* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Congenital Heart Diseases II
Survey
Document related concepts
Cardiac contractility modulation wikipedia , lookup
Heart failure wikipedia , lookup
Coronary artery disease wikipedia , lookup
Aortic stenosis wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Turner syndrome wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Atrial septal defect wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
Transcript
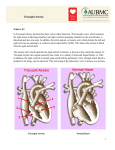
Congenital Heart Diseases II Samer Abbas, MD Cardiology UIC/Christ 02/27/2008 Classification Cyanosis with low pulm flow: - TOF - Ebstien Anamoly - Hypoplastic of RV - Tricuspid atresia - Pulm atresia Cyanosis with high pulm flow: - TGA - Double outlet V. - Double inlet V - TAPVD. Eisenmenger Syndrome In 1897 Victor Eisenmenger published a paper entitled “Congenital Defects of the Ventricular Septum.” In 1958, Paul Wood summarized Eisenmenger’s accounts: “The patient was a powerfully built man of 32 who gave a history of cyanosis and moderate breathlessness since infancy. He managed well until January of 1894 when dyspnea increased and edema set in. Seven months later he was admitted to the hospital in a state of heart failure……He improved with rest and digitalis, but collapsed and died suddenly on November 13 following a large hemoptysis” Eisenmenger Syndrome Large left to right shunt: – severe pulmonary vascular disease leading to shunt reversal Initial reversible changes: – Medial hypertrophy of the pulmonary vasculature – Intimal proliferation Eisenmenger Syndrome Progressive irreversible changes: – plexiform lesions – necrotizing arteritis As the increased PVR approaches or exceeds the SVR the shunt is reversed. As R to L shunting develops cyanosis appears. Most patients will develop exertional dyspnea and impaired exercise tolerance. Eisenmenger Syndrome Palpitations occur in >50% of patients – A. fib/flutter in 40% – VT in 10%) Hemoptysis in ~20% PE, angina, syncope, endocarditis ~10% Signs of PHTN – RV heave, palpable P2, and right sided S4 Pulmonary ejection click and a soft scratchy SEM – d/t dilated pulmonary trunk High pitched decrescendo diastolic murmur (Graham-Steele) audible in most patients Usually no peripheral edema until R HF ensues Eisenmenger Syndrome ECG shows RVH, RAE and RAD Atrial arrhythmias may be present Eisenmenger Syndrome CXR reveals prominent central pulmonary arteries and decreased vascular markings (pruning) of the peripheral vessels Eisenmenger Syndrome Large variation in life expectancy in adults with Eisenmenger syndrome Rate of survival among patients with Eisenmenger syndrome is: – 80% at 10 years – 77% at 15 years – 42% at 25 years Recent study of 109 adults revealed following as independent predictors of mortality: – Age at presentation – Supraventricular arrhythmias – Poor NYHA functional class (III or IV) Eisenmenger Syndrome Pregnancy is discouraged due to high maternal (50%) and fetal (60%) mortality. CVA may occur secondary to paradoxical emboli Cerebral abscesses Avoid intravascular volume depletion, heavy exertion, high altitudes, and use of vasodilators IV epoprostenol may be beneficial in decreasing PVR Phlebotomy with isovolumic replacement is recommended for patients with moderate to severe symptoms of hyperviscosity and an elevated hematocrit >65% Prevention of iron deficiency is important Supplemental oxygen reduces episodes of dyspnea Lung transplantation (with repair of cardiac defect) or heart/lung transplantation is an option Tetralogy of Fallot Etienne-Louis Arthur Fallot made the first published bedside diagnosis that was proven at postmortem in 1888 and called the condition of ‘maladie bleue’. Epidemiology Prevalence varies from 0.26-0.48/1000 live births. Exact incidence of TOF with PS versus pulmonary atresia or absent pulmonary valve is not known as most studies lump them together. Most recent study is the BWIS (Baltimore –Washington Infant Study) -Incidence 0.33/1000 live births -Fifth most common defect overall (6.8% of all CHD) -Most common cyanotic CHD -TOF with PS 0.26/1000 live births (5.4% of all CHD) -Suggestion of male predominance ( p=NS) Genetics Syndromes associated with TOF: – – – – – – DiGeorge’s Velocardiofacial Alagille’s Trisomy 21/18/13 Cat Eye San Luis Valley Mutations of Nkx 2.5 may be associated with TOF. Morphology Ventricular Septal Defect – 80% of cases fibrous continuation between the mitral, tricuspid, and aortic valves – 20% cases there is a muscular rim around the defect. – Rarely subarterial defect due to hypoplasia of the outlet septum. – The pulmonary valve annulus tends to be hypoplastic or atretic. Morphology Overriding Aorta – Degree of override can vary from an exclusive connection of the right ventricle to an exclusive connection to the left ventricle. Pulmonary Stenosis: – Infundibular – valvular Morphology Concentric right ventricular hypertrophy: – Secondary to RV outflow obstruction Associated Lesions Pulmonary valvar stenosis Pulmonary atresia Absence of pulmonary valve leaflets – pulmonary insufficiency in utero and marked dilatation of the pulmonary trunk. Right aortic arch Patent foramen ovale Atrial Septal Defect ( Pentalogy of Fallot) Anomalous origin of the LAD from the RCA. On Exam Cyanosis of varying degrees Clubbing Accentuated RV impulse Second heart sound usually single and loud. If mild PS with normal valve apparatus S2 normally split. Pulse pressure may be wide in the presence of aortopulmonary collaterals or PDA. On Exam Systolic Murmur: – Upper left sternal border, crescendo-decrescendo or plateau – Intensity inversely proportional to severity of the right to left shunt – In PS with intact septum murmur directly proportional to the severity of stenosis – Murmur decreases in intensity during hypercyanotic spells Diastolic Murmur: – If AI concomitantly heard in TOF with APV syndrome Tetralogy of Fallot Tetralogy of Fallot Catheterization Surgical repair Closure of VSD Relieving the RVOTO – – – – Pulmonary valvotomy Resection of infundibular muscle RVOT or subannular patch Transannular patch only when PV annulus is restrictive – Pulmonary valve implantation usually in adolescents and adults – Extracardiac conduit between the RV and PA Angioplasty or patch augmentation of the central pulmonary arteries – Closure of ASD or PFO Complications After Repair Mechanical problems Significant Pulmonary regurgitation RV Dilatation Restrictive RV Residual RVOTO Aneurysmal dilatation of the RVOT Residual VSD AR with or without root dilatation LV dysfunction Infective endocarditis Complications after repair Electrical Problems Heart block: RBBB, Bifascicular.Complete heart block is rare. Supraventricular arrythmia (A.flutter/A.fib) Relatively common. A.flutter-SCD,1:1. NSVT: Significance uncertain, ? SCD VT SCD Risks for SCD/VT Older age at repair Male gender Worse heart failure NYHA Class Chronic RV volume overload Presence of PR QRS duration >180 msec Transient complete heart block lasting more than 3 days after repair. Management of the complications Significant PR and RV dilatation – Pulmonic valve replacement with homograft or porcine bioprosthesis – Timing of surgery is debated. Selection of asymptomatic patient ?criteria. – Might TV annuloplasty if concomitant moderate to severe TR. Restrictive RV – Diuretics in the post-operative period. Residual RVOTO – Indicated if RVSP>2/3 systemic pressure. – Surgery or balloon angioplasty with stenting. Management of complications Aneurysmal dilatation of the RVOT Residual VSD: – Surgical correction if Qp/Qs>2:1 or 1.5-2:1 with LV dilatation or dysfunctionor H/O paradoxical emboli Dilatation of aortic root: – Aortic root replacement when ascending aorta>55 mm and/or in the presence severe AI. Management of the Complications Heart block: – Patients with bifascicular block with prolonged PR (>0.20 msec) should have holter or EPS. If true trifascicular block is documented then pacemaker. SVT – Correction of target residual hemodynamic lesions. – RF ablation for a.flutter or reentrant tachycardia either percutaneously or during surgery. – Biatrial maze for a.fib. – Anti-arrhythmic meds /antitachycardia pacemakers can be used as adjunctivetools. Management of Complications VT – – – – Correction of the hemodynamic lesions RF ablation of VT focus Role of AICD (?) Antiarrhythmic meds for symptomatic patients. SCD – Resuscitated SCD-AICD – Role of prophylactic AICD (?) Fontan procedure-requiring patient Tricuspid atresia Hypoplastic left ventricle Double inlet ventricle Isomerism Tricuspid atresia Tricuspid Atresia On Echo Hypoplastic Left Heart Double Inlet Ventricle Fontan procedure 10 year survival is 60-70% Poor prognosis – 5 year survival 46-59% Fontan Patient Long term complications include – Atrial flutter or fibrillation Incidence increases with time – Right atrial thrombus formation – Obstruction of the Fontan circuit Should be r/o in all pts presenting with atrial arrhythmias – Ventricular dysfunction – Protein losing enteropathy Etiology unclear: pleural effusions, ascites, edema and chronic diarrhea. Low S.Albumin, high α-1 antitrypsin stool clearance. Management of Complication In patients with atrial fibrillation and flutter – Prompt TEE followed by cardioversion – Maintenance of sinus with drugs ( Amiodarone) <50% success rate. – Transcatheter atrial ablation <50% success rate – Recalcitrant cases surgical revision with antiarrhythmic surgery Management of Complications In patients with established thrombus – Thrombolytic therapy – Surgical thrombectomy – Conversion of Fontan circuit – Long term anticoagulation – Many centers prophylactically anticoagulate all patients with Fontan. Management of complications In patients with Fontan obstruction – Surgical revision – Balloon angioplasty Protein losing enteropathy – – – – Creation of a fenestration in the atrial septum Heparin SQ IV Octreotide Prednisone In ECHO The presence or absence of right atrial stasis, thrombus, patency of a fenestration, and Fontan circuit obstruction should be sought. Superior and inferior vena cavae biphasic and pulmonary artery triphasic flow patterns suggest unobstructed flow in the Fontan circuit. Mean gradient between the Fontan circuit and the pulmonary artery of 2 mm Hg or more may represent significant obstruction. Conduit or Lateral Tunnel Fontan Fenestration Fontan TGA D loop L loop RV follow the Loop – D loop------RV on the right – L loop------RV on the left L TGA Congenitally corrected TGA. Ventricular inversion D TGA Incidence 20-30/1000,000 live births. Without treatment 30% die within 1st week, 50% within 1st month, 70% in 6 months and 90% in first year. With current medical and surgical interventions 90% early and midterm survival. In complete Transposition the connection between atria and ventricles are normal. The connections between the ventricles and the great arteries are discordant. D TGA Surgery Most commonly done procedures were atrial switch operations: – Blood redirected at atrial level with a baffle made of dacron or pericardium (Mustard) – With atrial flaps (Senning) to achieve physiological correction Surgery Current practice is the arterial switch operation developed in 1980’s. Blood is redirected at the level of the great artery – the morphological left ventricle becomes the subaortic ventricle – the morphological right ventricle becomes the subpulmonic ventricle. Atrial Switch Surgery (Mustard/Senning Procedure) Mustard Procedure Mustard Procedure Modified Arterial Switch Operation Consequences of Mustard Most patients reaching adulthood have NYHA I/II symptoms over the next 25 years. 50% develop moderate systolic dysfunction of the RV but only few present with CHF. 1/3rd have severe systemic TR. Atrial flutter arises in 20% by age 20. 50% patients have sinus node dysfunction by age 20. Baffle leak or obstruction can also occur. Baffle Leak Consequences of Arterial Switch Data about long term consequences still unavailable. Concerns include: – supra neopulmonary artery stenosis – ostial coronary artery disease – progressive neoaortic valve regurgitation Treatment Use of ACE inhibitors suggests a benefit in nonrandomized studies. – randomized study using Ramipril is underway. Failed Mustard or Senning might be a reason for heart transplantation. Alternatively, atrial baffle can be converted to an arterial switch after retraining the LV with a pulmonary band. – No data available for outcomes TV replacement is not recommended since it worsens RV function. Ebstein’s anomaly May be associated with: •PFO •ASD •Most commonly secundum rarely primum •VSD •with or without pulmonary atresia •Pulmonary outflow obstruction •PDA •Coarctation of the aorta •LV cardiomyopathy Ebstein’s Anomaly Prevalence 1:50,000 to 100,000. Occurs with higher frequency in infants of mothers who took Lithium in early pregnancy. Clinical Features Depends on the severity of the pathology and the magnitude of right to left intraatrial shunting. In milder forms of the condition incidental murmur and arrhythmia may be the only features. In severe forms of the disease cyanosis is seen and depends on the extent of right to left shunting. Increased incidence of WPW syndrome. Echo Increase in RV volume Paradoxical septal motion Increase in TV excursion Delay in TV closure compared to MV closure Decreased TV EF slope Abnormal anterior position of the TV in diastole Prognosis and Management Survival at 1 year 67% and 10 year 59 %. Survival after Tricuspid bioprosthesis: 10 yr 93% and 15 yr 81%. 94% in NYHA I/II. Management consists of surgical repair of the TV or replacement in older individuals. Pregnancy BP decrease early in pregnancy ~10 mmHg, usually secondary to decreased SVR This increases flow across right to left shunts. 30-50% increase in intravascular volume. Poorly tolerated by women with valvular lesions and LV/RV dysfunction. Marked fluctuations occur in CO during normal labor and delivery. Pregnancy Major maternal cardiac risk factors Pulmonary HTN -Maternal mortality 30-50% with Eisenmenger syndrome. Maternal Cyanosis -Cyanotic worse then acyanotic. -Erythrocytosis worsens outcome. -Goal Hct<65 Poor maternal functional class -Difficult to assess in cyanotic CHD. H/O Arrhythmia -Antiarrhythmic drugs can be used with accepted risk for debilitating symptoms Anticoagulation Specific Patients TOF – Uncorrected TOF associated with worsening cyanosis. Susceptible to Infective endocarditis. – Corrected TOF usually uncomplicated pregnancy Ebstein’s Anomaly -Worsening RV function in pregnancy -Atrial tachyarrhythmias/ WPW -Cyanosis might be manifest for the first time -Increased risk of paradoxical embolization and hypoxemia -Acyanotic women with Ebstein’s tolerate pregnancy well if sinus rhythm is maintained. -Repaired Ebstein’s significantly reduces risks Specific patients Congenitally corrected TGA (L-TGA) -Increased risk of ventricular volume overload -Increased risk of heart block Complete TGA -Atrial baffle- Risks are related to functional status of the RV, PAP and rhythm disturbances. -Not enough experience with arterial switch. Fontan -Usually leads to uncomplicated pregnancy. -Risk of thrombus formation. Labor and delivery Women with unoperated or operated CHD who are functionally normal can be allowed to progress to spontaneous labor If functional adequacy of heart and lungs in doubt: -Controlled induction -Oxytocin low dose for induction. -Avoid maternal pushing -Assisted delivery with low forceps or vacuum extraction. Anesthesia -IM/IV narcotics safe. -Epidural safe to use :caution systemic venous pooling and increased thromboembolism risk. Labor and delivery No indication for routine Swan Ganz Routine endocarditis prophylaxis Cesarean delivery reserved for obstetric indications -A/W twice the amount of blood loss -Increased risks of wound and uterine infection -Increased incidence of post-op thrombophlebitis. Breastfeeding -Affects cardiac reserve and risks mastitis and bacteremia. Thanks