* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Practice Questions
Chemical thermodynamics wikipedia , lookup
Gas chromatography wikipedia , lookup
Diamond anvil cell wikipedia , lookup
Acid dissociation constant wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Chemical reaction wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Catalytic reforming wikipedia , lookup
Click chemistry wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Thermomechanical analysis wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Atomic theory wikipedia , lookup
History of molecular theory wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Transition state theory wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Acid–base reaction wikipedia , lookup
Abiogenesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Stoichiometry wikipedia , lookup
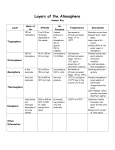
Practice Questions In addition to the following questions, all on-line Guiding & Embedded Powerpoint questions on the calendar since the previous exam should be considered as well as those questions in that period’s lab workheets. The class meeting’s weekly notes are a useful guide as to which topics & information are highly important. Which of these chemical processes adds to the carbon footprint? a. burning fossil fuels b. cement production c. flatulence (farting) from food digestion in certain animals d. breathing in all animals d. all of the above Compare the complete combustion of 1 mole of methane CH4 and 1 mole of butane C4H10. Circle all that apply. CH4 (g) + 2 O2 (g) → CO2 (g) + 2 H2O (g) 2 C4H10 (g) + 13 O2 (g) → 8 CO2 (g) + 10 H2O (g) The amount of oxygen that is needed for the reaction: a. is the same for both. b. is greater for butane. c. is greater for methane. The amount of CO2 produced: a. is the same for both. b. is greater for butane. c. is greater for methane. A given gas has a volume of 55 mL at a pressure of 1.2 atm. What will be its final volume if the pressure is increased to 2.4 atm (Temperature remains constant)? a. b. c. d. e. 27.5 mL 33 mL 91.7 mL 110 mL 55 mL A flexible walled balloon is heated. What happens to its volume? (Pressure remains constant.) a. b. c. d. e. Increases in proportion to the temperature change in Celsius Decreases in proportion to the temperature change in Celsius Increases in proportion to the temperature change in Kelvin Decreases in proportion to the temperature change in Kelvin Cannot determine Which gas is not a greenhouse gas? a. b. c. d. O2 CH4 CO2 H2O The lower atmosphere can be best described as being comprised of: a. Mostly oxygen with all other molecules present in very small amounts. b. Mostly nitrogen with all other molecules present in very small amounts. c. Mostly nitrogen and oxygen with more oxygen than nitrogen, and with some other trace molecules. d. Mostly nitrogen and oxygen with more nitrogen than oxygen, and with some other trace molecules. The Earth is relatively warm due to a. Ultraviolet radiation from the sun being absorbed by ozone molecules in the stratosphere. b. X-rays and gamma rays exciting nitrogen and oxygen gas molecules in the lower atmosphere. c. Infrared radiation being absorbed by certain trace gas molecules in the lower atmosphere. d. The very high temperature of the molten inner core of the Earth which is composed mostly of iron. Not all molecules absorb energy from sunlight. Which of the following does not absorb heat? a. carbon dioxide b. oxygen c. water vapor d. methane Without the atmosphere’s natural greenhouse effect, Earth’s temperature at the surface would be: a. 30-40 oF warmer b. 50-60 oF cooler c. the same A weather balloon is filled with helium to a volume of 10.0 L. What is the volume of the balloon when it rises to an altitude where the pressure has decreased by 80% (1 atm to 0.2 atm) and the Kelvin temperature decreased by 20% (300 K to 240 K)? a. b. c. d. e. 4.0 L 8.0 L 16.0 L 25.0 L 40.0 L What is occurring to the temperature if the molecules of a gas move more slowly on average? a. Increases b. Decreases c. Does not change d. Cannot determine What change in pressure occurs when molecules of a gas strike the walls of the container less often? a. Increases b. Decreases c. Does not change d. Cannot determine What is the correct formula for the compound formed by the combination of the ammonium ion (NH4)1+ ( a weak acid) and the phosphate ion (PO4)3- (a weak base)? a. b. c. d. NH4PO4 NH4(PO4)3 (NH4)3(PO4)2 (NH4)3PO4 Select all of the acids in the following list. a. b. c. d. e. f. NaOH (aq) C8H18 (l) Ba2SO4 (s) H2SO3(aq) CH3COOH(aq) CH3CH2OH (aq) A solution of unknown content had a pOH of 4.5, which of the following statements is correct a. b. c. d. e. The pH = 7 and the solution is neutral The pH = 4.5 and the solution is acidic The pH = 9.5 and the solution is basic The pH = 14 and the solution is basic The pH cannot be determined except with litmus paper In the reaction diagram above, which corresponds to the heat of reaction (ΔHrxn)? a) B b) D c) A d) C e) None of the above In the reaction diagram above, the reaction is: a) b) c) d) e) Isothermic Eurythermic Exothermic Stenothermic Endothermic Refer to the following reaction diagram below: Which of the following statements is true? a) b) c) d) The reaction is exothermic. The reaction is endothermic. The activation energy is a negative value. The reaction is neither exothermic nor endothermic. If an enzyme were added to the reaction, it would a) b) c) d) Decrease the rate of reaction. Increase the Energy of activation Eact Decrease the Energy of activation Eact Increase the heat produced ΔH. A B In the equilibrium above, every one minute 20% of reactants A react to form products B while during that same period of time 80% of the products B react in the opposite direction to form reactants A. This continues every minute over time. Equilibrium is reached at the end of : a) b) c) d) e) ~ 1 minute ~ 4 minutes ~ 40 minutes ~ 400 minutes Never The equilibrium constant K = [Products] / [Reactants] for this reaction is numerically: a) b) c) d) 0 <1 >1 There is no way to approximate the value. Which of the following is most important when determining the degree of unsaturation of a fat or an oil? a) b) c) d) Number of hydrogen atoms Number of double bonds Number of carbon atoms Number of single bonds Peptide bonds contain which of the following linkages? a) b) c) d) (-CH-) (-COO-) (-CONH-) (-NHHC-) Of the choices below, which is not found in the photosynthesis reaction? a) b) c) d) Glucose (C6H12O6) Water Nitrogen Oxygen Proteins are natural polymers composed of what monomers? a) b) c) d) e) Amino acids Carbohydrates Ethylene Cholesterol Amides TRUE/FALSE: Earth's lower atmosphere is comprised of a homogeneous mixture of molecules in the gas phase. Global warming, the long-term increase in world temperatures, occurs mainly because certain man made chemicals thin the ozone layer, allowing more solar radiation to reach Earth’s surface. Burning gasoline chemically produces water and carbon dioxide. The concentration of carbon dioxide in the atmosphere today is significantly higher than at the start of the Industrial Revolution, around 1750. Some kinds of particulate pollution in the atmosphere counteract global warming by reducing the solar radiation that reaches Earth’s surface. Ocean life is not threatened from the effects of carbon dioxide buildup and climate change. Scientists can derive evidence of the chemical composition of Earth’s atmosphere and temperature hundreds of thousands of years ago from ice cores and the gas bubbles trapped in ancient ice. Consider the following graph: Earth has been warmer in the past 100,000 years. Earth has been colder in the past 100,000 years. Using renewable biofuels like ethanol and biodiesel in place of gasoline will eliminate global warming. Molecules of different gases having the same volume, the same pressure, and the same temperature will have the same average kinetic energy. Molecules of different gases having the same volume, the same pressure, and the same temperature will have the same average speed. Molecules of different gases having the same volume, the same pressure, and the same temperature will have an average speed that is in proportion to their molecular mass. A balloon containing 22.4 liters of hydrogen gas at zero degrees Celsius and a pressure of one atmosphere will contain the same number of oxygen atoms in a balloon of the same size at the same pressure and temperature. A balloon containing 22.4 liters of hydrogen gas and a pressure of one atmosphere is at 25 degrees Celsius. If the temperature doubles to 50 degrees Celsius, the volume should increase to 24.3 liters; an increase of ~ 8%. A balloon containing 22.4 liters of hydrogen gas and a pressure of one atmosphere is at 25 degrees Celsius (298 K). If the temperature doubles to 596 K, the volume should double to 44.8 liters; an increase of 100%. Compare the complete combustion of 1 mole of methane CH4 and 1 mole of butane C4H10. Circle all that apply. Leptin is a carbohydrate that is involved with controlling appetite. Catabolism is the metabolic breakdown of large molecules. The primary protein structure is defined by alpha helices and beta sheets. Alpha helices and beta sheets are a result of hydrogen bonding. In the upper atmosphere, ozone absorbs infrared radiation which causes genetic mutation and skin cancer. For each of the four reactions, classify the reactants as an acid (A) or a base (B) and the products as the conjugate acid (CA) or conjugate base (CB). CN– + H2O → HCN + OH– NH3 + HCl → NH4+ + Cl The pH of five solutions were measured: A =1.5; B = 7.0; C = 2.5; D = 8.8; E = 12.1 Which are basic? __________ Which is(are) neutral? ________ Which is the weakest base? _______ Which is the strongest acid? _______ Refer to: http://chemconnections.org/general/chem106/106assign.html#CO2 http://chemconnections.org/general/chem106/Chem%20106%20Week%2012.2017.ppt.pdf http://chemconnections.org/general/chem106/Limiting%20Reactant-demo%20wks%202017s.pdf A set of three flasks with balloons attached were filled with 70.0 mL 1.0 M hydrochloric acid, HCl (aq). Then the balloons had different amounts of solid sodium bicarbonate added to the acid: 2.00g NaHCO3 (s) added from the green balloon, 4.00g NaHCO3 (s) added from the yellow balloon, and 6.00g NaHCO3 (s) added from the blue balloon. When mixed, the sodium bicarbonate reacted with the acid and produced carbon dioxide, which inflated the balloon in the following reaction: _____ HCl (aq) + ____ NaHCO3(s) _____ CO2(g) + _____ H2O(l) + _____ NaCl(aq) 1. 2. 3. 4. Balance the above equation. Identify the limiting reactant in each flask. Calculate the number of moles of sodium bicarbonate that were added to the respective flasks. (The moles of hydrochloric acid in all 3 flasks is equal to its molarity (1.0 mol / L) x 70.0 ml x 1.00 L / 1,000. mL) Calculate the number of moles of CO2 theoretically produced and then calculate the respective theoretical volume of CO2 produced in each flask assuming STP (Standard Temperature and Pressure) and that 1 mole of gas equals 22.4 liters. ___________________________ The following compound, methylparaben, 4-hydroxybenzoic acid methyl ester, is found in canine estrus secretions. Remarkably, it is used as a preservative in foods and cosmetics, and it is also an isomer of oil of wintergreen which can be prepared from salicylic acid, the same starting material used to prepare aspirin. Name the two functional groups each with one or more oxygen aroms that are present in methylparaben O . COCH3 OH ___________________________ What are the functions in aspirin and its molecular formula? ( CxHyOz) O CO-H O OCCH3 Bonus: A DVC chemistry student attempted to synthesize 0.500 g of aspirin, but obtained only an 80% yield. If a dose of 325 mg were required, did the student's synthesis provide a sufficient amount for one dose? Show your calculation. (1 g = 1000 mg) Match with the choices on the right. O A) amide C H CH3 B) trans-double bond O H3C C) cis-double bond CH3 O D) ether C H2NH2C E) aldehyde OH F) ketone O H3C H C H2 G) alcohol O2C-R H) fatty acid O2C-R' I) protein O2C-R'' H3C J) starch H K) disaccharide H CH3 L) amino acid HO HO M) triglyceride OH N) carboxylic acid O O O HO HO OH OH OH