* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download PDF UNIT 2A Organic Chem. Intro
Survey
Document related concepts
Transcript
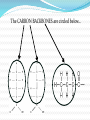
Today 1/21 Grab an iRemote Get your journal Turn in water lab and H2O R.A.F.T to basket In your journal for today, explain this picture using what we learned about properties of water yesterday: Quiz: Thurs 1/21 1. Your saliva measures 7 on the pH scale, it is a(n) ___ solution? a. acidic b. basic c. neutral 2. The ___ property of water helps in the formation of soil. a. capillary action b. expansion during freezing c. evaporative cooling 3. Laundry detergent typically has a pH of 3 which indicates a(n) ___ solution? a. acidic b. basic c. neutral 4. The use of ___ helps to maintain a certain pH in our bodies maintaining homeostasis. a. buffers b. bases c. acids 5. The ___ property of water removes heat helping to cool your skin through sweating. a. capillary action b. evaporation c. cohesion What are ORGANIC COMPOUNDS? Def: made by cells & contain CARBON. Inorganic= no carbon # of valence electrons in outer shell of electron cloud determines an elements chemical properties- how it reacts/behaves with other elements. Remember from Friday… Carbon can make 4 bonds Nitrogen can make 3 bonds Oxygen can make 2 bonds Hydrogen can make 1 bond Why is CARBON important? Carbon is a very diverse element because… It can make four bonds with other elements It can form single & double bonds It can have many types of arrangements (branched, unbranched, isomers) Carbon and other elements can bond together to make HYDROCARBONS… What are HYDROCARBONS? Def: Organic Compounds only made of H and C Usually has chains of C’s which form a CARBON BACKBONE. The CARBON BACKBONES are circled below… HYDROCARBONS can be: Branched Unbranched HYDROCARBONS can be: 1-Butene ISOMERS- have same formula but different structure. (Can you see why the first picture is 1-Butene and 2nd picture is 2Butene?) 2-Butene HYDROCARBONS can: Form ring structures Hydrocarbons are the “backbone” of most molecules. The thing that makes a molecule different is its FUNCTIONAL GROUP This is what gives the molecule its individualized properties. This is the hydrocarbon Think about it this way… The hydrocarbon is like your last name- most everyone in your immediate family shares the same last name but it’s your first name that makes you different. So your first name is like the functional group. It’s what makes the molecule unique. “backbone” This is a functional group that makes it an individual What are FUNCTIONAL GROUPS? Def: groups of atoms that participate in chemical reactions 4 important functional groups: 1. HYDROXYL GROUP O-H Oxygen end bonds to carbon skeleton Called Alcohols Found in sugars; water-soluble vitamins 2. CARBONYL GROUP Formaldehyde C=O Found in sugars Two kinds: ALDEHYDE carbonyl group is at end of chain Ex: formaldehyde KETONE carbonyl group is within the chain Ex: ketones in urine Acetone 3. CARBOXYL GROUP C=OOH Acts as acid donating H+ to solutions. Called carboxylic acids Found in amino acids, fatty acids, proteins, vitamins. Acetic Acid 4. AMINO GROUP NH2 Acts as a base picking up H+ from a solution. Called amines Found in amino acids, proteins, urea The CARBON BACKBONES and the FUNCTIONAL GROUPS combine to make MACROMOLECULES… PROTEIN LIPIDS NUCLEIC ACID CARBOHYDRATES What are MACROMOLECULES? Def: large, biological molecules that are used to build new cell parts, secretions, etc. Four types: Proteins- made up of amino acids Carbohydrates (a.k.a. Polysaccharides)- made up of monosaccharides Nucleic acids- made up of nucleotides Lipids- made up of glycerol and fatty acids WHAT DO ALL FOUR TYPES OF MACROMOLECULES HAVE IN COMMON? All are “made up” of something. Cells make macromolecules by joining long chains of organic molecules together to form POLYMERS. The subunits that make up polymers are called MONOMERS. The arrangement of and joining of monomers creates trillions of different types polymers. This entire chain is called a POLYMER… one piece (circled) is a MONOMER. ANALOGIES My colorful plastic baby toy on my desk is a representation of a polymer. Each individual toy would be a monomer subunit. Create your own analogy for a polymer and monomer. How are polymers created in the cell? Cells JOIN monomers to create polymers by dehydration synthesis. All unlinked monomers have H atoms at one end and hydroxyl groups (OH) at other end. When a monomer is added… an H and OH is removed. This creates a molecule of H2O (water) a covalent bond is formed btwn molecules. (glucose & fructose) New polymer is created (sucrose) Dehydration Synthesis How are monomers created in the cell? Cells BREAK polymers apart into monomers by hydrolysis. (How food is digested in your stomach) Cells break bonds by adding H2O to them. When a polymer is broken… Water is split into an OH group and H group OH group is added to one monomer & H is added to the other the covalent bond is broken (btween sucrose) 2 new products are formed (glucose & fructose) Hydrolysis Quiz A __ reaction involves the addition of water. a. dehydration b. hydrolysis c. polar 2. A polymer is made up of ___. a. monomers b. trionomers c. pentonomers 3. The ___ group is made up of OH. a. carbonyl b. carboxyl c. hydroxyl 4. The ___ group is made up of COOH. a. carbonyl b. carboxyl c. hydroxyl 5. When compounds have the same formula, but different structures they are ___. a. clones b. isomers c. isotopes 1.