* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download PDF
Survey
Document related concepts
Transcript
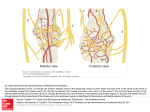
Pain Medicine 2015; 16: 13–17 Wiley Periodicals, Inc. EDITORIAL Long-Acting Local Anesthetic Agents and Additives: Snake Oil, Voodoo, or the Real Deal? Supported by the University of Florida. The authors disclose no conflicts of interest. The onset, spread, density, and duration of a nerve block are functions of what local anesthetic drug is injected, where it is injected, and for how long the nerve is exposed to it. Over the past 27 or more years, as far back as 1988 [1], researchers and companies, initially with topical tetracaine [1], and later as injectable liposomal bupivacaine [2], have been searching for the “magic bullet” to be injected somewhere near a nerve, or infiltrated into tissue, that will eliminate a patient’s acute or perioperative pain for as long as the pain lasts without unwanted side effects. The work of the Williams and colleagues, reported in this edition of Pain Medicine, is a further report on such efforts [3–5]. Although we have no or very little evidence of this, which points to a bigger problem eluded upon below, we instinctively know that most pain, especially surgical pain, outlasts our single-injection blocks, and we also know that we have to offer patients nerve blocks that last at least as long as the pain does without causing discomfort and unwanted side effects. Over the years, the challenge of developing a blocking drug that lasts long enough to outlast pain but that does not have similarly long-lasting unwanted side effects has been addressed by combining different drugs and developing new presentations of drugs. Few adjuvant agents, other than perhaps dexamethasone, have stood the test of time [6]. Another attempt was to add epinephrine to the existing arsenal of drugs [7] to cause vasoconstriction and decreased blood flow, thus slowing down the washout of the drugs and increasing the time that the nerve is exposed to the local anesthetic agent. We now know, that epinephrine does not increase the duration of action of bupivacaine or ropivacaine [7]; if anything, epinephrine can shorten the onset of a nerve block with bupivacaine, increase the density of such blocks, and increase the duration of some drugs like lidocaine, for example, by causing nerve ischemia [8] if used for nerve blocks. Workers like Dag Selander et al. [8], among many others, have conclusively shown years ago that this ischemia can cause nerve injury. Adding dexamethasone has been met with enthusiasm [6,9] because it seemed to extend the analgesic duration of a single-injection nerve block from an average of 730 minutes (12 hours) to around 1,306 minutes (21 hours), as reported in a recent meta-analysis of studies that involved 801 patients [9]. The authors also reported an increase in the duration of a motor block from 664 minutes (11 hours) to 1,102 minutes (18 hours). Desmut and colleagues [10], however, reported in a study of 150 patients that intravenous (IV) dexamethasone is equivalent to perineural dexamethasone in prolonging the analgesic duration of single-injection interscalene block with ropivacaine for arthroscopic shoulder surgery. Both IV and perineural dexamethasone almost doubled the duration from 757 (635–910) minutes of the sensory block. However, we already know that systemic (IV) dexamethasone has analgesic effects [11]. Systemic response to injury and its role in pain, the pain cascade, and pain mediators are important to mention [12]. Nerve blocks usually preempt (surgical) injury, whereas local infiltration of local anesthetic agents is usually administered after surgical trauma. It may well be that nerve blocks, combined with systemic steroids, are a more complete approach than a single injectable agent. In a recent large meta-analysis, De Oliveira and coworkers [11] showed that IV dexamethasone significantly reduces postoperative pain and opioid consumption. The question, therefore, was whether dexamethasone, if injected perineurally, could add any value without adding neurotoxicity. The study the Williams et al. reported in this edition of Pain Medicine [3] demonstrated that dexamethasone and other agents such as clonidine and buprenorphine, when combined with bupivacaine, caused no long-term motor or sensory deficits or damage to the sciatic nerves or dorsal root ganglia of rats. These findings, for dexamethasone at least, support the findings of a recent meta-analysis that reported no persistent nerve palsy in 180 patients who received perineural dexamethasone [8]. A further attempt at finding the “magic bullet” has been to engineer slow-release local anesthetic agents, for example, in liposomal spheres [2,13] (Exparel, Pacira Pharmaceuticals, Parsippany, NJ, USA), or in an organic matrix of sucrose acetate isobutyrate [14] (Posidur [SABERBupivacaine], Durect Corporation, Cupertino, CA, USA). Medication encapsulated within liposomes, including drugs such as ibuprofen, opioids, vitamins, cancer chemotherapeutic agents, and local anesthetic agents, is 13 Boezaart et al. slowly released because of the gradual breakdown of the lipid vesicles. Unlike their cousins, however, for whom slow release has been proven to be advantageous (liposomal vitamin C, liposomal glutathione, etc.), emerging literature [14] is unconvincing in proving the virtues of liposomal bupivacaine and suggests that it does not fulfill the promises it originally held for hemorrhoid [15,16] and bunion surgery [17]. In contrast to these findings, manufacturers heavily market it to surgeons. If it could extend the effects of a single-injection nerve block, for which it has not yet been approved by the Food and Drug Administration, for up to 72–96 hours, it would most probably be hugely unpopular and would probably self-destruct because few patients are likely to tolerate a 72- to 96hour motor, proprioception, and sensory nerve block. Similarly, very few patients would, for example, tolerate a 3- to 4-day phrenic nerve block or a 3- to 4-day quadriceps muscle block. Its use for tissue infiltration for surgery resulting in high pain levels such as knee and hip replacement surgery is also proving to be disappointing [18]. Market penetration and utilization has far outpaced science and data to support its use. In a recent study, the periarticular infiltration of 20 mL of 1.3% (266 mg) liposomal bupivacaine [18] after total knee arthroplasty provided inferior pain control compared with the 100 times cheaper 30 mL of 0.5% bupivacaine (150 mg) in 1:200,000 epinephrine. Another article by Barington and colleagues [19], which was supported by an educational grant from the manufacturers of liposomal bupivacaine, presents weak data for its successful use. In 2007, Parvataneni and coworkers [20] reported successful use of a multimodal periarticular injection consisting of bupivacaine (200–400 mg), morphine sulfate (4–10 mg), epinephrine (300 mcg), methylprednisolone acetate (40 mg), and cefuroxime (750 mg) in normal saline. However, when bupivacaine alone was injected into the hip joint capsule after total hip arthroplasty, it, similar to liposomal bupivacaine [19], faired no better, or only marginally better than placebo [21]. The data strongly suggest that it is most likely the other drugs (morphine, and steroidal and other multimodal agents) that have the analgesic effect and not the local anesthetic agent. Encapsulated morphine for epidural use was originally met with great enthusiasm [22], but because of the plethora of unwanted and even dangerous side effects that ranged from itching (51%), nausea (76%), vomiting (53%), and a large percentage (16%) of respiratory depression for a relatively long time in one study [20], it basically disappeared from general use. To put it bluntly, if a drug can cause a long-acting nerve block effect, it most likely can also cause long-acting unwanted side effects. Furthermore, even doubling the effective time of any short-acting local anesthetic agent by any means, no matter how safe, when the agent wears off, it will still unmask the untreated severe pain of surgical insults that usually last for days and even weeks, not just for hours. The work of the Williams et al. [3–5], as reported in this issue of Pain Medicine, is a further effort to minimize these unwanted side effects and maximize the positive 14 ones. This is a very noble effort that should be wholeheartedly supported and congratulated because if they succeed, the implications could be huge. For example, imagine the impact of empowering a medic on the battlefield who can do a quick, easy, and safe nerve block on a fellow soldier who is wounded, and the block can last 30– 72 hours, keeping the soldier free of pain until he/she can be transferred to a field hospital or other advanced medical facility. As with so many other advances in medicine that originated in the military, a long-acting local anesthetic agent may very well be another one of these developments. Even continuous nerve blocks were first performed in 1975 in an austere war situation [23]. To understand the onset and duration of nerve blocks, however, we need to understand the microanatomy of peripheral nerves. Figure 1 is a summary of the important recent work of Anderson and colleagues [24] in elegant dissections of peripheral nerves and Karmakar and colleagues [25] with their excellent work with high-definition ultrasound to help us understand the “sweet spot” [24] of a nerve. Unlike an electrical current that can readily cross over anatomical barriers [26], even over the skin [27], local anesthetic agents cannot easily cross over biological membranes [28]. If they could easily cross over anatomical and physiological barriers, they would be highly toxic and unusable [29]. Local anesthetic drugs complete their action on the sodium channels of nerve axons by diffusing to the axons (Figure 1, areas 13–16). This diffusion takes place against a concentration gradient following Fick’s Law [28] (simplified as dQ/dt 5 P 3 DC, where dQ/dt is the rate of passive diffusion, P is the permeability constant of the drug, and DC is the concentration gradient). A local anesthetic drug injected on the far side of a barrier or biological membrane, or a number of membranes, will take much longer to reach the axons (if at all) than the same drug injected on the same (near) side of the membrane or sheath as the axons, and the duration of action, will be significantly shorter. To illustrate, a drug injected into a nerve fascicle deep to the perineurium (Figure 1, area 11) would have a very fast, almost immediate, onset of action, and the action would last for weeks, if not months—the typical numb thumb 5 weeks after an intrafascicular injection during an axillary nerve block. The same drug, on the other hand, injected into a muscle near a nerve or in the subepimyseal space (Figure 1, area 6) would have a very slow onset (if at all), and the local blood flow would remove the drug so that the action would be very short. A drug injected into the subparaneural space (recently more correctly named the subcircumneural space [30]) (Figure 1, area 8), the “sweet spot” of the nerve [30], will have a short onset of action, and depending on the volume and concentration and type of drug, the density and duration of the single-injection block would vary. If, however, a drug that has a 3- to 4day action on the axons was injected into the subcircumneural space (Figure 1, area 8) and the drug is not readily removed by the local blood flow, all the axons would be blocked, causing a block not only of pain fibers but also Editorial Figure 1 Microanatomy of the ulnar nerve (1). Basilic vein (2), brachial vein (3), and brachial artery (4) among others share a common epimysium (5). Deep to this fascia layer is the subepimyseal space (6) that forms the well known “doughnut sign” on ultrasound if fluid is injected into it. The ulnar nerve is surrounded by the circumneural sheath (7) (also referred to as the “paraneural sheath”), which is a thin but tough layer (previously, especially in neurosurgical texts, referred to as the “gliding apparatus”), and deep to this sheath is the subcircumneural space (8)—the “sweet-spot” of the nerve. Each nerve is in turn surrounded by an epineurium (9), which houses the nerve fascicles (10). Each nerve fascicle is surrounded by a tough and relatively noncompliant perineurium (11), which is thought to be embryological remnants of the dural sheath that surround nerve roots at the paravertebral level. The fluid and fibrous collagen inside the fascicles forms the endoneurium (12), and the a-, c-, c-, and bfibers (13–16) are situated in the endoneurium (Reprinted with the kind permission of Mary K. Bryson). of sensory, motor, and proprioception fibers—something probably very few patients would tolerate. If that same drug were injected on the far side of a barrier or a number of barriers, the concentration of the drug, for example, released by liposomes, would probably be too low and would be removed too quickly to have any effect. What we desperately need today is a drug or treatment modality that can selectively block sensory nerves and pain fibers that can be turned on and off as the need exists and that can outlast surgical pain. We need blocks that we can increase the spread of (to block more nerves if we need to; for example, more epidural roots or roots or cords of the brachial plexus), that we can control the density of by changing the concentration of the drug used, and that we can increase the motor component if we need to, for example, in rotator cuff surgery, or decrease the motor component to facilitate earlier mobilization and 15 Boezaart et al. ambulation. We need to be able to control the density of the block to facilitate feeling but have it dense enough to block only the pain fibers of a nerve, and we need to be able to turn the block off when the pain has subsided and the block is no longer needed. We also need to be able to reinitiate the block if the pain returns or the block has been turned off prematurely. We believe our research efforts and energy and resources should be spent on the above by promoting the establishment of more subspecialty acute pain services, further developing, for example, continuous peripheral nerve blocks [31] and delivery devices (pumps and catheter systems), etc., that we as practitioners can control. Surgeons and especially patients are fearful of pain and are therefore willing to try almost anything given enough suffering, but this dilutes the science of perioperative pain management, and this should further bolster support for acute pain medicine as a subspecialty. The development of long-acting, single-injections blocks, except for exceptional use in mild to moderate intermediate pain situations that are perhaps better served by other systemic and enteral analgesic regimes, and military use of course, will most probably not advance our cause of properly caring for patients with acute pain and for patients with longerlasting, hideous pain such as pain caused by cancer [32]. Obviously, despite decades of research, the manufacture of products (many of which have fizzled), and the creation of techniques (including minimally invasive surgery), the treatment of pain remains a major issue, which means no clear winner has emerged. If the goal is to get the patient out of the recovery room, then most additives are useful. But if the goal is to treat patients with pain rather than pain itself, there is a good reason why many ideas and drugs have fizzled out over time. Ultimately, the truth will prevail and the patient with pain will be the final arbiter of this truth. The gallant, brave, and forward-looking efforts of Trip Buckenmaier, Patrick Tighe, and many others [33] to establish acute pain medicine as a subspecialty should receive the full and continuous support of all medical practitioners. A structured discipline of acute pain medicine would help to establish vibrant acute pain services and would contribute to the understanding of the pathophysiologic response to injury and its role in acute pain through systemic pathways that may trigger more inflammation, pain, swelling, and immobility for weeks (months in some patients), complex regional pain syndrome in some, and progression to chronic pain syndromes that develop after injury (including surgery) in others. Only then will we finally be able to offer patients nerve blocks and other treatment modalities that outlast their pain. ANDRÉ P. BOEZAART, MD, PhD,*† YURY ZASIMOVICH, MD,* and HARI K. PARVATANENI, MD† Departments of *Anesthesiology and † Orthopaedics, College of Medicine, University of Florida, Gainesville, Florida, USA 16 References 1 Gesztes A, Mezei M. Topical anesthesia of the skin by liposome-encapsulated tetracaine. Anesth Analg 1988;67:1079–81. 2 Boogaerts JG, Lafont ND, Declerq AG, et al. Epidural administration of liposome-associated bupivacaine for the management of postsurgical pain: A first study. J Clin Anesth 1994;6:315–20. 3 Williams B, Butt M, Zeller J, Coffee S, Pippi M. Multimodal perineural analgesia with combined bupivacaine-clonidinebuprenorphine-dexamethasone: Safe in vivo and chemically compatible in solution. Pain Med 2014; (in press). 4 Williams B, Ibinson J, Mangione M, et al. Research priorities regarding multimodal peripheral nerve blocks for postoperative analgesia and anesthesia, based on hospital quality data extracted from over 1300 cases (2011–2014). Pain Med 2014; (in press). 5 Williams B, Ibinson J, Mangione M, Scanlan R, Cohen P. Clinical benchmarks regarding multimodal peripheral nerve blocks for postoperative analgesia: Observations regarding combined perineural midazolam-clonidine-buprenorphine-dexamethasone. Pain Med 2014; (in press). 6 Choi S, Rodseth R, McCartney CJL. Effects of dexamethasone as a local anesthetic adjuvant for brachial plexus block: A systematic review and meta-analysis of randomized trials. Br J Anaesth 2014;112:427–39. 7 Butterworth JF. Clinical pharmacology of local anesthetic agents. In: Cousins MJ, Horlocker TT, Car DB, Bridenbaugh PO, eds. Neural Blockade in Clinical Anesthesia and Pain Medicine. Philadelphia: Wolters Kluwer/Lippencott Williams & Wilkins; 2009:101–2. 8 Selander D, Brattsand R, Lundborg G, Nordborg C, Olsson Y. Local anesthetics: Importance of mode of application, concentration and adrenaline for the appearance of nerve lesions. An experimental study of axonal degeneration and barrier damage after intrafascicular injection or topical application of bupivacaine (Marcain). Acta Anaesthesiol Scand 1979;23:127–36. 9 Rasmussen SB, Saied NN, Bowens C Jr, et al. Duration of upper and lower extremity peripheral nerve blockade is prolonged with dexamethasone when added ropivacaine: A retrospective database analysis. Pain Med 2013;14:1239–47. 10 Desmut M, Braems H, Reynvoet M, et al. I.V. and perineural dexamethasone are equivalent in increasing the analgesic duration of a single-shot interscalene block with ropivacaine for shoulder surgery: A prospective, randomized, placebo-controlled study. Br J Anaesth 2013;111:445–52. Editorial 11 De Oliveira GS Jr, Almeida MD, Benzon HT, et al. Perioperative single dose systemic dexamethasone for postoperative pain: A meta-analysis of randomized controlled trials. Anesthesiology 2011;115:575– 88. 22 Viscusi ER, Martin G, Hartrick CT, Single N, Manvelian G. Forty-eight hours of postoperative pain relief after total hip arthroplasty with a novel, extended-release epidural morphine formulation. Anesthesiology 2005;102:1014–22. 12 Jules-Elysee KM, Wilfred SE, Memtsoudis SG, et al. Steroid modulation of cytokine release and desmosine levels in bilateral total knee replacement. J Bone Joint Surg Am 2012;94:2120–7. 23 Buckenmaier CC III. Battlefield orthopaedic anesthesia. In: Boezaart AP, ed. Anesthesia and Orthopaedic Surgery. New York: McGraw Hill; 2006:435–6. 13 Grant GJ, Bansinath M. Liposomal delivery systems for local anesthetics. Reg Anesth Pain Med 2001; 26:61–3. 14 Skolnik A, Gan TJ. New formulations of bupivacaine for the treatment of postoperative pain: Liposomal bupivacaine and SABER-Bupivacaine. Expert Opin Pharmacother 2014;15:1535–42. 15 Haas E, Onel E, Miller H, et al. A double-blind, randomized, active-controlled study for posthemorrhoidectomy pain managed with liposome bupivacaine, a novel local anesthetic formulation. Am Surg 2012;78:574–81. 16 Gorfine SR, Onel E, Patou G, et al. Bupivacaine extended-release liposome injection for prolonged postsurgical analgesia in patients undergoing hemorrhoidectomy: A multicenter, randomized, doubleblind, placebo-controlled trial. Dis Colon Rectum 2011;54:1552–9. 17 Golf M, Daniels SE, Onel E. A phase 3, randomized, R bupivacaine placebo-controlled trial of DepoFoamV (extended-release bupivacaine local analgesic) in bunionectomy. Adv Ther 2011;28:776–88. 18 Bagsby DT, Ireland PH, Meneghini RM. Liposomal bupivacaine versus traditional periarticular injection for pain control after total knee arthroplasty. J Arthroplasty 2014;29:1687–90. 19 Barington JW, Dalury DF, Emerson RH Jr, et al. Improving patient outcomes through advanced pain management techniques in total hip and knee arthroplasty. Am J Orthoped 2013;XLII:S1–15. 20 Parvataneni HK, Shah VP, Howard H, et al. Controlling pain after total hip and knee arthroplasty using a multimodal protocol with local periarticular injections: A prospective randomized study. J Arthroplasty 2007;22(6 suppl 2):33–8. 21 Zoric L, Cuillon P, Alonso S, et al. Single-shot intraoperative local anaesthetic infiltration does not reduce morphine consumption after total hip arthroplasty: A double-blinded placebo-controlled randomized study. Br J Anaesth 2014;112:722–8. 24 Anderson HL, Anderson SL, Tranum-Jensen J. Injection inside the paraneural sheath of the sciatic nerve: Direct comparison among ultrasound imaging, macroscopic anatomy and histological analysis. Reg Anesth Pain Med 2012;37:410–4. 25 Karmakar MK, Shariat AN, Pangthipampai P, Chen J. High-definition ultrasound imaging defines the paraneural sheath and the fascial compartments surrounding the sciatic nerve at the popliteal fossa. Reg Anesth Pain Med 2013;38:447–51. 26 Tsui BC, Hopkins D. Electrical nerve stimulation in regional anesthesia. In: Boezaart AP, ed. Anesthesia and Orthopaedic Surgery. New York: McGraw Hill; 2006:249–56. €senberg AT, Raw R, Boezaart AP. Surface map27 Bo ping of peripheral nerves in children with a nerve stimulator. Pediatr Anaesth 2002;12:398–403. 28 Mather LE, Tucker GT. Properties, absorption and disposition of local anesthetic agents. In: Cousins MJ, Horlocker TT, Car DB, Bridenbaugh PO, eds. Neural Blockade in Clinical Anesthesia and Pain Medicine. Philadelphia: Wolters Kluwer/Lippencott Williams & Wilkins; 2009:53. 29 McLeod GA, Butterworth JF, Wildsmith JAW. Local anesthetic systemic toxicity. In: Cousins MJ, Horlocker TT, Car DB, Bridenbaugh PO, eds. Neural Blockade in Clinical Anesthesia and Pain Medicine. Philadelphia: Wolters Kluwer/Lippencott Williams & Wilkins; 2009:114–32. 30 Boezaart AP. The sweet spot of the nerve: Is the “paraneural sheath named correctly and does it matter? Reg Anesth Pain Med 2014;(In Press). 31 Richman JM, Liu SS, Courpas G, et al. Does continuous peripheral nerve block provide superior pain control to opioids? A meta-analysis. Anesth Analg 2006;102:248–57. 32 Esch AT, Esch A, Knorr JL, et al. Long-term ambulatory continuous nerve blocks for terminally ill patients: A case series. Pain Med 2010;11:1299–302. 33 Carr DB, Boezaart AP, Buckenmaier CC III, et al. The Acute Pain Medicine special interest group (APMSIG). Pain Med 2013;14:1117–8. 17