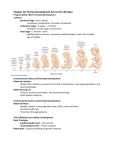
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Long-term pathological consequences of prenatal infection: beyond
Common cold wikipedia , lookup
Immune system wikipedia , lookup
Social immunity wikipedia , lookup
Inflammation wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Hepatitis B wikipedia , lookup
Innate immune system wikipedia , lookup
Sociality and disease transmission wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Infection control wikipedia , lookup
Neonatal infection wikipedia , lookup
Am J Physiol Regul Integr Comp Physiol 309: R1–R12, 2015. First published April 29, 2015; doi:10.1152/ajpregu.00087.2015. Review Long-term pathological consequences of prenatal infection: beyond brain disorders Marie A. Labouesse, Wolfgang Langhans, and Urs Meyer Physiology and Behavior Laboratory, ETH Zurich, Switzerland Submitted 6 March 2015; accepted in final form 21 April 2015 autism; cytokines; gut microbiota; maternal immune activation; metabolic syndrome; schizophrenia PRENATAL EXPOSURE TO INFECTIOUS pathogens or inflammatory stimuli is increasingly recognized to play an important etiological role in neuropsychiatric and neurological disorders with neurodevelopmental components (15, 21, 93, 99, 100). Significant associations between prenatal infection during pregnancy and increased disease risk in later life have been revealed for various brain disorders, including schizophrenia (19), autism (7, 117), bipolar disorder (22, 116), mental retardation (61), and cerebral palsy (31, 50). Hence, prenatal exposure to immune challenges may be best viewed as a general vulnerability factor for neurodevelopmental brain disorders rather than a disease-specific risk factor (52, 100). In this sense, the adverse effects induced by prenatal infection may reflect an early entry into a deviant neurodevelopmental route, but the specificity of subsequent disease or symptoms is likely to be influenced by the genetic and environmental context in which the prenatal infectious process occurs. This concept would, indeed, be consistent with the emerging evidence suggesting that seemingly remote disorders, such as schizophrenia, autism, attention-deficit/hyperactivity disorder, and major depression share considerable amounts of risk factors and brain dysfunctions (23, 92, 138). The presence of shared genetic and environ- Address for reprint requests and other correspondence: M. A. Labouesse, Physiology and Behavior Lab., Schorenstrasse 16, 8603 Schwerzenbach, Switzerland (e-mail: [email protected]). http://www.ajpregu.org mental risks among those illnesses has led to the proposal that they might lie along a continuum of genetically and environmentally induced neurodevelopmental causalities (103, 113), wherein prenatal infection may be one of the many factors that shape the eventual pathological outcomes. While the importance of prenatal immunological adversities has been widely acknowledged in the fields of developmental neuropsychiatry and neurology, less attention has been paid to the possibility that prenatal exposure to infection may also play an etiological role beyond central nervous system (CNS) disorders. Hence, the possible long-term effects of prenatal infection on disorders that are not primarily associated with CNS dysfunctions may be somewhat underestimated. This seems surprising for various reasons. The prenatal period is not only a highly sensitive period for early brain development (99), but also for other biological systems that develop in utero, including the innate and adaptive immune systems (47, 70), the cardiovascular system (46, 60), the renal system (55, 80), adipose tissue (140), and skeletal bones and muscles (20, 68). It is, in fact, rather unlikely that maternal infection during pregnancy would specifically disrupt fetal brain development; instead, it may more generally represent a developmental stressor for the entire organism. The underlying assumption for this hypothesis is that infection leads to the secretion and circulation of various immune system-related factors in the maternal host and fetal environment, which, in turn, are likely 0363-6119/15 Copyright © 2015 the American Physiological Society R1 Downloaded from http://ajpregu.physiology.org/ by 10.220.32.246 on June 17, 2017 Labouesse MA, Langhans W, Meyer U. Long-term pathological consequences of prenatal infection: beyond brain disorders. Am J Physiol Regul Integr Comp Physiol 309: R1–R12, 2015. First published April 29, 2015; doi:10.1152/ajpregu.00087.2015.—Prenatal immunological adversities such as maternal infection have been widely acknowledged to contribute to an increased risk of neurodevelopmental brain disorders. In recent years, epidemiological and experimental evidence has accumulated to suggest that prenatal exposure to immune challenges can also negatively affect various physiological and metabolic functions beyond those typically associated with primary defects in CNS development. These peripheral changes include excessive accumulation of adipose tissue and increased body weight, impaired glycemic regulation and insulin resistance, altered myeloid lineage development, increased gut permeability, hyperpurinergia, and changes in microbiota composition. Experimental work in animal models further suggests that at least some of these peripheral abnormalities could directly contribute to CNS dysfunctions, so that normalization of peripheral pathologies could lead to an amelioration of behavioral deficits. Hence, seemingly unrelated central and peripheral effects of prenatal infection could represent interrelated pathological entities that emerge in response to a common developmental stressor. Targeting peripheral abnormalities may thus represent a valuable strategy to improve the wide spectrum of behavioral abnormalities that can emerge in subjects with prenatal infection histories. Review R2 PERIPHERAL EFFECTS OF PRENATAL INFECTION Obesity and Metabolic Dysfunctions Epidemiological investigations in humans and experimental work in animals both emphasize a critical role of the early-life environment in shaping postnatal metabolic functions. Stimulated by the seminal work of David Barker and colleagues, the concept of “early-life priming of adult disease” is now widely accepted for various adverse health outcomes (37). In the context of obesity and metabolic disorders, this concept refers to the phenomenon that exposure to a specific environmental factor during early prenatal or perinatal periods can induce lifelong changes in metabolic functions, thereby predisposing the organism to excessive adiposity and associated pathophysiological conditions, including impaired glucose homeostasis, insulin resistance, Type 2 diabetes, and cardiovascular disease (81, 89). One of the most noticeable early-life adversities precipitating such metabolic dysfunctions is maternal obesity during pregnancy (54, 141). Indeed, a robust association between prenatal maternal obesity and an enhanced risk for metabolic disorder in the offspring has been established by a plethora of human epidemiological studies and translational animal models (41, 77). Because obesity is accompanied by chronic low-grade inflammation (105, 131), it has been suggested that enhanced systemic and placental inflammation in obese mothers may represent one of the mediating factors underlying the developmental disruption of metabolic functions in the offspring (121, 127). Only relatively recently, however, epidemiologists have begun to explore more directly whether discrete maternal exposure to infectious or inflammatory stimuli is similarly associated with increased risk for metabolic disorder in the offspring. A first line of evidence supporting this hypothesis comes from a human epidemiological study that used a cross-sectional cohort design, in which more than 17,000 male singletons were included (25). The findings from this study suggested a 34% increased risk of obesity [defined on the basis of a body mass index (BMI) of 30 kg/m2 or more] after being born to a mother with infection during pregnancy (25). Interestingly, a similar positive association has also been found following childhood infections (25), suggesting that the developmental period for infection-induced changes in adiposity extends to the early postnatal life. Work in developmental rodent models further supports the hypothesis of causal effects between maternal immune challenge during pregnancy and long-term metabolic dysfunctions in the offspring. For example, it has been shown that maternal treatment with the bacterial endotoxin LPS during midpregnancy in rats induces a wide spectrum of metabolic disturbances in the adult offspring, including increases in body weight and adipose tissue, hyperphagia, and insulin resistance (111). These effects were sex-specific and emerged in male but not female rats, indicating that male offspring are more vulnerable than females in terms of developing metabolic abnormalities following maternal endotoxemia during pregnancy. Findings from other rodent models of maternal immune activation further suggest that the association between prenatal immune challenge during pregnancy and development of metabolic disturbances is not dependent on the precise identity of the infectious or inflammatory pathogen. In fact, such metabolic effects can even be induced by prenatal exposure to specific inflammatory cytokines (29). Moreover, long-term metabolic abnormalities have also been observed in mouse offspring of mothers that were exposed to the viral mimetic polyriboinosinic-polyribocytidilic acid (polyI:C), a synthetic analog of double-stranded RNA that induces a virus-like acute phase response (93). In this virus-like immune activation model, offspring of polyI:C-treated mouse dams were shown to develop altered glycemic regulation and abnormal AJP-Regul Integr Comp Physiol • doi:10.1152/ajpregu.00087.2015 • www.ajpregu.org Downloaded from http://ajpregu.physiology.org/ by 10.220.32.246 on June 17, 2017 to affect peripheral organs and brain structures in the developing organism to the same extent. Another reason why we should not ignore CNS-remote effects of prenatal infection relates to the clinical observations that individuals with major psychiatric disorders often display signs of physiological and metabolic dysfunctions, including insulin resistance, Type 2 diabetes, obesity, and cardiovascular disease (90). Even though chronic psychopharmacotherapy may facilitate or even induce such abnormalities (40, 139), it is becoming increasingly evident that they cannot solely be accounted for by chronic drug exposure. Indeed, certain metabolic and physiological dysfunctions often occur (albeit in a somewhat less severe form) in drug-naïve or minimally medicated first-episode patients with mental illnesses (63, 124, 143, 153). These findings have been taken as circumstantial evidence that at least some of the metabolic and pathophysiological comorbidities in major brain disorders may have a developmental origin and, thus, emerge prior to the onset of fullblown psychiatric illness. Finally, neurodevelopmentally acquired brain abnormalities in response to maternal infection may also facilitate the development of metabolic and physiological dysfunctions across the postnatal life span. For example, the development of homeostatic brain regions, such as the hypothalamus, encompasses a number of steps that begin early in fetal life and continue postnatally (135). Infection-induced maldevelopment of such brain regions could thus impair the homeostatic control of various physiological functions, including food intake, renal functions, and reproduction (81, 89). Here, we review the existing evidence indicating that the long-term pathological consequences of prenatal immune challenges are not solely confined to primary CNS disorders. Instead, they extend more globally to other physiological and metabolic dysfunctions in peripheral systems. Relevant studies were identified and selected using PubMed. All articles published before December 2014 were screened for relevance and were searched using the following search criteria (in alphabetical order) in various combinations: “animal model,” “autism,” “bipolar disorder,” “body weight,” “cytokines,” “diabetes,” “dopamine,” “epidemiology,” “food intake,” “glucose,” “gut,” “homeostasis,” “hypothalamus,” “immune activation,” “infection,” “inflammation,” “LPS,” “maternal,” “metabolism,” “metabolic syndrome,” “microbiota,” “microglia,” “obesity,” “poly(I:C),” “pregnancy,” “prenatal,” and “schizophrenia”. In addition to reviewing the available literature, we also provide a conceptual framework suggesting that several apparently unrelated central and peripheral effects of prenatal infection may not represent separate pathological entities but could, in fact, result from interrelated pathological mechanisms that are engaged in response to a common developmental stressor. Review PERIPHERAL EFFECTS OF PRENATAL INFECTION Peripheral (and Central) Inflammation Various rodent models demonstrate that maternal exposure to infectious or immune system-activating agents leads to robust post-acute immune changes at the maternal-fetal interface, including the placenta, amniotic fluid, and fetal organism (2, 6, 96, 97, 151, 154). The nature and/or severity of these changes are influenced by various factors, most notably the identity and/or intensity of the pathogen (51, 94), the gestational timing of exposure (97), and the genetic background of the infected host (2, 96, 154). Despite this, it seems that maternal exposure to distinct infectious or immune system-activating agents leads to partially overlapping immune responses in the fetal system. These overlapping effects are mostly characterized by increased fetal expression of inflammatory factors, such as proinflammatory cytokines and chemokines (5). It is believed that abnormal expression of inflammatory factors in the fetal brain contribute to, or even mediate, abnormal brain and behavioral development following prenatal exposure to infection (79, 136). Indeed, as reviewed extensively elsewhere (95, 102), acute inflammation during early fetal brain development may negatively affect ongoing neurodevelopmental processes, such as neuronal/glial cell differentiation, proliferation, migration, and survival, and, thus, predispose the developing offspring to long-term brain and behavioral dysfunctions. Since signs of subchronic systemic (and central) inflammation are often present in at least a subset of patients with neurodevelopmental disorders (64, 86, 92, 101, 150), considerable research has been undertaken to address the question of whether maternal exposure to infectious or inflammatory agents may lead to persistent inflammatory changes in the offspring postnatally. The evidence for this hypothesis is, at best, equivocal. Some studies using rodent models of bacterial or viral maternal immune challenge have reported a significant upregulation of circulating inflammatory factors such as proinflammatory cytokines or chemokines in the juvenile or adult offspring (17, 43, 69). Other studies using the same animal models, however, failed to find clear signs of systemic inflammation in juvenile or adult offspring born to immunologically challenged mothers (96, 157), or they even reported opposite effects that were characterized by blunted systemic inflammatory activity following prenatal infection (115). Inconclusive data also exist with respect to central inflammation. In the CNS, microglia and astrocytes are the major immunocompetent cells, which drive both the induction and limitation of inflammatory processes (122). This is achieved through the synthesis of proinflammatory and anti-inflammatory cytokines, upregulation, or downregulation of various cell surface receptors, such as pathogen recognition receptors, cytokine receptors, and numerous receptors crucial for antigen presentation. Enhanced microglia activation in brain parenchyma, along with increased central production of secreted inflammatory factors, is often taken as a sign of ongoing inflammation in the CNS (48). The possibility that prenatal infection leads to chronic signs of brain inflammation has been supported only by some studies in rats and mice (17, 69), whereas other rodent studies failed to find evidence for such neuroinflammatory processes extending into neonatal or adult life (5, 43, 96, 119, 151, 157). The inconsistency surrounding the long-term effects of prenatal infection on the persistence of inflammatory processes across the postnatal life span may be explained by various factors, most notably the severity and/or chronicity of the infectious process targeting the maternal host. Indeed, it appears that more marked postnatal inflammatory changes are seen following relatively severe forms of maternal immune challenge, such as chronic exposure to immune system-activating agents throughout the entire gestational period (17). In contrast, acute or subchronic prenatal exposure to immune system-activating stimuli in mice and rats appears to be largely devoid of systemic and central inflammatory effects persisting into the juvenile or adult period (5, 43, 96, 119, 151, 157). The latter may not seem surprising because inflammation is typically counteracted by homeostatic processes that mount antiinflammatory and/or immunosuppressive responses upon the induction of inflammation (132), which, in turn, dampen and finally resolve the post-acute fetal inflammatory responses to maternal immune activation (91). Still, the developing organism might sustain latent inflammatory abnormalities that may not become apparent until reexposure to specific immunogens postnatally. In support of this hypothesis, it has been shown that prenatal virus-like immune activation in mice leads to a persistent decrease in the number of regulatory T cells (Tregs) in the spleen and mesenteric lymph nodes (58). Besides other functions, Tregs can potently suppress innate and adaptive immune responses, so that a disruption of normal Tregs functions can lead to exacerbated inflammatory reactions (24). Such an exacerbation is seen in mouse offspring born to immunologically challenged mothers, which mount a potentiated proinflammatory T-cell response to immunogenic T-cell stimulation (58). Prenatal virus-like immune activation in mice has further been shown to induce long-term changes in macrophage function that persist into adulthood. More specifically, bone marrow-derived macrophages of prenatally poly(I:C)-exposed mice show an augmented proinflammatory cytokine response to in vitro LPS stimulation alone, or in combination with interferon (IFN)-␥ (112). The costimulation with LPS and IL-4 does not result in a similar proinflammatory cytokine signature (112). Collectively, these results indicate that prenatal viruslike immune activation potentiates the polarization of macrophages toward an M1 phenotype, which, in turn, is typically associated with larger production of proinflammatory cytokines, such as IL-12, at the expense of reduced production of anti-inflammatory cytokines, such as IL-10 (85, 106). Thus, it AJP-Regul Integr Comp Physiol • doi:10.1152/ajpregu.00087.2015 • www.ajpregu.org Downloaded from http://ajpregu.physiology.org/ by 10.220.32.246 on June 17, 2017 ingestive behavior in adolescence and excess fat deposition in adulthood (115). Similar results were obtained in a mouse model, in which pregnant mice were infected with Ljungan virus (LV), a virus that belongs to the Picornavirus family and is virulent for laboratory rodents (126). Maternal LV exposure in mice has been shown to predispose the offspring to signs of Type 2 diabetes and obesity (110). These effects, however, were particularly manifest in LV-exposed mice that experienced additional stress during adolescence, suggesting that the severity of metabolic abnormalities following prenatal immune activation can be exacerbated by exposure to other environmental adversities, such as adolescent stress (110). These findings are particularly relevant for the multifactorial etiology of obesity and Type 2 diabetes, which are likely caused by a combination of environmental (and genetic) factors (38, 81). R3 Review R4 PERIPHERAL EFFECTS OF PRENATAL INFECTION Gastrointestinal Abnormalities and Dysbiosis In recent years, there has been an increasing interest in the relative potential of gastrointestinal (GI) functions to modulate neuropsychological traits implicated in psychiatric disorders (28, 87, 88). Epidemiological and clinical studies have repeatedly demonstrated a high comorbidity between GI inflammatory disorders and stress-related psychiatric symptoms, such as anxiety or depressive behavior (28, 36). These findings have been complemented by experimental studies in rodents showing that abnormal development of the gut microbiota leads to neuroendocrine, neurochemical, and emotional abnormalities, some of which are reminiscent of hormonal and behavioral aberrations typically seen in depression and/or anxiety disorders. For example, ablation of the commensal GI microbiota in germ-free mice or by chronic antimicrobial compounds can lead to functional changes in the hypothalamus-pituitary-adrenal (HPA) axis and in the central dopaminergic, serotonergic, and GABAergic neurotransmitter systems, and these effects are further linked to the emergence of increased anxiety-like behavior (11, 35). Other clinical populations, for which GI abnormalities and dysbiosis seem clinically relevant, comprise individuals suffering from neurodevelopmental psychiatric illnesses. Indeed, in addition to the pathological symptoms traditionally attributed to CNS dysfunctions, neurodevelopmental psychiatric illnesses, such as autism and schizophrenia, are also associated with a number of GI dysfunctions. Such abnormalities include chronic intestinal low-grade inflammation, increased intestinal permeability (“leaky gut”), allergic reactions to dietary proteins, diarrhea, gastric dysmobility, and alterations in gut microbiota (16, 26, 133, 136). By further undermining general physical health and daily life quality, GI distress induces an additional clinical burden to patients suffering from psychiatric disorders (9, 53). In view of the etiological contribution of prenatal infection to neurodevelopmental diseases, experimental research has begun to explore whether early-life immune challenges may be a relevant environmental risk factor for the development of GI abnormalities. In a seminal recent study, Hsiao et al. (58) demonstrated that prenatal polyI:C-induced immune activation in mice can indeed cause long-term defects in intestinal integrity and alterations in the composition of the commensal microbiota. The former effect was evident by increased translocation of dextran across the intestinal epithelium and was associated with decreased colonic expression of tight junction components. Interestingly, signs of intestinal disintegrity in polyI:C-exposed mouse offspring were already present by the age of 3 wk, suggesting that these abnormalities are established during early life. Furthermore, the colons from prenatally infected mice displayed increased expression of the inflammatory cytokine IL-6, which is in line with the notion that increased gut permeability is commonly associated with altered immune responses in the GI tract (148). Offspring of immunologically exposed mouse dams also markedly differed from control offspring in terms of their gut microbiota composition, and this difference was mostly accounted for by differences in the relative abundance of Clostridia and Bacteroidia classes (58). The study by Hsiao et al. (58), which used a mouse model of virus-like immune activation, is, thus far, the only one that directly assessed the influence of prenatal immune challenge on the development of GI abnormalities in the offspring. Hence, it remains essentially unknown whether similar effects can also be induced by prenatal exposure to other infectious or inflammatory agents. It should be noted, however, that remarkable changes in the gut microbiota composition and associated intestinal abnormalities have also been identified in another experimental model of neurodevelopmental disease, namely prenatal exposure to valproic acid (VPA) in mice (34). Similar to the polyI:C model (83), the VPA model is frequently used to induce autism-related brain and behavioral abnormalities in experimental rodents (123). Thus, it appears that prenatal exposure to distinct environmental factors does not only induce overlapping neurobehavioral phenotypes, but further induce similar changes in GI functions and microbiota. It remains currently unknown whether the latter commonalities may be explained by mutual pathogenic mechanisms, whereby neurodevelopmentally acquired abnormalities could increase the vulnerability to GI pathology through mechanisms involving a AJP-Regul Integr Comp Physiol • doi:10.1152/ajpregu.00087.2015 • www.ajpregu.org Downloaded from http://ajpregu.physiology.org/ by 10.220.32.246 on June 17, 2017 seems that prenatal immune activation can prime the offspring’s peripheral immune system in such a way that it mounts more excessive proinflammatory responses in the event of postnatal pathogen (re-)exposures. It should also be pointed out that prenatal immune activation can similarly lead to latent immune abnormalities in the CNS. As reviewed in detail elsewhere (14, 50), numerous experimental studies in rodents show that immunological exposure in early (prenatal or neonatal) life can cause the organism to mount differential (and often more vigorous) CNS inflammatory responses to subsequent immunological or nonimmunological challenges. For example, even though prenatal virus-like immune activation per se does not cause overt glial abnormalities and associated inflammatory changes in the brain parenchyma of mouse offspring (5, 43, 157), signs of microglia overactivation and exacerbated inflammatory cytokine secretion in CNS areas can be induced in these offspring when they are additionally exposed to subchronic stress postnatally (44). This form of immune sensitization or priming suggests that prenatal immune activation can markedly increase the vulnerability of the offspring to brain immune changes in response to stress. A similar microglia-priming effect by prenatal immune challenge has been demonstrated in a virus-like immune activation model, in which mouse offspring were subjected to either prenatal poly(I:C) treatment alone, or in combination with a second poly(I:C) treatment regimen in late adulthood (69). In this model, mice exposed to both prenatal and postnatal poly(I:C) treatment showed more extensive microglia activation and astrogliosis compared with either treatment alone (69). Taken together, even though the long-term effects of prenatal immune activation on peripheral and central inflammatory responses may not be apparent under basal conditions, they may become so when the offspring are exposed to additional environmental challenges, such as stress or acute infection during the postnatal life. In addition, persistent inflammatory abnormalities may develop more locally and may thus emerge in specific peripheral organs. The latter possibility is discussed in more detail in the subsequent section. Review PERIPHERAL EFFECTS OF PRENATAL INFECTION R5 disruption of the normal top-down (CNS to GI tract) signaling. This possibility is discussed in more detail below. Do Peripheral Abnormalities Contribute to Neurobehavioral Deficits Following Prenatal Infection? Fig. 1. Graphical illustration of main top-down [central nervous system (CNS) to periphery] and bottom-up (periphery to CNS) communication pathways and summary of peripheral and central pathologies emerging following prenatal infection. Top-down and bottom-up communication pathways are represented by solid and dashed lines, respectively. CNS, central nervous system; CORT, cortisol/corticosterone; NA, noradrenaline; Treg, regulatory T cells. (58). On the basis of their subsequent findings showing that prenatal virus-like immune challenge in mice leads to impaired GI functions and dysbiosis, Hsiao et al. (59) went on to show that oral treatment with the human commensal Bacteroides fragilis normalizes gut permeability and microbial composition and further corrects autism-related behavioral deficits in mouse offspring born to immunologically challenged mothers. Together, these findings support the hypothesis that gut- and immune system-related peripheral abnormalities following prenatal infection have a direct impact on CNS functions, so that normalization of the former leads to beneficial effects on the latter. A similar conclusion can be drawn from a recent investigation in mice demonstrating a link between prenatal virus-like immune activation and the emergence of altered purine metabolism in plasma (108). Besides other metabolic changes, offspring of immunologically challenged mouse dams showed marked signs of hyperpurinergia, a pathophysiological condition that is characterized by increased (plasma) levels of purines. Importantly, Naviaux et al. (108) showed that an antipurinergic therapy using acute administration of suramin not only normalizes the peripheral metabolic abnormalities in prenatally infected mice, but further corrects several of the behavioral deficits typically observed in untreated offspring. In AJP-Regul Integr Comp Physiol • doi:10.1152/ajpregu.00087.2015 • www.ajpregu.org Downloaded from http://ajpregu.physiology.org/ by 10.220.32.246 on June 17, 2017 Even though the evidence for altered peripheral functions in prenatally infected subjects is emerging, it remains largely elusive whether these abnormalities functionally contribute to the manifestation of neurobehavioral deficits. In other words, do peripheral and central abnormalities, which can both be precipitated by early-life adversities such as prenatal infection, represent separate pathological entities? Or is there a pathological connection between the two, so that functional abnormalities in the former can lead to pathological changes in the latter? It is obvious that the latter scenario would require bottom-up (periphery to CNS) and top-down (CNS to periphery) pathways that allow a bidirectional communication between the brain and the peripheral systems. As extensively reviewed elsewhere (12, 27, 28, 88), such pathways, indeed, exist and are evolutionarily well conserved across species. In fact, this bidirectional communication between the CNS and periphery is pivotal for body homeostasis and adaptive responses to changes in environmental conditions. Various peripheral signals such as GI peptides, cytokines, neuroactive bacterial metabolites, and adipose-derived factors can be relayed to the CNS via multiple routes, including the vagal afferents and the blood circulation (12, 27, 28, 88). The latter allows transport of systemic signals into the CNS through specialized transport systems embedded in the blood-brain barrier (BBB), or at sites lacking a complete BBB, such as the circumventricular organs. Afferent vagal nerve fibers are the main neuronal pathways through which peripheral signals can be conveyed to the brain (Fig. 1). Vagal afferent neurons synapse bilaterally on the nucleus tractus solitarii, from where neuronal signals are transmitted to other brain stem nuclei and to various forebrain structures, such as the hypothalamus, nucleus accumbens, amygdala, and prefrontal cortex (12). On the other hand, the periphery is connected with and modulated by the CNS through neuronal efferent branches of the sympathetic and parasympathetic nervous systems, which regulate visceral functions, both at rest and in response to activating stimuli (134). The periphery is further regulated by factors secreted by various neuroendocrine systems, such as the HPA axis, which has a central role in regulating many homeostatic processes, particularly in response to stress-related stimuli (10). In view of these elaborated bidirectional communication pathways, it may not be surprising that interventions directed at peripheral targets have the potential to ameliorate neurobehavioral deficits in offspring exposed to prenatal infection. A proof of concept for this notion has recently been realized using prenatal virus-like immune activation models in mice. In a first study, Hsiao et al. (58) demonstrated that autism-like behavioral abnormalities, which typically emerge in prenatally polyI:Cinfected mouse offspring, can be rescued by transplantation of bone marrow derived from control offspring. This elegant rescue experiment was conducted on the basis of the aforementioned findings showing that prenatal virus-like immune activation in mice impairs development and functions of Tregs, and further alters the differentiation of the myeloid cell lineage Review R6 PERIPHERAL EFFECTS OF PRENATAL INFECTION Do Neurodevelopmental Deficits Contribute to Peripheral Abnormalities Following Prenatal Infection? While a direct influence of peripheral abnormalities to CNS dysfunctions is supported by at least some animal studies, it remains essentially unknown whether the opposite scenario holds true as well. Hence, the role of neurodevelopmentally acquired CNS dysfunctions in promoting peripheral abnormalities following prenatal infection still awaits thorough examination. In view of the elaborated top-down (CNS to periphery) communication pathways (Fig. 1), however, it seems feasible that abnormal functioning of discrete neuronal and neuroendocrine systems can, at least to some extent, facilitate the development and/or maintenance of peripheral abnormalities. Perhaps one of the most obvious examples in this context relates to the crucial role of the hypothalamus and interconnected structures in controlling food intake and energy balance (104, 129). Given that maternal immune activation alters hypothalamic functions in rodent offspring (42, 78, 158), neurodevelopmentally acquired abnormalities in the hypothalamus may contribute to the emergence of a hyperphagic phenotype in offspring with prenatal infection (111, 115). An alternative (but not mutually exclusive) mechanism underlying such changes in food intake may be related to changes in the mesocorticolimbic dopamine system. A plethora of investigations support a key role of dopamine in reward and incentive values on the one hand, and in the associations between reward and eating behavior on the other hand. These functional associations are highly complex and likely involve intricate interactions among homeostatic, hedonic, motivational, and associative processes (62, 104, 125). Prenatal immune activation in rats and mice is well known to induce primary defects in early dopaminergic development (91, 155) and to lead to long-term dopaminergic abnormalities in the adult offspring (4, 98, 114, 155, 160). It seems, therefore, plausible that such dopaminergic dysfunctions may be involved in the development of altered food intake and energy balance, be it because of changes in homeostatic, hedonic, motivational, and/or associative processes. Another possible association that warrants careful examination in future studies is the potential impact of altered autonomic nervous system (ANS) functions in prenatally infected animals. Initial evidence suggests that prenatal immune activation can cause hyperactivity of the sympathetic nervous system, perhaps at the expense of diminished parasympathetic nervous system functions. Hyperactivity of the sympathetic nervous system typically leads to increased secretion of noradrenaline and corticosterone, both of which have been observed in mouse offspring that were exposed to prenatal immune challenge (115, 158). Prolonged secretion of these stressrelated factors can negatively influence GI physiology, including gut motility, secretion, permeability, and composition of the gut microbiota (13, 67). Given that some of these GI abnormalities have been shown to develop in mice exposed to prenatal immune challenge (59), it would appear highly warranted to explore whether they may be causally related to a hyperactivity of the sympathetic nervous system. At the same time, diminished activity of the parasympathetic branch of the ANS may contribute to the development of altered inflammatory responses in the GI tract and other peripheral organs (120). Indeed, efferent vagal pathways originating from the dorsal motor nucleus of the vagus possess anti-inflammatory activity by releasing ACh, which, in turn, inhibits proinflammatory cytokine secretion by peripheral immune cells upon binding to ␣7 nicotinic receptors (118, 145). This parasympathetic mechanism of neuronal anti-inflammatory signaling has been termed “the cholinergic antiinflammatory pathway” and seems to play an important role in dampening peripheral inflammatory responses (118, 145). Whether this pathway is altered by prenatal exposure to infection still awaits direct examination, but it could provide a contributing factor for the prenatal infection-induced disturbances in local inflammatory responses, including intestinal inflammation (59). Possible Mechanisms Mediating the Effects of Maternal Infection on the Offspring The precise mechanisms responsible for mediating the pathological effects of maternal infection on the developing organism in utero remain to be determined. In fact, several plausible mechanisms exist, whereby maternal infection can negatively affect the normal development of peripheral (and central) organs. As summarized in Fig. 2, the different pathological mechanisms induced by infection may not be mutually exclusive, but may rather interact with each other to affect various developmental processes that take place in prenatal life. One prevalent hypothesis suggests that common immunological factors, in general, and inflammatory cytokines, in particular, are key mediating factors changing developmental trajectories in the offspring (4, 45, 79, 96, 102, 137). Inflammatory cytokines are typically induced during the acute phase response to infection (146, 147) and may represent a major developmental stressor for the organism (18, 57, 95). In the event of maternal infection, an increase in fetal cytokine levels may be caused by transplacental transfer of maternally pro- AJP-Regul Integr Comp Physiol • doi:10.1152/ajpregu.00087.2015 • www.ajpregu.org Downloaded from http://ajpregu.physiology.org/ by 10.220.32.246 on June 17, 2017 addition to immunological and gut-related factors (58, 59), alterations in blood purine metabolism, thus, seem to be another peripheral pathology that readily contributes to the emergence of behavioral deficits following prenatal infection (108). Thus far, it remains unknown whether other metabolic disturbances that are induced by prenatal infection, including alterations in glycemic regulation, insulin insensitivity, and increased adiposity, may also directly influence behavioral functions in subjects with a history of prenatal infection. Hence, it remains to be determined whether normalization of these metabolic abnormalities may exert beneficial effects on behavioral deficits in this population. Furthermore, the precise link between individual peripheral abnormalities also remains elusive. For example, it is not known whether the excessive accumulation of adipose tissue in offspring of infected mothers may facilitate excessive proinflammatory signaling. Such a link has been demonstrated in various models of obesity, but it still awaits direct verification in models of prenatal immune challenge. Given the emerging functional relationships between the gut microbiome and obesity and between the gut microbiome and Type 2 diabetes (144), it would also seem warranted to explore such possible connections in the context of pathologies that are associated with prenatal exposure to infection. Review PERIPHERAL EFFECTS OF PRENATAL INFECTION duced cytokines (30, 159), by placental production of cytokines (6, 56, 128), or by increased fetal cytokine synthesis (97). In addition to its effects on inflammatory cytokine secretion, infection and the subsequent induction of inflammatory responses are also strongly associated with numerous other pathophysiological effects, including oxidative stress. Oxidative stress is referred to as an imbalance between the production and elimination of reactive oxygen species (ROS), some of which are highly cytotoxic and promote tissue injury (66). Upon activation, innate immune cells secrete ROS and reactive nitrogen species (RNS) as a central part of killing invading pathogens (107). Production of ROS and RNS is, thus, an important downstream mechanism of inflammation-mediated immune responses. Several lines of evidence from rodent models suggest that infection-induced increases in fetal ROS and RNS levels may, indeed, be involved in changing development trajectories in the offspring (74, 75). Besides its effects on oxidative stress systems, exposure to infection and the subsequent cytokine-associated inflammatory reactions further lead to the activation of the HPA axis by stimulating the release of corticotropin-releasing factor from the hypothalamus and of adrenocorticotropic hormone from the pituitary gland; this results eventually in an increase of glucocorticoid levels in the peripheral bloodstream (49). The cytokine-mediated effects on glucocorticoid secretion may be of special interest because it has been suggested that prenatal physiological stress triggered by high glucocorticoid levels can interfere with normal physiological and metabolic development (130, 156). Activation of the innate immune system (in response to infection) also changes the maternal and fetal availability of several micronutrients, including iron and zinc, both of which are highly important for the normal development of peripheral and central organs (3, 71, 72, 149). In the case of iron, it is well established that infection leads to a temporary depletion of iron in the infected host. This process is mediated to a great extent by the proinflammatory cytokines IL-1 and IL-6 (76, 109) and serves to reduce the availability of this essential nutrient to the invading pathogens as part of the host’s inherent defense system (65). As part of the acute-phase response to infection, proinflammatory cytokines also trigger the induction of the zinc-binding protein metallothionein (152). During the course of pregnancy, this process leads to maternal and fetal zinc deficiency, which has further been associated with teratogenicity and abnormal developmental processes in utero (33, 142). In addition to its effects on micronutrient availability, maternal infection during pregnancy may also impair the fetal supply for macronutrients. Indeed, it is well established that peripheral cytokine elevation in response to infection induces a set of behavioral and physiological changes collectively referred to as sickness behavior (32). Sickness behavior typically includes fever, malaise, and reduced exploratory, and social investigation, as well as decreased food and water intake, accompanied usually by weight loss. The effects of immune challenge on weight loss may be particularly relevant because prenatal malnutrition has also been implicated as a risk factor for various developmental disturbances (73, 84). Finally, it should also be noted that at least part of the changes to the microbiome that emerge following prenatal infection (59) may have an early prenatal origin. The conventional view is that microbial colonization begins at birth when the neonate is first exposed to the microbiome of the mother and the surrounding environment (39, 82), implying furthermore that the fetal environment is sterile and, therefore, lacks a microbiome before birth. This view has recently been challenged by findings showing that the human placenta is not sterile but, in fact, is colonized with nonpathogenic commensal microbiota (1). Perhaps even more important are the findings suggesting that the microbial composition of the human placenta can be modified by maternal infection during pregnancy, even if the infectious process takes place during the time of conception or in early gestation (1). One important implication from these recent findings is that modifications of the placental microbiome, be it as a result of maternal infection or by other environmental factors, can critically shape the development of the offspring’s microbiome and, thus, predispose the developing organism to dysbiosis and other microbiome-associated abnormalities. Defining the Next Steps Prenatal immunological adversities, such as maternal infection, have been widely acknowledged to contribute to an AJP-Regul Integr Comp Physiol • doi:10.1152/ajpregu.00087.2015 • www.ajpregu.org Downloaded from http://ajpregu.physiology.org/ by 10.220.32.246 on June 17, 2017 Fig. 2. Possible mechanisms mediating the pathological effects of maternal infection on the developing organism in utero. Maternal infection during pregnancy induces a number of pathophysiological responses in the maternal host, including production of soluble immune factors, such as cytokines, reactive oxygen species, and soluble endocrine factors, such as stress hormones. Some of these factors might cross the placental barrier and enter the fetal environment, thereby causing fetal inflammation and oxidative stress. Abnormal fetal expression of these factors might impair the normal development of peripheral and central organs by modifying the differentiation, proliferation, and/or migration of target cells. These processes may involve alterations in gene expression via epigenetic modifications. Maternal infection during pregnancy can further induce inflammatory response in the placenta and cause placental insufficiency, which, in turn can cause fetal hypoxemia. In addition, infection can cause (temporary) states of macronutrient and micronutrient deficiency, which limit the fetal availability of essential nutrients necessary for normal fetal development and growth. Finally, maternal infection during pregnancy can modify the microbial composition of the placenta, which might alter the development of the offspring’s microbiome and thus predispose the developing organism to dysbiosis and other microbiome-associated abnormalities. R7 Review R8 PERIPHERAL EFFECTS OF PRENATAL INFECTION Perspectives and Significance Several recent epidemiological and animal studies suggest that infection-related prenatal adversities can negatively affect various physiological and metabolic functions beyond those typically associated with primary defects in CNS development. These include excessive accumulation of adipose tissue and increased body weight, impaired glycemic regulation and insulin resistance, altered myeloid lineage development in favor of proinflammatory signaling, increased gut permeability, and changes in microbiota composition. The existing data suggest that these peripheral abnormalities can directly contribute to the emergence of CNS abnormalities, so that a normalization of peripheral symptoms effectively alleviates behavioral deficits induced by prenatal infection. Thus, it follows that seemingly unrelated central and peripheral effects of prenatal infection may not represent separate pathological entities, but rather, they may be considered as pieces of the same puzzle. In fact, there might be a “vicious circle” involving constant pathological interactions between developmentally acquired brain abnormalities and peripheral dysfunctions, in which deficient peripheral functions facilitate or maintain CNS abnormalities, and vice versa. Thus, targeting peripheral abnormalities may represent a valuable strategy to improve the multitude of behavioral abnormalities that can emerge in subjects with prenatal infectious histories. ACKNOWLEDGMENTS Related work by the authors has been supported by grants from the Swiss National Science Foundation (310030_146217; to U. Meyer) and from ETH Zurich, Switzerland (ETH Research Grant 25_13-2; to U. Meyer and W. Langhans). Present address: U. Meyer, Institute of Pharmacology and Toxicology, University of Zürich-Vetsuisse, Zürich, 8057 Switzerland. DISCLOSURES No conflicts of interest, financial or otherwise, are declared by the authors. AUTHOR CONTRIBUTIONS Author contributions: M.A.L. and U.M. prepared figures; M.A.L. and U.M. drafted manuscript; M.A.L., W.L., and U.M. edited and revised manuscript; M.A.L., W.L., and U.M. approved final version of manuscript. REFERENCES 1. Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med 6: 237ra65, 2014. 2. Abazyan B, Nomura J, Kannan G, Ishizuka K, Tamashiro KL, Nucifora F, Pogorelov V, Ladenheim B, Yang C, Krasnova IN, Cadet JL, Pardo C, Mori S, Kamiya A, Vogel MW, Sawa A, Ross CA, Pletnikov MV. Prenatal interaction of mutant DISC1 and immune activation produces adult psychopathology. Biol Psychiatry 68: 1172– 1181. 3. Aguilar-Valles A, Flores C, Luheshi GN. Prenatal inflammation-induced hypoferremia alters dopamine function in the adult offspring in rat: relevance for schizophrenia. PLoS One 5: e10967, 2010. 4. Aguilar-Valles A, Jung S, Poole S, Flores C, Luheshi GN. Leptin and interleukin-6 alter the function of mesolimbic dopamine neurons in a rodent model of prenatal inflammation. Psychoneuroendocrinology 37: 956 –969, 2012. 5. Arsenault D, St-Amour I, Cisbani G, Rousseau LS, Cicchetti F. The different effects of LPS and poly I:C prenatal immune challenges on the behavior, development and inflammatory responses in pregnant mice and their offspring. Brain Behav Immun 38: 77–90, 2014. 6. Ashdown H, Dumont Y, Ng M, Poole S, Boksa P, Luheshi GN. The role of cytokines in mediating effects of prenatal infection on the fetus: implications for schizophrenia. Mol Psychiatry 11: 47–55, 2006. 7. Atladóttir HO, Thorsen P, Østergaard L, Schendel DE, Lemcke S, Abdallah M, Parner ET. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord 40: 1423–1430, 2010. 8. Basu S, Haghiac M, Surace P, Challier JC, Guerre-Millo M, Singh K, Waters T, Minium J, Presley L, Catalano PM, Hauguel-de Mouzon S. Pregravid obesity associates with increased maternal endotoxemia and metabolic inflammation. Obesity (Silver Spring) 19: 476 –482, 2011. 9. Bauman ML. Medical comorbidities in autism: challenges to diagnosis and treatment. Neurotherapeutics 7: 320 –327, 2010. 10. Baumann N, Turpin JC. Neurochemistry of stress. An overview. Neurochem Res 35: 1875–1879, 2010. AJP-Regul Integr Comp Physiol • doi:10.1152/ajpregu.00087.2015 • www.ajpregu.org Downloaded from http://ajpregu.physiology.org/ by 10.220.32.246 on June 17, 2017 increased risk of neurodevelopmental brain disorders. Somewhat less attention has been given to the long-term consequences of prenatal infection on peripheral functions. Hence, additional work is clearly warranted to further define the putative effects of infection-related prenatal adversities on postnatal physiology and metabolism. What is perhaps most urgently needed to achieve this goal is more comprehensive evidence from human epidemiological studies. To our knowledge, only one epidemiological study has yet directly explored the putative association between maternal infection during pregnancy and increased risk of obesity in the offspring (25). Even though this study included a large number of subjects, it requires replication and further extension to other physiological and metabolic parameters. It should also be mentioned that exposures were broadly defined as “infections requiring hospitalization” in this first epidemiological study, so that the possible relevance of pathogen specificity remained unexplored. Hence, we do not know whether long-term metabolic abnormalities can similarly be induced by prenatal exposures to various infectious pathogens, or alternatively, whether this association is dependent on the precise identity of the pathogen. One powerful approach to overcome this limitation would be the use of prospective epidemiological designs, in which a specific infectious pathogen or inflammatory marker in prenatal life can be measured quantitatively. Such prospective epidemiological research has been proven indispensible for the establishment of associations between prenatal exposure to specific infectious pathogens and later risk of neuropsychiatric disease (19, 22, 116). Hence, the future identification of possible long-term effects of prenatal infection on general physiology and metabolism might strongly benefit from such prospective epidemiological research. For ethical and technical reasons, however, human epidemiological research will not be able to establish causality for such associations and will be limited in its capacity to unravel the downstream cellular and molecular mechanisms affecting normal physiological development. The examination of epidemiologically relevant risk factors in animal models, thus, remains an important field of research to overcome these limitations. As outlined above, several animal models of prenatal immune challenge exist, so that their continuous implementation will be pivotal to extend our knowledge on pathophysiological mechanisms underlying the association between prenatal infection and peripheral abnormalities. In these attempts, a special emphasis should be placed on elucidating causal (and bidirectional) relationships between peripheral abnormalities and neurobehavioral deficits. These latter investigations may help to establish holistic therapeutic interventions with beneficial effects on both central and peripheral dysfunctions. Review PERIPHERAL EFFECTS OF PRENATAL INFECTION 35. Diaz Heijtz R, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA 108: 3047–3052, 2011. 36. Dinan TG, Cryan JF. Melancholic microbes: a link between gut microbiota and depression? Neurogastroenterol Motil 25: 713–719. 37. Dover GJ. The Barker hypothesis: how pediatricans will diagnose and prevent common adult-onset diseases. Trans Am Clin Climatol Assoc 120: 199 –207, 2009. 38. Drong AW, Lindgren CM, McCarthy MI. The genetic and epigenetic basis of type 2 diabetes and obesity. Clin Pharmacol Ther 92: 707–715, 2012. 39. Fanaro S, Chierici R, Guerrini P, Vigi V. Intestinal microflora in early infancy: composition and development. Acta Paediatr Suppl 91: 48 –55, 2003. 40. Fernandez-Egea E, Miller B, Garcia-Rizo C, Bernardo M, Kirkpatrick B. Metabolic effects of olanzapine in patients with newly diagnosed psychosis. J Clin Psychopharmacol 31: 154 –159, 2011. 41. Freeman DJ. Effects of maternal obesity on fetal growth and body composition: implications for programming and future health. Semin Fetal Neonatal Med 15: 113–118, 2010. 42. Galvão MC, Chaves-Kirsten GP, Queiroz-Hazarbassanov N, Carvalho VM, Bernardi MM, Kirsten TB. Prenatal zinc reduces stress response in adult rat offspring exposed to lipopolysaccharide during gestation. Life Sci 120: 54 –60, 2015. 43. Garay PA, Hsiao EY, Patterson PH, McAllister AK. Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development. Brain Behav Immun 31: 54 –68, 2013. 44. Giovanoli S, Engler H, Engler A, Richetto J, Voget M, Willi R, Winter C, Riva MA, Mortensen PB, Feldon J, Schedlowski M, Meyer U. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science 339: 1095–1099, 2013. 45. Girard S, Tremblay L, Lepage M, Sébire G. IL-1 receptor antagonist protects against placental and neurodevelopmental defects induced by maternal inflammation. J Immunol 184: 3997–4005, 2010. 46. Gittenberger-de Groot AC, Bartelings MM, Poelmann RE, Haak MC, Jongbloed MR. Embryology of the heart and its impact on understanding fetal and neonatal heart disease. Semin Fetal Neonatal Med 18: 237–244, 2013. 47. Golub R, Cumano A. Embryonic hematopoiesis. Blood Cells Mol Dis 51: 226 –231, 2013. 48. Graeber MB. Neuroinflammation: no rose by any other name. Brain Pathol 24: 620 –622, 2014. 49. Haddad JJ, Saadé NE, Safieh-Garabedian B. Cytokines and neuroimmune-endocrine interactions: a role for the hypothalamic-pituitaryadrenal revolving axis. J Neuroimmunol 133: 1–19, 2002. 50. Hagberg H, Gressens P, Mallard C. Inflammation during fetal and neonatal life: implications for neurologic and neuropsychiatric disease in children and adults. Ann Neurol 71: 444 –457, 2012. 51. Harvey L, Boksa P. A stereological comparison of GAD67 and reelin expression in the hippocampal stratum oriens of offspring from two mouse models of maternal inflammation during pregnancy. Neuropharmacology 62: 1767–1776, 2012. 52. Harvey L, Boksa P. Prenatal and postnatal animal models of immune activation: relevance to a range of neurodevelopmental disorders. Dev Neurobiol 72: 1335–1348, 2012. 53. Heald A. Physical health in schizophrenia: a challenge for antipsychotic therapy. Eur Psychiatry 25 Suppl 2: S6 –S11, 2010. 54. Heerwagen MJ, Miller MR, Barbour LA, Friedman JE. Maternal obesity and fetal metabolic programming: a fertile epigenetic soil. Am J Physiol Regul Integr Comp Physiol 299: R711–R722, 2010. 55. Hei MY, Yi ZW. Environmental factors for the development of fetal urinary malformations. World J Pediatr 10: 17–23, 2014. 56. Holmlund U, Cebers G, Dahlfors AR, Sandstedt B, Bremme K, Ekström ES, Scheynius A. Expression and regulation of the pattern recognition receptors Toll-like receptor-2 and Toll-like receptor-4 in the human placenta. Immunology 107: 145–151, 2002. 57. Howerton CL, Bale TL. Prenatal programing: at the intersection of maternal stress and immune activation. Horm Behav 62: 237–242, 2012. 58. Hsiao EY, McBride SW, Chow J, Mazmanian SK, Patterson PH. Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc Natl Acad Sci USA 109: 12,776 –12,781, 2012. AJP-Regul Integr Comp Physiol • doi:10.1152/ajpregu.00087.2015 • www.ajpregu.org Downloaded from http://ajpregu.physiology.org/ by 10.220.32.246 on June 17, 2017 11. Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD, Verdu EF, Collins SM. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 141: 599 –609, 2011. 12. Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci 85: 1–17, 2000. 13. Bhatia V, Tandon RK. Stress and the gastrointestinal tract. J Gastroenterol Hepatol 20: 332–339, 2005. 14. Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci 3: 14, 2009. 15. Boksa P. Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav Immun 24: 881–897, 2010. 16. Borre YE, O’Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med 20: 509 –518, 2014. 17. Borrell J, Vela JM, Arévalo-Martin A, Molina-Holgado E, Guaza C. Prenatal immune challenge disrupts sensorimotor gating in adult rats. Implications for the etiopathogenesis of schizophrenia. Neuropsychopharmacology 26: 204 –215, 2002. 18. Bronson SL, Bale TL. Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment. Endocrinology 155: 2635– 2646, 2014. 19. Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry 167: 261– 280, 2010. 20. Brown LD. Endocrine regulation of fetal skeletal muscle growth: impact on future metabolic health. J Endocrinol 221: R13–R29, 2014. 21. Brown AS. The environment and susceptibility to schizophrenia. Prog Neurobiol 93: 23–58, 2011. 22. Canetta SE, Bao Y, Co MD, Ennis FA, Cruz J, Terajima M, Shen L, Kellendonk C, Schaefer CA, Brown AS. Serological documentation of maternal influenza exposure and bipolar disorder in adult offspring. Am J Psychiatry 171: 557–563, 2014. 23. Cheung C, Yu K, Fung G, Leung M, Wong C, Li Q, Sham P, Chua S, McAlonan G. Autistic disorders and schizophrenia: related or remote? An anatomical likelihood estimation. PLoS One 5: e12233, 2010. 24. Chow Z, Banerjee A, Hickey MJ. Controlling the fire - tissue-specific mechanisms of effector regulatory T-cell homing. Immunol Cell Biol 93: 355–363, 2015. 25. Cocoros NM, Lash TL, Nørgaard M, Farkas DK, DeMaria A Jr, Sørensen HT. Hospitalized prenatal and childhood infections and obesity in Danish male conscripts. Ann Epidemiol 23: 307–313, 2013. 26. Critchfield JW, van Hemert S, Ash M, Mulder L, Ashwood P. The potential role of probiotics in the management of childhood autism spectrum disorders. Gastroenterol Res Pract 2011: 161358, 2011. 27. Critchley HD, Harrison NA. Visceral influences on brain and behavior. Neuron 77: 624 –638, 2013. 28. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 13: 701–712, 2012. 29. Dahlgren J, Nilsson C, Jennische E, Ho HP, Eriksson E, Niklasson A, Björntorp P, Albertsson Wikland K, Holmäng A. Prenatal cytokine exposure results in obesity and gender-specific programming. Am J Physiol Endocrinol Metab 281: E326 –E334, 2001. 30. Dahlgren J, Samuelsson AM, Jansson T, Holmäng A. Interleukin-6 in the maternal circulation reaches the rat fetus in mid-gestation. Pediatr Res 60: 147–151, 2006. 31. Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res 42: 1–8, 1997. 32. Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9: 46 –56, 2008. 33. Daston GP, Overmann GJ, Baines D, Taubeneck MW, LehmanMcKeeman LD, Rogers JM, Keen CL. Altered Zn status by alphahederin in the pregnant rat and its relationship to adverse developmental outcome. Reprod Toxicol 8: 15–24, 1994. 34. de Theije CG, Wopereis H, Ramadan M, van Eijndthoven T, Lambert J, Knol J, Garssen J, Kraneveld AD, Oozeer R. Altered gut microbiota and activity in a murine model of autism spectrum disorders. Brain Behav Immun 37: 197–206, 2014. R9 Review R10 PERIPHERAL EFFECTS OF PRENATAL INFECTION 81. Luo ZC, Xiao L, Nuyt AM. Mechanisms of developmental programming of the metabolic syndrome and related disorders. World J Diabetes 1: 89 –98, 2010. 82. Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr 69: 1035S–1045S, 1999. 83. Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun 26: 607–616, 2012. 84. Marques AH, Bjørke-Monsen AL, Teixeira AL, Silverman MN. Maternal stress, nutrition and physical activity: Impact on immune function, CNS development and psychopathology. Brain Res In press. 85. Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci 13: 453–461, 2008. 86. Masi A, Quintana DS, Glozier N, Lloyd AR, Hickie IB, Guastella AJ. Cytokine aberrations in autism spectrum disorder: a systematic review and meta-analysis. Mol Psychiatry 20: 440 –446, 2015. 87. Mayer EA, Knight R, Mazmanian SK, Cryan JF, Tillisch K. Gut microbes and the brain: paradigm shift in neuroscience. J Neurosci 34: 15,490 –15,496, 2014. 88. Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci 12: 453–466, 2011. 89. McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev 85: 571–633, 2005. 90. Meyer JM, Stahl SM. The metabolic syndrome and schizophrenia. Acta Psychiatr Scand 119: 4 –14, 2009. 91. Meyer U, Engler A, Weber L, Schedlowski M, Feldon J. Preliminary evidence for a modulation of fetal dopaminergic development by maternal immune activation during pregnancy. Neuroscience 154: 701–709, 2008. 92. Meyer U, Feldon J, Dammann O. Schizophrenia and autism: both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatr Res 69: 26R–33R, 2011. 93. Meyer U, Feldon J, Fatemi SH. In-vivo rodent models for the experimental investigation of prenatal immune activation effects in neurodevelopmental brain disorders. Neurosci Biobehav Rev 33: 1061–1079, 2009. 94. Meyer U, Feldon J, Schedlowski M, Yee BK. Towards an immunoprecipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev 29: 913–947, 2005. 95. Meyer U, Feldon J, Yee BK. A review of the fetal brain cytokine imbalance hypothesis of schizophrenia. Schizophr Bull 35: 959 –972, 2009. 96. Meyer U, Murray PJ, Urwyler A, Yee BK, Schedlowski M, Feldon J. Adult behavioral and pharmacological dysfunctions following disruption of the fetal brain balance between pro-inflammatory and IL-10-mediated anti-inflammatory signaling. Mol Psychiatry 13: 208 –221, 2008. 97. Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci 26: 4752–4762, 2006. 98. Meyer U, Nyffeler M, Schwendener S, Knuesel I, Yee BK, Feldon J. Relative prenatal and postnatal maternal contributions to schizophreniarelated neurochemical dysfunction after in utero immune challenge. Neuropsychopharmacology 33: 441–456, 2008. 99. Meyer U, Yee BK, Feldon J. The neurodevelopmental impact of prenatal infections at different times of pregnancy: the earlier the worse? Neuroscientist 13: 241–256, 2007. 100. Meyer U. Prenatal poly(I:C) exposure and other developmental immune activation models in rodent systems. Biol Psychiatry 75: 307–315, 2014. 101. Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Metaanalysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry 70: 663–671, 2011. 102. Miller BJ, Culpepper N, Rapaport MH, Buckley P. Prenatal inflammation and neurodevelopment in schizophrenia: a review of human studies. Prog Neuropsychopharmacol Biol Psychiatry 42: 92–100, 2013. 103. Moreno-De-Luca A, Myers SM, Challman TD, Moreno-De-Luca D, Evans DW, Ledbetter DH. Developmental brain dysfunction: revival and expansion of old concepts based on new genetic evidence. Lancet Neurol 12: 406 –414, 2013. 104. Morton GJ, Meek TH, Schwartz MW. Neurobiology of food intake in health and disease. Nat Rev Neurosci 15: 367–378, 2014. AJP-Regul Integr Comp Physiol • doi:10.1152/ajpregu.00087.2015 • www.ajpregu.org Downloaded from http://ajpregu.physiology.org/ by 10.220.32.246 on June 17, 2017 59. Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, Patterson PH, Mazmanian SK. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155: 1451–1463, 2013. 60. Huhta J, Linask KK. Environmental origins of congenital heart disease: the heart-placenta connection. Semin Fetal Neonatal Med 18: 245–250, 2013. 61. Johnson J, Anderson B, Pass RF. Prevention of maternal and congenital cytomegalovirus infection. Clin Obstet Gynecol 55: 521–530, 2012. 62. Kenny PJ. Common cellular and molecular mechanisms in obesity and drug addiction. Nat Rev Neurosci 12: 638 –651, 2011. 63. Kirkpatrick B, Miller BJ, Garcia-Rizo C, Fernandez-Egea E, Bernardo M. Is abnormal glucose tolerance in antipsychotic-naive patients with nonaffective psychosis confounded by poor health habits? Schizophr Bull 38: 280 –284, 2012. 64. Kirkpatrick B, Miller BJ. Inflammation and schizophrenia. Schizophr Bull 39: 1174 –1179, 2013. 65. Kluger MJ, Rothenburg BA. Fever and reduced iron: their interaction as a host defense response to bacterial infection. Science 203: 374 –376, 1979. 66. Kohen R, Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol 30: 620 –650, 2002. 67. Konturek PC, Brzozowski T, Konturek SJ. Stress and the gut: pathophysiology, clinical consequences, diagnostic approach and treatment options. J Physiol Pharmacol 62: 591–599, 2011. 68. Kovacs CS. Bone development and mineral homeostasis in the fetus and neonate: roles of the calciotropic and phosphotropic hormones. Physiol Rev 94: 1143–1218, 2014. 69. Krstic D, Madhusudan A, Doehner J, Vogel P, Notter T, Imhof C, Manalastas A, Hilfiker M, Pfister S, Schwerdel C, Riether C, Meyer U, Knuesel I. Systemic immune challenges trigger and drive Alzheimerlike neuropathology in mice. J Neuroinflammation 9: 151, 2012. 70. Kunzmann S, Collins JJ, Kuypers E, Kramer BW. Thrown off balance: the effect of antenatal inflammation on the developing lung and immune system. Am J Obstet Gynecol 208: 429 –437, 2013. 71. Kwik-Uribe CL, Gietzen D, German JB, Golub MS, Keen CL. Chronic marginal iron intakes during early development in mice result in persistent changes in dopamine metabolism and myelin composition. J Nutr 130: 2821–2830, 2000. 72. Kwik-Uribe CL, Golub MS, Keen CL. Chronic marginal iron intakes during early development in mice alter brain iron concentrations and behavior despite postnatal iron supplementation. J Nutr 130: 2040 –2048, 2000. 73. Lakshmy R. Metabolic syndrome: role of maternal undernutrition and fetal programming. Rev Endocr Metab Disord 14: 229 –240, 2013. 74. Lanté F, Meunier J, Guiramand J, De Jesus Ferreira MC, Cambonie G, Aimar R, Cohen-Solal C, Maurice T, Vignes M, Barbanel G. Late N-acetylcysteine treatment prevents the deficits induced in the offspring of dams exposed to an immune stress during gestation. Hippocampus 18: 602–609, 2008. 75. Lanté F, Meunier J, Guiramand J, Maurice T, Cavalier M, de Jesus Ferreira MC, Aimar R, Cohen-Solal C, Vignes M, Barbanel G. Neurodevelopmental damage after prenatal infection: role of oxidative stress in the fetal brain. Free Radic Biol Med 42: 1231–1245, 2007. 76. Lee P, Peng H, Gelbart T, Wang L, Beutler E. Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc Natl Acad Sci USA 102: 1906 –1910, 2005. 77. Li M, Sloboda DM, Vickers MH. Maternal obesity and developmental programming of metabolic disorders in offspring: evidence from animal models. Exp Diabetes Res 2011: 592408, 2011. 78. Lin YL, Lin SY, Wang S. Prenatal lipopolysaccharide exposure increases anxiety-like behaviors and enhances stress-induced corticosterone responses in adult rats. Brain Behav Immun 26: 459 –468, 2012. 79. Lipina TV, Zai C, Hlousek D, Roder JC, Wong AH. Maternal immune activation during gestation interacts with Disc1 point mutation to exacerbate schizophrenia-related behaviors in mice. J Neurosci 33: 7654 – 7666, 2013. 80. Lumbers ER, Pringle KG, Wang Y, Gibson KJ. The renin-angiotensin system from conception to old age: the good, the bad and the ugly. Clin Exp Pharmacol Physiol 40: 743–752, 2013. Review PERIPHERAL EFFECTS OF PRENATAL INFECTION 128. Schaefer TM, Fahey JV, Wright JA, Wira CR. Innate immunity in the human female reproductive tract: antiviral response of uterine epithelial cells to the TLR3 agonist poly(I:C). J Immunol 174: 992–1002, 2005. 129. Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 404: 661–671, 2000. 130. Seckl JR, Holmes MC. Mechanisms of disease: glucocorticoids, their placental metabolism and fetal ‘programming’ of adult pathophysiology. Nat Clin Pract Endocrinol Metab 3: 479 –488, 2007. 131. Sell H, Eckel J. Adipose tissue inflammation: novel insight into the role of macrophages and lymphocytes. Curr Opin Clin Nutr Metab Care 13: 366 –370, 2010. 132. Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol 6: 1191–1197, 2005. 133. Severance EG, Yolken RH, Eaton WW. Autoimmune diseases, gastrointestinal disorders and the microbiome in schizophrenia: more than a gut feeling. Schizophr Res In press. 134. Shields RW Jr. Functional anatomy of the autonomic nervous system. J Clin Neurophysiol 10: 2–13, 1993. 135. Shimogori T, Lee DA, Miranda-Angulo A, Yang Y, Wang H, Jiang L, Yoshida AC, Kataoka A, Mashiko H, Avetisyan M, Qi L, Qian J, Blackshaw S. A genomic atlas of mouse hypothalamic development. Nat Neurosci 13: 767–775, 2010. 136. Short DD, Hawley JM, McCarthy MF. Management of schizophrenia with medical disorders: cardiovascular, pulmonary, and gastrointestinal. Psychiatr Clin North Am 32: 759 –773, 2009. 137. Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci 27: 10,695–10,702, 20107. 138. Smoller W, Craddock N, Kendler K, Lee PH, Neale BM, CrossDisorder Group of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: A genome-wide analysis. Lancet 381: 1371–1379, 2013. 139. Stahl SM, Mignon L, Meyer JM. Which comes first: atypical antipsychotic treatment or cardiometabolic risk? Acta Psychiatr Scand 119: 171–179, 2009. 140. Symonds ME, Mostyn A, Pearce S, Budge H, Stephenson T. Endocrine and nutritional regulation of fetal adipose tissue development. J Endocrinol 179: 293–299, 2003. 141. Symonds ME, Sebert SP, Hyatt MA, Budge H. Nutritional programming of the metabolic syndrome. Nat Rev Endocrinol 5: 604 –610. 142. Taubeneck MW, Daston GP, Rogers JM, Gershwin ME, Ansari A, Keen CL. Tumor necrosis factor-alpha alters maternal and embryonic zinc metabolism and is developmentally toxic in mice. J Nutr 125: 908 –919, 1995. 143. Thakore JH, Mann JN, Vlahos I, Martin A, Reznek R. Increased visceral fat distribution in drug-naive and drug-free patients with schizophrenia. Int J Obes Relat Metab Disord 26: 137–141, 2002. 144. Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest 121: 2126 –2132, 2011. 145. Tracey KJ. The inflammatory reflex. Nature 420: 853–859, 2002. 146. Traynor TR, Majde JA, Bohnet SG, Krueger JM. Intratracheal double-stranded RNA plus interferon-gamma: a model for analysis of the acute phase response to respiratory viral infections. Life Sci 74: 2563– 2576, 2004. 147. Triantafilou M, Triantafilou K. Lipopolysaccharide recognition: CD14, TLRs, and the LPS-activation cluster. Trends Immunol 23: 301–304, 2002. 148. Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 9: 799 –809, 2009. 149. Unger EL, Paul T, Murray-Kolb LE, Felt B, Jones BC, Beard JL. Early iron deficiency alters sensorimotor development and brain monoamines in rats. J Nutr 137: 118 –124, 2007. 150. Upthegrove R, Manzanares-Teson N, Barnes NM. Cytokine function in medication-naive first episode psychosis: a systematic review and meta-analysis. Schizophr Res 155: 101–108, 2014. 151. Urakubo A, Jarskog LF, Lieberman JA, Gilmore JH. Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain. Schizophr Res 47: 27–36, 2001. 152. Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev 73: 79 –118. 153. Verma SK, Subramaniam M, Liew A, Poon LY. Metabolic risk factors in drug-naive patients with first-episode psychosis. J Clin Psychiatry 70: 997–1000, 2009. AJP-Regul Integr Comp Physiol • doi:10.1152/ajpregu.00087.2015 • www.ajpregu.org Downloaded from http://ajpregu.physiology.org/ by 10.220.32.246 on June 17, 2017 105. Mraz M, Haluzik M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J Endocrinol 222: R113–R127, 2014. 106. Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41: 14 –20, 2014. 107. Nathan C, Shiloh MU. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl Acad Sci USA 97: 8841–8848, 2000. 108. Naviaux JC, Schuchbauer MA, Li K, Wang L, Risbrough VB, Powell SB, Naviaux RK. Reversal of autism-like behaviors and metabolism in adult mice with single-dose antipurinergic therapy. Transl Psychiatry 4: e400, 2014. 109. Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest 113: 1271–1276, 2007. 110. Niklasson B, Samsioe A, Blixt M, Sandler S, Sjöholm A, Lagerquist E, Lernmark A, Klitz W. Prenatal viral exposure followed by adult stress produces glucose intolerance in a mouse model. Diabetologia 49: 2192–2129, 2006. 111. Nilsson C, Larsson BM, Jennische E, Eriksson E, Björntorp P, York DA, Holmäng A. Maternal endotoxemia results in obesity and insulin resistance in adult male offspring. Endocrinology 142: 2622–2630, 2001. 112. Onore CE, Schwartzer JJ, Careaga M, Berman RF, Ashwood P. Maternal immune activation leads to activated inflammatory macrophages in offspring. Brain Behav Immun 38: 220 –226, 2014. 113. Owen MJ. Intellectual disability and major psychiatric disorders: A continuum of neurodevelopmental causality. Br J Psychiatry 200: 268 – 269, 2012. 114. Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, Iyo M. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol Psychiatry 59: 546 –554, 2006. 115. Pacheco-López G, Giovanoli S, Langhans W, Meyer U. Priming of metabolic dysfunctions by prenatal immune activation in mice: relevance to schizophrenia. Schizophr Bull 39: 319 –329, 2013. 116. Parboosing R, Bao Y, Shen L, Schaefer CA, Brown AS. Gestational influenza and bipolar disorder in adult offspring. JAMA Psychiatry 70: 677–685, 2013. 117. Patterson PH. Maternal infection and immune involvement in autism. Trends Mol Med 17: 389 –394, 2011. 118. Pavlov VA, Tracey KJ. The vagus nerve and the inflammatory reflex— linking immunity and metabolism. Nat Rev Endocrinol 8: 743–754, 2012. 119. Pineda E, Shin D, You SJ, Auvin S, Sankar R, Mazarati A. Maternal immune activation promotes hippocampal kindling epileptogenesis in mice. Ann Neurol 74: 11–19, 2013. 120. Pongratz G, Straub RH. Role of peripheral nerve fibres in acute and chronic inflammation in arthritis. Nat Rev Rheumatol 9: 117–126, 2013. 121. Radaelli T, Uvena-Celebrezze J, Minium J, Huston-Presley L, Catalano P, Hauguel-de Mouzon S. Maternal interleukin-6: marker of fetal growth and adiposity. J Soc Gynecol Investig 13: 53–57, 2006. 122. Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol 27: 119 –145, 2009. 123. Roullet FI, Lai JK, Foster JA. In utero exposure to valproic acid and autism—a current review of clinical and animal studies. Neurotoxicol Teratol 36: 47–56, 2013. 124. Ryan MC, Collins P, Thakore JH. Impaired fasting glucose tolerance in first-episode, drug-naive patients with schizophrenia. Am J Psychiatry 160: 284 –289, 2003. 125. Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron 76: 470 –485, 2012. 126. Salisbury AM, Begon M, Dove W, Niklasson B, Stewart JP. Ljungan virus is endemic in rodents in the UK. Arch Virol 159: 547–551, 2014. 127. Sanders TR, Kim DW, Glendining KA, Jasoni CL. Maternal obesity and IL-6 lead to aberrant developmental gene expression and deregulated neurite growth in the fetal arcuate nucleus. Endocrinology 155: 2566 – 2577, 2014. R11 Review R12 PERIPHERAL EFFECTS OF PRENATAL INFECTION 154. Vuillermot S, Joodmardi E, Perlmann T, Ögren SO, Feldon J, Meyer U. Prenatal immune activation interacts with genetic Nurr1 deficiency in the development of attentional impairments. J Neurosci 32: 436 –451, 2012. 155. Vuillermot S, Weber L, Feldon J, Meyer U. A longitudinal examination of the neurodevelopmental impact of prenatal immune activation in mice reveals primary defects in dopaminergic development relevant to schizophrenia. J Neurosci 30: 1270 –1287, 2010. 156. Welberg LA, Seckl JR. Prenatal stress, glucocorticoids and the programming of the brain. J Neuroendocrinol 13: 113–128, 2001. 157. Willi R, Harmeier A, Giovanoli S, Meyer U. Altered GSK3 signaling in an infection-based mouse model of developmental neuropsychiatric disease. Neuropharmacology 73: 56 –65. 158. Zager A, Andersen ML, Tufik S, Palermo-Neto J. Maternal immune activation increases the corticosterone response to acute stress without affecting the hypothalamic monoamine content and sleep patterns in male mice offspring. Neuroimmunomodulation 21: 37–44, 2014. 159. Zaretsky MV, Alexander JM, Byrd W, Bawdon RE. Transfer of inflammatory cytokines across the placenta. Obstet Gynecol 103: 546 – 550, 2004. 160. Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology 28: 1778 –1789, 2003. Downloaded from http://ajpregu.physiology.org/ by 10.220.32.246 on June 17, 2017 AJP-Regul Integr Comp Physiol • doi:10.1152/ajpregu.00087.2015 • www.ajpregu.org