* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Energy - TeacherWeb
Open energy system models wikipedia , lookup
Energy subsidies wikipedia , lookup
100% renewable energy wikipedia , lookup
Energy storage wikipedia , lookup
Public schemes for energy efficient refurbishment wikipedia , lookup
Low-Income Home Energy Assistance Program wikipedia , lookup
Potential energy wikipedia , lookup
World energy consumption wikipedia , lookup
Zero-energy building wikipedia , lookup
Energy Charter Treaty wikipedia , lookup
Low-carbon economy wikipedia , lookup
Alternative energy wikipedia , lookup
Gibbs free energy wikipedia , lookup
International Energy Agency wikipedia , lookup
Life-cycle greenhouse-gas emissions of energy sources wikipedia , lookup
Energy policy of the United Kingdom wikipedia , lookup
Regenerative brake wikipedia , lookup
Energy returned on energy invested wikipedia , lookup
Energy policy of Finland wikipedia , lookup
Energy efficiency in transport wikipedia , lookup
Distributed generation wikipedia , lookup
Kinetic energy wikipedia , lookup
Energy harvesting wikipedia , lookup
Negawatt power wikipedia , lookup
Energy in the United Kingdom wikipedia , lookup
Internal energy wikipedia , lookup
Energy policy of the European Union wikipedia , lookup
United States energy law wikipedia , lookup
Energy efficiency in British housing wikipedia , lookup
Energy Independence and Security Act of 2007 wikipedia , lookup
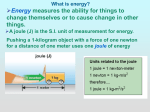
Unit: Types of Energy Sections: Potential Energy Kinetic Energy Heat and Temperature Key Words: Mechanical energy Hydroelectric power Degree Mechanical advantage Chemical reaction Calorie (heat energy) Kinetic energy Stored energy Radiant energy fahrenheit Celsius Boiling point Physical property Thermometer (weather) Friction Potential energy Entropy Diffusion Energy (physical) Melting point Thermal energy Essential Questions (by Section): Potential Energy - What is potential energy and what are some examples of it? - What are types of potential energy? - What is the difference between potential energy and kinetic energy? - How does potential energy turn into kinetic energy? Kinetic Energy - What is kinetic energy? - What are some examples of kinetic energy? - How does kinetic energy turn into potential energy? Heat and Temperature - What is the difference between heat and temperature? - What happens to the molecules of a substance when heat is added to it? - Which types of measurements are used to describe quantities of heat and temperature? - How can heat be transferred from one object to another? - How is energy transferred between objects or systems?** Potential Energy Energy – the ability to do work or cause change o Potential energy energy an object has due to its position or chemical composition energy that is “stored” until it can be converted to kinetic energy System - all the parts of an energy system o uses energy ability to do work o uses “forces” a push or pull - has both PE and KE - PE depends upon position and condition Energy transfer Conduct Convection (heat) energy stored between bonds of atoms CPE - chemical PE changes to heat when an object burns changes based on location of elevation Potential Energy GPE - gravitational PE ex. skier on top of a mountain stored energy by electron movement ElPE - electric PE ex. batteries - to + poles stored energy in the nucleus of atoms NPE - nuclear PE fission - splitting of atoms fusion - combining of atoms energy stored as an object is stretched (tension force) EPE - elastic PE ex. rubber bands Kinetic Energy Kinetic energy energy an object has due to its motion includes: heat (vibrational, rotational, translational motion of atoms) electrical (motion of electrons) Sound (wave motion of oscillating particles of matter) Any motion of objects Even when “standing still” molecular movement is still vibrating Absolute zero – where all molecular motion stops Temperature – a measure of kinetic energy o DIRECTLY PROPORTIONAL Higher temps Higher KE KE – object has due to motion - KE = ½ mv2 o KE = one half * mass of the object * the object’s velocity squared o Ball is 100g (mass), moving at 25 m/s – what is the KE? ½ (100g)(25)2 ½ (100g)(625) ½ (62500) KE = 31,250 J (J = Joules = Kg*m/s –> units of energy) - Law of conservation of energy o KE is transferred from one object to another o Ex. bat hits a ball -- > the KE of swinging the bat transfers to the ball Ball goes flying through the air o Ex. skier going up a lift to the top of a mountain the lift provides the KE building GPE due to height of the mountain GPE increases as any object increases altitude - other forms of KE o electricity generators move water or steam by spinning turbines converts mechanical energy to electrical energy Law of conservation of energy o How it applies Energy is continually changing from kinetic to potential and potential to kinetic During conversions, energy is always “lost” Never 100% efficient Lost as “heat” (another form of energy) Ex. Picking up a ball from the ground o Some energy from your body is converted to GPE (gravitational potential energy) in the ball o o o o Drop the ball, as it hits the ground, KE is converted to sound and heat Shape of ball has PE called “elastic PE” when ball deforms from impact Ball springs back into shape converts EPE to KE Ball does not go as high on next bounce because of energy lost as sound and heat Heat and Temperature Heat – form of energy transferred between objects of different temps - Transferred or “flows” between objects by: Conduction Transfer of heat between 2 objects in physical contact with each other Convection A vertical circulation pattern is established Radiation Heat energy travels through any medium (atmosphere or space) Warmer regions expand and become less dense (rise) Faster moving molecules of the hotter object collide with the slower moving molecules of the cooler object – causing them to move faster and the hotter to move slower - Cooler, more dense material will move under warmer (sinking) amount of work needed to raise temp of 1 lb of water 1 degree Fahrenheit Calorie small - amount of energy required to raise 1 g or water by 1 degree Celsius Joules BTU Calorie Can be quantified (given a value) British Thermal Units - Ex. Infrared radiation Joules equal to the energy transferred or work done to move 1 Newton a distance of 1 meter Often confused with temp o Temp – measure of the average kinetic energy of the particles in matter Transfer of thermal energy o Thermal energy – kinetic energy or motion energy of particles of matter - o Particles can take the form of: Translation Rotation Vibration Melting and Boiling Points o Refers to states of matter Solid Liquid Gas o Unique points for each element, molecule, or compound Melting Point solid to liquid Boiling Point liquid to gas