myers Squibb, Johnson and Johnson, Sanofi-Aventis, Takeda, Portola Stockholder None Speakers Bureau Pfizer, GSK Scientific Advisory Board Bayer HealthCare, Johnson and Johnson, New anticoagulants Direct Thrombin Inhibitors - Dabigatran Factor Xa Inhibitors - Rivaroxaban - Apixaban - Edoxaban Future challenges • Management of bleeding - Prevention - Treatment • Measurement • Antidotes All anticoagulants can cause bleeding Bleeding in VKA anticoagulated patients • Is common – Major bleeding 1-5% per year in AF – Intracranial bleeding 0.5-1.2% per year in AF • Associated with adverse outcomes – 3 to 5-fold increase in thrombotic events and death • Rapid and timely control of bleeding is likely to improve clinical outcomes but the efficacy of anticoagulant reversal is unproven Management of bleeding • Prevention • Treatment Prevention of bleeding • Anticoagulant selection • Patient and dose selection • Appropriate management of interruption Perioperative Management of NOAC-treated Patients Management of interruption of treatment Drug Patient Procedure Drug half-life Renal function Bleeding risk Route of clearance Concomitant drugs (e.g., aspirin) Thrombosis risk Last intake of drug before elective surgical intervention Creatinine clearance Dabigatran Apixaban Rivaroxaban (CrCl) No important bleeding risk and/or adequate local haemostasis possible: Perform at trough level (i.e. 12 h or 24 h after last intake) Low High Low High Low risk High risk risk risk risk risk CrCl ≥80 ml/min ≥24 h ≥48 h ≥24 h ≥48 h ≥24 h ≥48 h CrCl 50-80 ml/min ≥36 h ≥72 h ≥24 h ≥48 h ≥24 h ≥48 h CrCl 30-50 ml/min* ≥48 h ≥96 h ≥24 h ≥48 h ≥24 h ≥48 h CrCl 15-30 ml/min* Not indicated Not indicated ≥36 h ≥48 h ≥36 h ≥48 h CrCl <15ml/min No official indication for use *Many of these patients may be on lower dose of NOAC Low risk = surgery with low risk of bleeding, high risk = surgery with high risk of bleeding Heidbuchel et al, 2013 Resumption of NOAC Procedure Action Procedures with immediate and complete haemostasis: Resume 6–8 h after surgery Atraumatic spinal/ epidural anaethesia Clean lumbar puncture Procedures associated with immobilization Initiate reduced venous or intermediate dose of LMWH 6– 8 h after surgery if haemostasis achieved Procedures with post-operative risk of bleeding Restart NOACs 48–72h after surgery upon complete haemostasis Thromboprophylaxis (e.g. with LMWH) can be initiated 6-8 h after surgery Heidbuchel et al, 2013 Monitoring vs measuring • Monitoring implies dose adjustment according to test result • Measuring the drug or drug effect may be useful in: • • • • • • • Bleeding Overdosage Questions of compliance Urgent surgery, interventions, thrombolysis Extreme body weights Children Renal insufficiency Measurement of anticoagulant effects of NOACs Test Dabigatran Rivaroxaban Apixaban Specific Assay Hemoclot Anti-Xa Anti-Xa aPTT ↑↑↑ ↑ ↑ PT ↑ ↑↑ ↑ TT ↑↑↑↑ No effect No effect Non-specific assays Future Point of care testing Management of VKA bleeding • Hold drug(s) • Vitamin K • Resuscitation (i.v. access, fluid administration, blood product transfusion) • Maintain diuresis to clear drug • Mechanical compression and surgical methods to stop bleeding Replace clotting factors Characteristic Frozen plasma PCC Constituents All clotting factors II, (VII), IX, X (C, S) Dose 10-15 ml/kg 25-50 IU factor IX/kg Onset Duration of infusion 15-30 min Fluid overload, febrile Adverse effects & allergic reactions, infection, TRALI Other Vitamin K to sustain reversal* Possible excess thromboembolic complications Vitamin K to sustain reversal* *Half life of factor VII is 6 hours Quinlan D, et al. Circulation 2013; 128:1179-81. Frozen plasma or PCC? Sarode R, et al. Circulation 2013; 128:1234-1243. Management of NOAC bleeding • Hold drug(s) • No Vit K • Resuscitation (i.v. access, fluid administration, blood product transfusion) • Maintain diuresis to clear drug • Mechanical compression and surgical methods to stop bleeding Reversal of NOACs • Activate coagulation to overcome the effect of the drug • Remove drug • Neutralize drug Lauw MN, et al. Can J Cardiol. 2014;30:381-384. Activate coagulation • Recombinant factor VIIa (rVIIa) • Prothrombin complex concentrates (PCC) – II, VII, IX, X, C, S, – 25-50 units per kg • Activated prothrombin complex concentrates (aPCC) • Antifibrinolytic agents (e.g., tranexamic acid) Effect of NOACs on prothrombin time and endogenous thrombin potential with PCC Rivaroxaban: Effect on prothrombin time and endogenous thrombin potential with PCC Prothrombin time (PT) Endogenous thrombin potential (ETP) PCC demonstrated the potential to reverse rivaroxaban effects on PT and ETP in humans Eerenberg et al, 2011 Antidotes to anticoagulants • • • • Bleeding Emergency Intervention Elective Intervention Overdose Specific antidotes to NOACs Idarucizumab PER977 Andexanet alpha Structure Humanized Fab fragment Synthetic small molecule Human rXa variant Target Dabigatran Universal FXa inhibitors Binding Non-competit. High affinity ? Competitive ? Rapid, near complete reversal Clinical studies Rapid complete reversal Lauw M, et al. Can J Cardiol 2014 (accepted). Andexanet Alpha - Phase 2 clinical study overview Double blind, randomized 2:1 (9 healthy subjects per cohort) Factor Xa Inhibitor PRT064445/ Placebo IV Days 1-6 Day 6 (to steady state) 3h after last fXa inhibitor dose Checkout Inpatient Unit Day 13 Study 1 - Apixaban Cohort 1: 90 mg IV x 1 Cohort 2: 210 mg IV x 1 Cohort 3: 420 mg IV x 1 Cohort 4: TBD Study 1: Apixaban 5 mg PO Q12 Study 2: Rivaroxaban 20 mg PO QD Study 3: Enoxaparin 1 mg/kg SQ Q12 Study 4: Betrixaban 80 mg PO QD Study 5: Edoxaban TBD Last Safety f/u Day 48 Dose-dependent reversal of Apixaban-induced Anti-FactorXa activity correlates with reduction in Apixaban plasma free fraction Anti-fXa activity Mean ± SEM Apixaban free fraction Andexanet Alpha • FDA designated breakthrough therapy • Phase III Clinical Trials - ANNEXA-A: apixaban - ANNEXA-R: rivaroxaban Conclusions • NOACs provide opportunity to minimize growing burden of potentially preventable thromboembolism (especially AF) • Reductions in both stroke and bleeding translate into important benefits for patients • Most bleeding can be managed without specific antidotes • Specific antidotes in development will provide reassurance to physicians • Education to overcome the fear of bleeding as a barrier to appropriate anticoagulant use important