* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download PATHOPHYSIOLOGY COURSE - ENDOCRINE MODULE
Growth hormone therapy wikipedia , lookup
Hormone replacement therapy (menopause) wikipedia , lookup
Androgen insensitivity syndrome wikipedia , lookup
Hypothalamus wikipedia , lookup
Hormone replacement therapy (male-to-female) wikipedia , lookup
Hypopituitarism wikipedia , lookup
Kallmann syndrome wikipedia , lookup
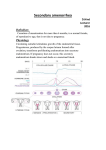
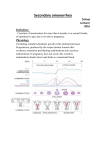
PATHOPHYSIOLOGY COURSE - ENDOCRINE MODULE DISORDERS OF THE OVARY AND AMENORRHEA Raymond W. Ke, M.D. Friday, December 4, 2009, 10:00-10:50a.m. In order to understand ovarian disorders it is first necessary to establish the two primary functions of the ovary. The human female ovary has only two responsibilities: 1. the release of mature oocytes (ovulation) and 2. production of sex hormones (steroidogenesis). Therefore, it follows that functional disorders of the ovary will likely result in anovulation and abnormal steroidogenesis. Since the primary unit of ovarian steroidogenesis is the egg follicle, if ovulation is disrupted then so is hormone synthesis resulting in either amenorrhea or dysfunctional uterine bleeding. Dysfunctional uterine bleeding should be discussed in the context of abnormal uterine bleeding and will not be covered here. A missed menses is common as an isolated, transient event but warrants further evaluation and treatment when it persists and meets the diagnostic criteria of amenorrhea. Amenorrhea simply means the absence of menstrual flow and can be classified into two categories: 1. Primary amenorrhea refers to young women or adolescents with secondary sexual characteristics that have not experienced menarche by age 16 or, by age 14, if there is no evidence of secondary sexual characteristics such as breast development. 2. Secondary amenorrhea applies to previously menstruating women who have missed the equivalent of their last three menses or have had no menses for six months. By far, this is the more common presentation. Menstrual Physiology Every month for 35 years or more, during the active reproductive phase of a woman’s life and in the absence of pregnancy, menstrual bleeding should occur. The initiation of reproductive life is heralded by the first occurrence of uterine bleeding, called menarche, and terminated at menopause when the major part of ovarian activity ceases. Normal human menstrual function is dependent upon an intricate series of hormone actions linking the neuronal nuclei of the hypothalamus to the pituitary gland which subsequently stimulates the ovaries to produce sex steroids that act upon the endometrial lining of the uterus. Appropriately, this pathway is known as the hypothalamic-pituitary-ovarian (HPO) axis. Along the HPO axis there is an ingenious system of positive and negative feedback signals which allow the end organs to communicate with the higher centers. To complicate matters, the ovary is subjected to numerous, and as yet undefined, autocrine and paracrine actions within itself which modulate external hormonal actions. Only with proper function of this complex interplay do normal ovulation and menses occur. Disorders of the Ovary and Amenorrhea (Female Gonadal Disorders) 7-1 Uterus Late in the menstrual cycle, the arcuate nucleus of the hypothalamus generates carefully timed pulses of gonadotropin-releasing hormone (GnRH), which stimulates cells of the anterior pituitary gland to produce follicle stimulating hormone (FSH) and, to a smaller extent, luteinizing hormone (LH). In the right proportion, FSH will recruit a cohort of ovarian follicles (eggs) to develop. At the same time, FSH/LH circulates back to the hypothalamus exerting a negative feedback control on GnRH pulses, limiting recruitment to the initial cohort. From the cohort of recruited ovarian follicles, a dominant follicle is selected by cycle day 7 (remember, it is customary to designate the first day of menses as “cycle day 1” of the menstrual cycle). It is the destiny of this one follicle to mature and proceed to ovulation, usually on cycle day 14. While achieving maturity, the dominant follicle secretes ever increasing levels of the estrogen, estradiol, which will eventually initiate an ovulation trigger (LH surge) by a positive feedback influence on the hypothalamus/pituitary. Increasing estradiol levels also serves to prevent other follicles from interfering with ovulation by inhibiting FSH release through negative feedback. Two smaller peptide hormones called inhibin and activin are also produced by the developing follicle to assist with ovarian feedback. Estradiol produced by the follicle, stimulates proliferative growth within the endometrial lining of the uterus so that embryo implantation can occur later in the cycle. Once the LH trigger has been sent by the pituitary, the ovum is released and the follicle collapses to become the corpus luteum. The corpus luteum is a sub-organ within the ovary that produces high amounts of progesterone and has a life-span of approximately 10 days. Progesterone Disorders of the Ovary and Amenorrhea (Female Gonadal Disorders) 7-2 transforms the already proliferative endometrium, increasing glandular production and preparing for embryo implantation. If implantation does not occur, then the corpus luteum will involute at 10 days and progesterone production is withdrawn. With progesterone withdrawal, the endometrial lining collapses, resulting in a menses. Once progesterone and estradiol levels have reached a nadir, the hypothalamus/pituitary escapes negative feedback and FSH values will again rise for the subsequent cycle. Ovulation Menses Progesterone Estradiol Day 2 8 14 20 26 Endometrial Height A normal menses is characterized by an orderly sloughing of the entire functional endometrium, resulting in self-limited bleeding. Only when the endometrium is first primed by estradiol, followed by progesterone, will the uterine lining be prepared for menses. Assuming that there is a patent outflow tract, the withdrawal of both estradiol/progesterone influences will result in a normal menses. Etiology of Amenorrhea Clearly the important players in this process include the hypothalamus, pituitary, ovary, and uterus/outflow tract. Because of the wide range of etiologies, it may be helpful to organize them into one of these four areas. Uterine Outflow Causes of Amenorrhea Young women with primary amenorrhea but otherwise normal gonadal function most commonly suffer from an anatomic etiology. Congenital absence of the vagina and uterus (Meyer-Rokitansky-Kuster-Hauser syndrome) is the most common followed by congenital vaginal defects and Androgen Insensitivity Syndrome (AIS). In Meyer-Rokitansky-Kuster-Hauser syndrome, pubertal landmarks occur at the appropriate age and secondary sexual characteristics are normal. However, the mullerian ducts fail to Disorders of the Ovary and Amenorrhea (Female Gonadal Disorders) 7-3 fuse leading to varying degrees of hypoplasia of the vagina, uterus, and fallopian tubes. Most commonly, there is complete absence or a very short vaginal pouch. The incidence is 1:4000 female births and one third will have associated renal and/or skeletal anomalies. Imperforate hymen and transverse vaginal septum cause an obstruction and accumulation of menstrual blood. The classic presentation is monthly episodes of cyclical pain in a pubertal girl with an associated abdominal/pelvic mass. AIS use to be known as testicular feminization (TFM). These patients present with primary amenorrhea, a blind vaginal pouch, absent uterus, diminished or absent pubic and axillary hair, and normal female secondary sexual characteristics. They are male pseudohermaphrodites with XY karyotype. The disorder is transmitted by an X-link recessive gene leading to a congenital absence of androgen receptors and thus they are insensitive to the action of androgens. The male gonads, which are intra-abdominal, must be removed after puberty because of the high risk of tumor formation. Patients with secondary amenorrhea do not commonly present with uterine or outflow tract obstruction. Except for the occasional case of genital mutilation or post-surgical obstruction, these patients may have Asherman’s syndrome. This is destruction of the endometrial lining usually following over-aggressive dilatation and curettage immediately after a pregnancy. Pituitary Causes of Amenorrhea A pituitary etiology for amenorrhea is usually due to a pituitary commonly prolactin-secreting, although any adenoma may Hyperprolactinemia leads to suppression of GnRH pulsatility as estrogenic effect on the endometrium. It is a common cause reproductive-age woman. adenoma. These are lead to amenorrhea. well as a direct antiof amenorrhea in the Infrequently, hypothyroidism may present as amenorrhea although more commonly as dysfunctional uterine bleeding. It may act secondarily through hyperprolactinemia as elevated thyrotropin-releasing hormone (TRH) stimulates prolactin release. Pituitary failure, empty sella syndrome and CNS tumors such as craniopharyngioma are rare explanations for amenorrhea. Sheehan’s syndrome is a condition which causes amenorrhea due to loss of FSH/LH secretion. It is a form of pituitary failure associated with multiple pituitary hormone deficiency secondary to postpartum hemorrhage and shock. The degree of pituitary hypofunction is often variable. The classic presentation is of a patient who cannot lactate after a delivery complicated by massive post-partum hemorrhage. Hypothalamic Causes of Amenorrhea Hypogonadotropic hypogonadism (also referred to as hypothalamic amenorrhea) is a diagnosis of exclusion. The possibilities include pubertal delay, anorexia nervosa, excessive exercise, GI malabsorption, psychosocial stress, malnutrition, and organic brain disease. As the hypothalamus is extremely sensitive to the environment, many supra-tentorial factors can Disorders of the Ovary and Amenorrhea (Female Gonadal Disorders) 7-4 affect the release of GnRH pulses. Since it is generally thought to be unwise for a woman to ovulate and be fertile during times of high stress or malnutrition, one can understand the delicacy of hypothalamic function. A classic example of hypogonadotropic hypogonadism is that associated with anorexia nervosa. Clinically, these women present with low FSH/LH and very low levels of estrogen production. Similarly, pubertal delay is an example of primary hypogonadotropic hypogonadism with an identical hormone profile. Kallmann’s syndrome is a congenital lack of GnRH neurons and is typically associated with an inability to smell (anosmia). It is rare and occurs in males with greater frequency than females. Hypothalamic tumors are a rare cause of primary or secondary amenorrhea. Ovarian Causes of Amenorrhea While an intrinsic disorder of the ovary is often associated with amenorrhea the list of possible etiologies is rather short. It includes gonadal dysgenesis, premature ovarian failure, surgical castration and rarely, ovarian neoplasm. It also includes polycystic ovarian syndrome (PCOS), a condition characterized by anovulation and inappropriate androgen secretion by the ovary. Whether PCOS is a primary derangement of the ovary, or a combined disorder of the HPO axis, or a systemic disorder encompassing many organ systems is yet to be fully elucidated. However, without question, this is the most common ovarian disorder and is probably the leading cause of amenorrhea in reproductive-age women. Gonadal dysgenesis presents with an abnormal karyotype, either systemically or as part of a mosaic within the germ cells themselves. The most common form of gonadal dysgenesis in the female is Turner’s syndrome (45X0). Gonadal dysgenesis is a cause of amenorrhea because it induces premature ovarian failure (premature menopause) as accelerated oocyte atresia in these patients leads to early depletion of the egg store, and thus ovulation and steroidogenesis cease. All patients with either primary or premature ovarian failure, particularly if they are under five feet tall, should have a karyotype analysis to confirm the diagnosis of gonadal dysgenesis and more importantly, to rule out the presence of a Y chromosome. This is because a Y bearing gonad in the intra-abdominal position is at risk for neoplastic transformation. Some premature ovarian failure patients are thought to suffer from an autoimmune etiology. This is often part of a multi-glandular autoimmune endocrinopathy and often ovarian failure is the first endocrine system to present. In these patients, yearly screening for thyroid, parathyroid, adrenal, etc. dysfunction is important. Patients suffering with ovarian failure or gonadal dysgenesis will require indefinite hormone replacement therapy since they have no endogenous ovarian estrogen production. Without therapy, long term estrogen deprivation results in osteoporosis and accelerated risk for cardiovascular disease. In addition to gonadal dysgenesis and autoimmunity, there are other forms of ovarian failure such as viral (mumps oophoritis) and post-radiotherapy. Polycystic Ovarian Syndrome PCOS classically presents as anovulation, hirsutism and obesity. However, only 70% of patients with PCOS will present with all three symptoms. The classic radiological Disorders of the Ovary and Amenorrhea (Female Gonadal Disorders) 7-5 appearance of a polycystic ovary on transvaginal ultrasound is only readily apparent in 60% of patients. Anovulation does appear to be the most consistent symptom. The pathophysiology of PCOS is not clear and the initial insult has not been identified. PCOS involves a cycle of events with the inability to ovulate leading to the production of excess androgens by the ovary. The androgens are converted to estrogens by peripheral adipose tissue providing PCOS patients with a rich estrogenic environment. Unfortunately, without ovulation, there is no progesterone effect and thus the patient rarely menstruates. The estrogenic milieu also induces more LH to be secreted by the pituitary which promotes further anovulation and androgen production by the ovary thereby worsening the condition. Hirsutism Insulin resistance and obesity Increased androgens Increased LH PCOS Anovulation Anovulation Amenorrhea Acyclic estrogen PCOS patients need to be treated and counseled carefully. Chronic anovulation and amenorrhea may often lead to endometrial hyperplasia and even endometrial carcinoma because the proliferating lining is not being shed. This may even occur in young patients under the age of 25. Secondly, while the hyperandrogenism experienced by these patients is often moderate, hirsutism can be a debilitating complaint that leads often to social isolation and distress. Lastly, PCOS patients have an increased risk of other metabolic diseases such as diabetes and need to be counseled regarding their risk of glucose intolerance and possible accelerated cardiovascular disease. Whether this is linked to their obesity, a high androgen level, or a striking association with insulin resistance is still not known. Evaluation of Amenorrhea Clearly there are multiple etiologies encompassing almost all the organ systems in the body. Therefore, a thorough history is vital and should direct the clinician to appropriate investigations. However, for all patients with amenorrhea an algorithm for evaluation starts with four investigations. 1. Pregnancy Test. Pregnancy is still the leading reason for amenorrhea in the reproductive age group. In the evaluation of this symptomology, pregnancy should always be assumed until effectively ruled out. Modern immunoassays for the ß-hCG molecule are highly sensitive pregnancy tests and should be performed on every patient Disorders of the Ovary and Amenorrhea (Female Gonadal Disorders) 7-6 2. Physical Exam. While a thorough exam goes without question, the clinician should be aware of two areas, particularly in the adolescent with primary amenorrhea. The first is to stage the patient’s breast development. Abnorm al bre ast developme nt Tanne r sta ge 1 or 2 -- m ea sure FSH FSH high FSH low Gonada l fa ilure or dysgene sis CT sca n or MRI Abnorm al -trea t a ccordingly Norma l -hypotha la mic or pituita ry puberta l dela y If an adolescent exhibits only Tanner stage I or II breast development, she is not likely to have been exposed to significant circulating levels of estradiol. An elevated FSH measurement at this time indicates ovarian failure and gonadal dysgenesis is the likely diagnosis. A low or normal FSH would be inappropriate given the relative hypoestrogenism and suggests a pituitary or hypothalamic etiology. Imaging of the head is required to rule out a serious neoplastic origin. Assuming the CT scan is normal and serum TSH and prolactin are normal, then this patient should be treated as pubertal delay. The second step is to perform a vaginal examination. This needs to be performed carefully in a virginal female and is best left to an experienced pediatric gynecologist in the young. If the physical exam reveals an absent vagina or a vagina that ends in a blind pouch without evidence of cervix, then the presence of pubic and axillary hair should be evaluated. If axillary and pubic hair is well developed, then the patient has mullerian tract agenesis or Rokitansky-Kuster-Hauser syndrome. However, if there is a lack of pubic hair in this patient, AIS is suspected. This can be confirmed by demonstrating a circulating testosterone level in the normal male range. Disorders of the Ovary and Amenorrhea (Female Gonadal Disorders) 7-7 Abnormal or absent vagina Pubic hair present Pubic hair absent Imperforate hymen, Trans. vaginal septum, or Rokitansky Androgen insensitivity syndrome 3. Thyroid stimulating hormone (TSH)/Prolactin. Hyperprolactinemia affects 5-10% of amenorrheic patients and often presents with galactorrhea. It should be first evaluated with a CT scan or MRI of the head to look for a large pituitary adenoma. Presently, even large macroadenomas of the pituitary can be very successfully treated with medical therapy. In the absence of an adenoma many medications, especially psychotropic drugs, may elevate prolactin levels and cause amenorrhea and even galactorrhea. Abnormal TSH/prolactin Hyperprolactinemia High TSHhypothyroidism Suppressed TSHhyperthyroidism CT scan or MRI Normalidiopathic hyperprolactinemia Abnormaltreat accordingly While thyroid disease is an uncommon direct etiology for amenorrhea (< 3%), the serum TSH level is a useful screen because hypothyroidism can be a common cause of hyperprolactinemia and because it is relatively inexpensive. In addition, the treatment of hypothyroidism is simple and very gratifying to both patient and clinician. A suppressed TSH level indicates a hyperthyroid condition and deserves further endocrinology workup. 4. Progestin Challenge Test. In the woman of reproductive age who is experiencing secondary amenorrhea the clinician needs to assess the estrogen level in the patient. While one can certainly draw a blood test, a much more useful evaluation is to perform the progestin challenge test. Not unlike evaluating breast development, the progestin challenge test is an effective bioassay for not only endogenous estrogen but for the presence of a patent uterus and lower outflow tract. Remember that in order for a woman to menstruate normally, her endometrium must first be primed with estrogen and then be exposed to progesterone (or progestin) Disorders of the Ovary and Amenorrhea (Female Gonadal Disorders) 7-8 followed by withdrawal of both hormones. The progestin challenge test is given to an amenorrheic woman with a negative pregnancy test by administering medroxyprogesterone acetate (Provera) 10 mg/day for 10 days. If the patient has a menses within 7 days after the Provera then she has a positive result. It implies that her endometrium has been primed by adequate circulating levels of estrogen. It also implies that the uterus and lower outflow tract is intact. If ovarian estrogen production is intact, the diagnosis of polycystic ovarian syndrome is made. Supporting evidence may include obesity, hirsutism, and other features of hyperandrogenism. Progestin Challenge Test After administration of progestin, wait for onset of menses Positive menses Negative menses Anovulation (PCOS) Estrogen+progestin challenge Positive menses Negative menses FSH Uterine/outflow problem FSH low or normal FSH high CT or MRI scan Premature ovarian failure Abnormal -treat accordingly Normal -Hypogonadotropic Hypogonadism If the patient fails to experience a menses following progestin withdrawal, then she either has: 1. inadequate levels of circulating estradiol or 2. an obstructed outflow tract. The next step would be to provide the woman with an estrogen/progestin challenge (conjugated estrogens 1.25 mg/day for 25 days combined with Provera 10 mg/day for the last 10 days). If she again fails to have a menses then the reproductive tract is either absent or obstructed. If, however, she does have a menses, then again the cause of inadequate circulating estradiol levels needs to be assessed. Therefore, an FSH value is measured with a high level implying premature ovarian failure. A low or normal FSH value again implies a pituitary or hypothalamic lesion and imaging of the brain again has to be undertaken. A normal brain image in this instance suggests that the woman has hypogonadotropic hypogonadism. Disorders of the Ovary and Amenorrhea (Female Gonadal Disorders) 7-9 Treatment of Amenorrhea The treatment of amenorrhea falls under three principles: 1. What is the etiology? While amenorrhea itself is not a cause of morbidity, it is usually a symptom of an underlying pathophysiology that needs to be treated. Thus, the treatment of amenorrhea has to be tailored according to the etiology. 2. All pathological causes of amenorrhea need to be treated. If the patient is amenorrheic because she is taking hormone replacement or oral contraceptives, that is not, in itself, harmful. Apart from this scenario and pregnancy, however, most pathological causes of amenorrhea have severe consequences. Depending on the cause, they include lack of secondary sexual characteristics, identification with a feminine gender role, endometrial neoplasia, central nervous system neoplasia, other endocrinopathies, osteoporosis, cardiovascular disease, diabetes, etc. Treatment therefore should not be unduly delayed. Most treatments are aimed at restoring regular menses or physiologic hormone replacement, even if the underlying etiology has not been corrected. 3. Is fertility an issue? For those patients that wish to conceive, clearly the first goal would be to initiate ovulation induction. In some cases, such as hyperprolactinemia, medical or surgical correction of the underlying problem will be the only therapy required. For PCOS, the patients will require some form of ovulation induction with medications such as clomiphene citrate but others may require more intensive assisted reproduction such as in vitro fertilization (IVF). Pregnancy is difficult but not impossible in women with ovarian failure when her oocytes have been depleted. Donor oocyte in vitro fertilization techniques, while expensive, offer these women the opportunity for pregnancy. Third party parenting is indicated for women with an absent uterus. Disorders of the Ovary and Amenorrhea (Female Gonadal Disorders) 7-10 References 1. Padilla SL, McDonough PG. Sexual abnormalities. In: Unwanted Hair-Ancestral Curse or Gland Disorder. Greenblatt RB ed. New York: Parthenon Press, 1985. 2. Chiazze L, Brayer FT, Micisco JJ, Parker MP, Duffy BJ. The length and variability of the human menstrual cycle. JAMA 203:337, 1968. 3. Reindollar RH, Byrd JR, McDonough PG. Delayed sexual development-a study of 252 patients. Am J Obstet Gynecol 140:371-380, 1981. 4. Griffin JE, Wilson JD. The syndromes of androgen resistance. N Engl J Med 302:198204, 1980. 5. Shangold M, Tomai T, Chin S, Zinamin M, Cook J, Simon J. Factors associated with withdrawal bleeding following administration of oral micronized progesterone in women with secondary amenorrhea. Fertil Steril 56:1040-1047, 1992. 6. Vollman RF. The menstrual cycle. Philadelphia:WB Saunders, 1977. 7. Rarick LD, Shangold M, Ahmed SW. Cervical mucus and serum estradiol as predictors of response to progestin challenge. Fertil Steril 54:353-359, 1990. 8. Frisch RE. Body fat, menarche, and reproductive ability.Semin Reprod Endocrinol 3:4557, 1985. Suggested Reading 1. McDonough PG. Amenorrhea - etiology approach to diagnosis. Fertil Steril 30:1 - 15, 1978. 2. Speroff L, Fritz MA. Clinical Gynecologic Endocrinology and Infertility. Seventh edition. Chap. 11, 12. Baltimore: Lippincott, Williams & Wilkins. 2005. 3. Yen SSC, Jaffe RB (eds). Reproductive Endocrinology. Third edition. Chap. 6, 17,18. Philadelphia: W.B.Saunders. 1991. Disorders of the Ovary and Amenorrhea (Female Gonadal Disorders) 7-11













![4-Amenorrhea [Dr.Mandeel]. - King Saud University Medical Student](http://s1.studyres.com/store/data/008318431_1-2f431d9b56a0e06930dc30cd21126053-150x150.png)