* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Answer key
California Green Chemistry Initiative wikipedia , lookup
Registration, Evaluation, Authorisation and Restriction of Chemicals wikipedia , lookup
Thermomechanical analysis wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Drug discovery wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
History of chemistry wikipedia , lookup
Al-Shifa pharmaceutical factory wikipedia , lookup
Chemical industry wikipedia , lookup
Chemical plant wikipedia , lookup
Chemical weapon proliferation wikipedia , lookup
Chemical weapon wikipedia , lookup
Chemical potential wikipedia , lookup
Safety data sheet wikipedia , lookup
Chemical Corps wikipedia , lookup
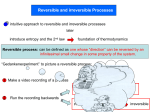
WORKSHEET Year: 2015-16 Subject: Science Class: VII (A & B) Topic: Physical and Chemical Changes 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. Choose the correct option from the following questions: Melting of ice is a a. Chemical change b. Physical change Which of these is the smallest particle? a. An atom b. A molecule Formation of a compound is a. Physical change b. Temporary change Sea water is a a. Element b. Compound Which of the following is a reversible change? a. Melting of ice b. Burning of match stick Which of the following is an example of chemical change? a. Melting of wax b. Burning of candle wick In a chemical change a. Molecule of substance do not change b. Molecule of substance change Which of the following is an example of physical change? a. A bud turning into a flower b. Rusting of iron Physical changes are a. Irreversible b. Periodic Chemical changes are characterised by a. Formation of new substance b. Release of energy Symbol that represent sodium is a. S b. K Crystallization is a process of obtaining a. solids only b. pure liquids only Rusting of iron can be prevented by a. Galvanising b. Painting c. Periodic change d. Both physical and chemical change c. A speck of dust d. A water drop c. Chemical change d. Reversible change c. Mixture d. Molecule c. Changing of milk into curd d. Germination of seed c. Heating of iron rod d. Woolen yarn to knitted sweater c. Substance remain the same d. Change is reversible c. Ripening of a tomato d. Boiling of water c. Reversible d. All of these c. Absorption of energy d. Reversible c. Na d. Fe c. pure gas only d. all of these c. Alloying d. All of these 14. When an iron nail is dipped in the blue copper sulphate solution i. Colour of the solution changes to green ii. Brown deposit is observed on the iron nail iii. Brown deposits is observed at the bottom of the beaker iv. Which of the above statements are correct? a. [i] & [ii] are correct c. [ii] & [iv] are correct b. [i] & [iii] are correct d. [i] &[iv] are correct 15. Dissolution of sugar is a/an a. Irreversible physical changes c. reversible chemical changes b. Irreversible chemical changes d. reversible physical changes 16. A chemical change among the following occurs when a. Salt is mixed with water c. A paper boat is made by paper cutting b. Wax is melted d. A wax candle is burnt 17. Which of the following is not a chemical change? a. Burning of log of wood c. Curdling of milk b. Chopping of trees d. Heating of milk 18. On addition of baking soda to vinegar, we come to know that a chemical reaction has occurred as a. Hydrogen gas is evolved c. There is evolution of heat and light b. Carbon dioxide gas is evolved d. There is a change in color NSO/HOTS QUESTIONS 1. Rohant observed formation of the green substance on a copper statue in his home. This formation is due to: a. chemical change c. periodic change b. physical change d. both (B) and (C) 2. Karan cuts a piece of wire and then bends to form pattern. Identify the change undergone by the wire. a. Irreversible physical change c. Chemical change b. Reversible physical change d. Initially chemical change later physical change 3. Look at the figure of whipping an egg white: What kind of change is undergone by egg white? a. Reversible chemical change c. Physical change b. Irreversible chemical change d. Both physical as well as chemical change 4. Identify the following as a chemical (C) or physical property (P): 1. 2. 3. 4. 5. 6. 7. blue color density flammability (burns) solubility (dissolves) reacts with acid supports combustion sour taste P P C P C C P 8. melting point 9. reacts with water 10. Hardness 11. boiling point 12. Luster 13. Odour 14. reacts with air P C P P P C C 5. Read each scenario. Decide whether a physical or chemical change has occurred and give evidence for your decision. The first one has been done for you to use as an example. Scenario Physical or Chemical Change? Evidence… No change in substances. 1. Umm! A student removes a loaf of bread hot from the oven. The student cuts a slice off the loaf and spreads butter on it. 2. Your friend decides to toast a piece of bread, but leaves it in the toaster too long. The bread is black and the kitchen if full of smoke. Chemical Toast has been burnt. New substance formed, smoke is formed, change is irreversible 3. Food color is dropped into water to give it color Physical Only change in colour,no new substance is formed 4. You blow dry your wet hair. Physical No change in substance, water changes to water vapur[change of state has taken place. 5. In baking biscuits and other quick breads, the baking powder reacts to release carbon dioxide bubbles. The carbon dioxide bubbles cause the dough to rise. Chemical New substance CO2 is formed, 6. In a fireworks show, the fireworks explode giving off heat and light. Chemical Heat & light is produced Physical No unexpected color change, temperature change or gas given off.