* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Ch. 2: Biochemistry
Survey
Document related concepts
Transcript
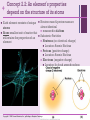
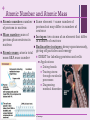
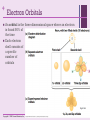
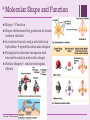
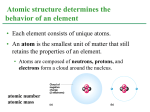
+ Chapter 2 The Chemical Context of Life + Overview: A Chemical Connection to Biology • Living organisms follow basic laws of physics and chemistry • use of formic acid by ants to maintain “devil’s gardens,” stands of Duroia trees Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings + • • Concept 2.1: Matter consists of chemical elements in pure form and in combinations called compounds Organisms composed of matter Matter: anything that takes up space and has mass • Made up of elements Element: substance that cannot be broken down to other substances by chemical reactions Compound: substance consisting of two or more elements in a fixed ratio Compound may not have same characteristics as elements it’s made of Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Essential Elements + of Life Only 25 of 92 elements essential to life 96% of living matter: Carbon, hydrogen, oxygen, and nitrogen Remaining 4%: Calcium, phosphorus, potassium, and sulfur Trace elements: those required by an organism in minute quantities Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Fig. 2-4 + (a) Nitrogen deficiency (b) Iodine deficiency + Concept 2.2: An element’s properties depend on the structure of its atoms Each element consists of unique atoms Atom: smallest unit of matter that still retains the properties of an element Neutron mass & proton mass are almost identical measured in daltons Subatomic Particles Neutrons (no electrical charge) Location: Atomic Nucleus Protons (positive charge) Location: Atomic Nucleus Electrons (negative charge) Location: In cloud around nucleus Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings + Atomic Number and Atomic Mass Atomic number: number of protons in nucleus Mass number: sum of protons plus neutrons in nucleus Atomic mass: atom’s total mass AKA mass number Same element = same number of protons but may differ in number of neutrons Isotopes: two atoms of an element that differ in number of neutrons Radioactive isotopes: decay spontaneously, giving off particles and energy GREAT for labeling proteins and cells Applications: Dating fossils Tracing atoms through metabolic processes Diagnosing medical disorders Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings + The Energy Levels of Electrons • • • • Energy: capacity to cause change Potential energy: that matter has because of its location or structure Electrons of atom differ in amounts of potential energy Electron’s state of potential energy is called its energy level, or electron shell Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings +Electron Distribution and Chemical Properties Valence electrons: in the outermost shell, or valence shell Elements with full valence shell are chemically inert Chemical behavior of atom determined by distribution of electrons in electron shells, MOSTLY by valence electrons Periodic table of the elements shows electron distribution for each element Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings + Electron Orbitals An orbital is the three-dimensional space where an electron is found 90% of the time Each electron shell consists of a specific number of orbitals Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings + Concept 2.3: The formation and function of molecules depend on chemical bonding between atoms • Incomplete valence shells can share/transfer valence electrons with other atoms • usually result in chemical bonds • atoms staying close together, held by attraction Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Covalent bond: sharing of a pair of valence electrons by two atoms + Molecule: consists of two or more atoms held together by covalent bonds A single covalent bond, or single bond, is the sharing of one pair of valence electrons A double covalent bond, or double bond, is the sharing of two pairs of valence electrons Structural formula: notation used to represent atoms and bonding Ex: H–H Molecular formula: indicates the amount and type of atoms in a molecule Ex: H2 Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings + Covalent bonds can form between same OR different elements Compound: combination of two or more different elements Valence: Bonding capacity of atom Electronegativity: atom’s attraction for electrons in covalent bond More electronegative an atom = the more strongly it pulls electrons Nonpolar covalent bond: electrons equally shared Polar covalent bond: one atom is more electronegative electrons unequally shared Unequal sharing partial positive or negative charge Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Fig. 2-14-2 + Na Cl Na Cl Na Sodium atom Cl Chlorine atom Na+ Sodium ion (a cation) Cl– Chloride ion (an anion) Sodium chloride (NaCl) + Ionic Bonds Ion: charged atom or molecule After the transfer of an electron both atoms have charges Cation: positively charged ion Anion: negatively charged ion Ionic bond: attraction between anion and cation Ionic compounds, or salts: compounds formed by ionic bonds Salts like NaCl often found in nature as crystals Animation: Ionic Bonds Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings + Weak Chemical Bonds Most of the strongest bonds in organisms are covalent bonds form a cell’s molecules Weak chemical bonds: reinforce shapes of large molecules help molecules adhere to each other Hydrogen bond: forms when a hydrogen atom covalently bonded to one electronegative atom is also attracted to another electronegative atom In living cells electronegative partners usually oxygen or nitrogen atom Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings + Van der Waals Interactions Electrons are distributed asymmetrically“hot spots” of positive or negative charge Van der Waals interactions: attractions between molecules that are close together as a result of these charges Together can be strong, as Ex: between molecules of a gecko’s toe hairs and a wall surface Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings + Molecular Shape and Function Shape = Function Shape determined by positions of atoms’ valence orbitals In covalent bond s and p orbitals may hybridize specific molecular shapes Biological molecules recognize and interact based on molecular shape Similar shapes = similar biological effects Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings + Concept 2.4: Chemical reactions make and break chemical bonds Chemical reactions: making and breaking of chemical bonds Reactants: starting molecules of chemical reaction Products: final molecules of a chemical reaction Photosynthesis IMPORTANT chemical reaction Sunlight powers the conversion of carbon dioxide + water glucose + oxygen 6 CO2 + 6 H20 → C6H12O6 + 6 O2 All chemical reactions reversible products of the forward reaction become reactants for the reverse reaction Chemical equilibrium reached when the forward and reverse reaction rates are equal Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings + You should now be able 1. Identify to: the four major elements Distinguish between the following pairs of terms: 2. 1. 2. 3. neutron and proton, atomic number and mass number, atomic weight and mass number Distinguish between and discuss the biological importance of the following: 3. 4. nonpolar covalent bonds, polar covalent bonds, ionic bonds, hydrogen bonds, and 5. van der Waals interactions 1. 2. 3. Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Fig. 2-UN3 + Nucleus Protons (+ charge) determine element Neutrons (no charge) determine isotope Electrons (– charge) form negative cloud and determine chemical behavior Atom Fig. 2-UN5 + Single covalent bond Double covalent bond Fig. 2-UN9 + Fig. 2-UN10 + Fig. 2-UN11 +