* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download A)€€€€ The Formula For The Chemical Compound Magnesium
Survey
Document related concepts
Water splitting wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Acid–base reaction wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Rate equation wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Electrolysis of water wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Stoichiometry wikipedia , lookup
Hydrogen atom wikipedia , lookup
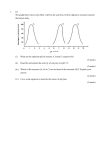
Transcript
Q1. (a) The formula for the chemical compound magnesium sulphate is MgSO4. Calculate the relative formula mass (Mr)of this compound. (Show your working.) ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (2) (b) Magnesium sulphate can be made from magnesium and dilute sulphuric acid. This is the equation for the reaction. Mg + H2SO4 → MgSO 4 + H2 Calculate the mass of magnesium sulphate that would be obtained from 4g of magnesium. (Show your working.) ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... Answer..................................... g (2) (Total 4 marks) Q2. The formula for the chemical compound magnesium sulphate is MgSO4. Calculate the relative formula mass (Mr) of this compound. (Show your working.) ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... (Total 2 marks) Page 1 of 71 Q3. Calculate the formula mass (Mr), of the compound calcium hydroxide, Ca (OH)2. (Show your working) ............................................................................................................................................ ............................................................................................................................................ ............................................................................................................................................ ............................................................................................................................................ ............................................................................................................................................ ............................................................................................................................................ (Total 3 marks) Q4. The information on the Data Sheet will be helpful in answering this question. (a) Calculate the formula mass (Mr) of the compound iron (III) oxide, Fe2O3. (Show your working.) ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (3) Page 2 of 71 (b) Calculate the mass of iron produced when 32g of iron (III) oxide is completely reduced by aluminium. The reaction is shown in the symbol equation: Fe2O3 + 2Al → 2Fe + Al 2O3 (Show your working.) ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... Answer = ..................................... grams (3) (Total 6 marks) Q5. (a) The formula for ammonia is NH3. What does the formula tell you about each molecule of ammonia? ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (3) (b) Ammonia is used to make nitric acid (HNO3). Calculate the formula mass (Mr) for nitric acid. (Show your working). ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (3) (Total 6 marks) Page 3 of 71 ## In this question you will need to use the following information: Relative atomic masses: H 1; O 16; Mg 24. The volume of one mole of any gas is 24 dm3 at room temperature and atmospheric pressure. The diagram shows a chemical reaction taking place in a conical flask. The balanced equation for this reaction is: Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g) (a) Write a balanced ionic equation for this reaction. .................................................................................................................................... (2) (b) Calculate the mass of magnesium required to produce 0.50 g of hydrogen. Show clearly how you work out your final answer and give the unit. ..................................................................................................................................... ..................................................................................................................................... Mass = ............................... (2) (c) (i) Draw a diagram to show how the electrons are arranged in a hydrogen molecule. (1) Page 4 of 71 (ii) What is the name of the type of chemical bond between the hydrogen atoms in a hydrogen molecule? ........................................................................................................................... (1) (d) The chemical formula for hydrogen peroxide is H2O2. Calculate, to the nearest whole number, the percentage, by mass, of hydrogen in hydrogen peroxide. Show clearly how you work out your answer. .................................................................................................................................... .................................................................................................................................... Percentage = ................................. % (2) (Total 8 marks) Q7. The balanced symbol equation for the reaction is H2 (g) + Cl2 (g) → 2HCl (g) Starting with 2 g of hydrogen, what mass of hydrogen chloride would be produced? (Relative atomic masses: H = 1; Cl = 35.5) ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... Mass of hydrogen chloride = ...................................... g (Total 3 marks) Q8. Ammonium chloride, NH4Cl, is made up of nitrogen, hydrogen and chlorine atoms. (i) Complete the table to show the number of atoms of each element present in NH4Cl. Element Number of atoms in NH4Cl nitrogen 1 hydrogen chlorine (1) Page 5 of 71 (ii) Calculate the relative formula mass of ammonium chloride, NH4Cl. (Relative atomic masses: H = 1, N = 14, Cl = 35.5) ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... Relative formula mass = ................................................. (2) (Total 3 marks) Q9. Calculate the percentage of iron in iron sulphate (FeSO4). (Relative atomic masses: Fe = 56, O = 16, S = 32) ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... Percentage of iron in iron sulphate = ..........................% (Total 3 marks) Q10. Iron is the most commonly used metal. Iron is extracted in a blast furnace from iron oxide using carbon monoxide. Fe2O3 (a) + 3CO → Fe + 3CO2 A sample of the ore haematite contains 70% iron oxide. Calculate the amount of iron oxide in 2000 tonnes of haematite. ..................................................................................................................................... ..................................................................................................................................... Amount of iron oxide = ......................................... tonnes (1) Page 6 of 71 (b) Calculate the amount of iron that can be extracted from 2000 tonnes of haematite. (Relative atomic masses: O = 16; Fe = 56) .................................................................................................................................... .................................................................................................................................... .................................................................................................................................... .................................................................................................................................... .................................................................................................................................... .................................................................................................................................... Amount of iron = .................................................... tonnes (4) (Total 5 marks) Q11. Nitrates, such as ammonium nitrate, are added to soil to help plant growth. (a) When rain falls nitrates dissolve and can end up in drinking water. Nitrates in drinking water can stop respiration in babies. This only happens if there is a lot of nitrate in the drinking water. Plants use nitrates for growth. Humans need plants. Should large amounts of nitrates be added to soil? Give two reasons for your answer. Answer ...................................................................................................................... Reason 1 .................................................................................................................... .................................................................................................................................... Reason 2 .................................................................................................................... .................................................................................................................................... (2) Page 7 of 71 (b) The amount of nitrogen in a nitrate compound is important. (i) How many nitrogen atoms are there in the formula of ammonium nitrate, NH4NO3 .......................................................................................................................... (1) (ii) Calculate the percentage of nitrogen in ammonium nitrate, NH4NO3. (Relative atomic masses: H = 1; N = 14; O = 16) .......................................................................................................................... .......................................................................................................................... .......................................................................................................................... Percentage of nitrogen in ammonium nitrate = ........................................... % (3) (Total 6 marks) Q12. Ammonia is a very important chemical. (a) The table shows the percentage of ammonia used to make different substances. SUBSTANCES MADE FROM AMMONIA PERCENTAGE (%) OF AMMONIA USED fertilisers 75 nitric acid 10 nylon 5 others 10 Page 8 of 71 Shade on the pie chart the percentage of ammonia used to make nitric acid. (1) (b) Ammonia gas is made by the reaction between nitrogen gas and hydrogen gas. Write a word equation to represent this reaction. .............................. + .............................. .............................. (1) (c) Nitrogen is one of the raw materials used to make ammonia. Nitrogen is obtained from air. This pie chart shows the proportion of nitrogen, oxygen and other gases in air. Label the area which represents the proportion of nitrogen in air. (1) Page 9 of 71 (d) An artificial fertiliser contains compounds with the formulae: NH4NO3 and (i) KCl Use the Data Sheet to help you answer this question. Name the elements in the compound NH4NO3. 1 .......................................................... 2 .......................................................... 3 .......................................................... (2) (ii) Use the Data Sheet to help you answer this question. Name the compound KCl. .......................................................................................................................... (1) (e) (i) Ammonium nitrate is one type of artificial fertiliser. Calculate the relative formula mass of ammonium nitrate NH4NO3. (Relative atomic masses: H = 1, N = 14, O = 16.) .......................................................................................................................... .......................................................................................................................... (1) (ii) Use your answer to part (f)(i) to help you calculate the percentage by mass of nitrogen present in ammonium nitrate NH4NO3. .......................................................................................................................... .......................................................................................................................... .......................................................................................................................... (2) (Total 9 marks) Page 10 of 71 Q13. The electrolysis of sodium chloride solution is an important industrial process. Three useful substances are produced: • chlorine gas is formed at the positive electrode; • hydrogen gas is formed at the negative electrode; • an alkali is left in the solution. The reactions which take place at the electrodes are represented by the equations shown below: 2Cl– – 2e– → Cl2 2H+ + 2e– → H2 (a) Name the important alkali which is left in the solution. .................................................................................................................................... (1) (b) State why chloride ions move towards the positive electrode. .................................................................................................................................... (1) (c) Why is the formation of chlorine at this electrode said to be an oxidation reaction? .................................................................................................................................... (1) (Total 3 marks) Q14. Quicklime can be converted to slaked lime. The equation which represents this reaction is shown below. CaO(s) + H2O(l) → Ca(OH)2(s) quickline (i) slaked line Why do farmers sometimes add slaked lime to acidic soil? ..................................................................................................................................... ..................................................................................................................................... (1) (ii) Use these relative atomic masses: H = 1; O = 16; Ca = 40 to calculate the relative formula mass (M r) of quicklime CaO ........................................................................................................... slaked lime Ca(OH)2 .................................................................................................. (2) Page 11 of 71 (iii) Calculate the mass of slaked lime that could be made from 1000 kg of quicklime. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... Mass of slaked lime .................... kg (2) (Total 5 marks) Q15. Use these relative atomic masses: H = 1; O = 16; Ca = 40 to calculate the relative formula mass (Mr ) of quicklime CaO ...................................................................................................................... slaked lime Ca(OH)2 ............................................................................................................. (Total 2 marks) Q16. Sodium hydroxide, hydrogen and chlorine can all be made in one industrial process. Electricity is passed through aqueous sodium chloride solution (brine). The diagram below shows a cell that can be used for this process. (a) Name A, B and C. Gas A ......................................................................................................................... Gas B ......................................................................................................................... Solution C .................................................................................................................. (2) (b) Balance the equations for the reactions at the electrodes. (i) .......... Cl– – ............ e– → Cl2 (ii) .......... H+ + ............ e– → H2 (2) Page 12 of 71 (c) Name the compound in this cell which produces the hydrogen ions. ..................................................................................................................................... (1) (d) Which type of particles must be able to pass through the barrier to allow the electrolysis to take place? ..................................................................................................................................... (1) (Total 6 marks) Q17. The following passage was taken from a chemistry textbook. Germanium is a white, shiny, brittle element. It is used in the electronics industry because it is able to conduct a small amount of electricity. It is made from germanium oxide obtained from flue dusts of zinc and lead smelters. The impure germanium oxide from the flue dusts is changed into germanium by the process outlined below. STEP 1 The germanium oxide is reacted with hydrochloric acid to make germanium tetrachloride. This is a volatile liquid in which the germanium and chlorine atoms are joined by covalent bonds. STEP 2 The germanium tetrachloride is distilled off from the mixture. STEP 3 The germanium tetrachloride is added to an excess of water to produce germanium oxide and hydrochloric acid. STEPS 1 to 3 are repeated several times. STEP 4 The pure germanium oxide is reduced by hydrogen to form germanium. (a) Balance the equation below which represents the reaction in step 1. GeO2 + ............ HCl → GeCl 4 + ............ H2O (1) (b) Write a word equation for the reaction in step 3. ..................................................................................................................................... (1) (c) Suggest why steps 1 to 3 are repeated several times. ..................................................................................................................................... ..................................................................................................................................... (1) (d) The equation which represents the reaction in step 4 is shown below. GeO2 + 2H2 → Ge + 2H 2O Page 13 of 71 (i) Explain what is meant by the term ‘reduced’. ........................................................................................................................... ........................................................................................................................... (1) (ii) Calculate the mass of germanium which could be made from 525 g of germanium oxide. (Relative atomic masses: Ge = 73; O = 16). ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... Mass ......................................... g (3) (e) Germanium is difficult to classify as either a metal or a non-metal. (i) Give as much evidence as you can from the information in this question to support the view that germanium is a metal. Explain your answer as fully as you can. ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... (3) (ii) Give as much evidence as you can from the information in this question to support the view that germanium is a non-metal. Explain your answer as fully as you can. ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... (3) (Total 13 marks) Q18. Calcium oxide (quicklime) is made by heating calcium carbonate (limestone). calcium carbonate → calcium oxide + carbon dioxide 100g ? 44g Page 14 of 71 (a) 44 grams of carbon dioxide is produced when 100 grams of calcium carbonate is heated. Calculate the mass of calcium oxide produced when 100 grams of calcium carbonate is heated. .................................................................................................................................... mass ......................... g (1) (b) What mass of carbon dioxide could be made from 100 tonnes of calcium carbonate? mass ....................... tonnes (1) (Total 2 marks) Q19. Ammonium nitrate is an important fertiliser. It is made by reacting nitric acid with the alkali ammonia. (i) State the type of reaction taking place. ........................................................................................................................... (1) (ii) The equation for this reaction is: NH3 + HNO3 → NH 4NO3 Calculate the number of tonnes of ammonium nitrate that can be made from 68 tonnes of ammonia. (Relative atomic masses: H = 1, N = 14, O = 16) ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... (3) (Total 4 marks) Page 15 of 71 Q20. 280 000 tonnes of magnesium are produced in the world each year. The pie chart below shows the ways in which magnesium is used. (a) (i) Use the pie chart to calculate the percentage of magnesium used to make aluminium alloys. ....................................... % (1) (ii) How many tonnes of magnesium are used to make aluminium alloys each year? ....................................... tonnes (1) (b) Magnesium is produced by the electrolysis of molten magnesium chloride. The reactions which take place at the electrodes are represented by the equations below. Mg2+ + 2e– → Mg 2Cl– – 2e– → Cl2 (i) Calculate the mass of chlorine produced when one kilogram of magnesium is made. (Relative atomic masses: Mg = 24, Cl = 35.5) ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... (3) Page 16 of 71 (ii) Give a use for chlorine. ........................................................................................................................... (1) (Total 6 marks) Q21. Limestone (CaCO3) is a raw material. On strong heating it is converted to calcium oxide which is a very useful substance. (a) Calculate the formula mass (Mr) of calcium carbonate. ..................................................................................................................................... Mr of calcium carbonate = ............................................... (2) (b) About 60 million tonnes of calcium oxide is made in Britain each year. Calculate the mass of calcium carbonate needed to make this amount of calcium oxide. .................................................................................................................................... .................................................................................................................................... .................................................................................................................................... .................................................................................................................................... .................................................................................................................................... .................................................................................................................................... Mass of calcium carbonate needed = .............................. million tonnes (4) (c) Water is added to some of the calcium oxide produced in a process known as ‘slaking’. The product of this reaction is used to make plaster. CaO(s) + H2O(1)→ Ca(OH)2(s) (i) Give the chemical name of Ca(OH)2. .......................................................................................................................... (1) (ii) What is the physical state of the Ca(OH)2 formed in the reaction? .......................................................................................................................... (1) (Total 8 marks) Page 17 of 71 Q22. Titanium is a transition metal used as pins and plates to support badly broken bones. Titanium is extracted from an ore that contains the mineral titanium oxide. This oxide is converted into titanium chloride. Titanium chloride is heated with sodium to form titanium metal. This reaction takes place in an atmosphere of a noble gas, such as argon. 4Na(s) + TiCl4(l) → Ti(s) + 4NaCl(s) Calculate the mass of titanium that can be extracted from 570 kg of titanium chloride. Relative atomic masses: Cl 35.5; Ti 48. ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... Mass of titanium = ............................ kg (Total 3 marks) Q23. Follow the steps to find the percentage of iron in iron oxide. Relative atomic masses: O 16; Fe 56. (i) Step 1 Calculate the relative formula mass of iron oxide, Fe2O3. ..................................................................................................................................... ..................................................................................................................................... (1) (ii) Step 2 Calculate the total relative mass of just the iron atoms in the formula, Fe2O3. ..................................................................................................................................... (1) Page 18 of 71 (iii) Step 3 Calculate the percentage (%) of iron in the iron oxide, Fe2O3. ..................................................................................................................................... ..................................................................................................................................... Percentage of iron ................................. % (1) (Total 3 marks) Q24. Limestone is a useful mineral. Every day, large amounts of limestone are heated in limekilns to produce lime. Lime is used in the manufacture of iron, cement and glass and for neutralising acidic soils. CaCO3 (i) CaO + CO2 The decomposition of limestone is a reversible reaction. Explain what this means. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (2) Page 19 of 71 (ii) Calculate the mass of lime, CaO, that would be produced from 250 tonnes of limestone, CaCO3. Relative atomic masses: C 12; O 16; Ca 40. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... Mass of lime = ........................................ tonnes (3) (Total 5 marks) Q25. Uranium metal can be produced by reacting uranium hexafluoride with calcium. UF6 + 3Ca → 3CaF2 + U (a) Describe how calcium and fluorine bond together to form calcium fluoride. The electron arrangement of each atom is shown. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (5) Page 20 of 71 (b) Uranium has two main isotopes, meant by the word isotope. and . Use these as examples to explain what is ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (4) (c) At the start of a reaction there was 174.5 g of uranium hexafluoride, UF6. Relative atomic masses: F 19; U 235 (i) Calculate the relative formula mass of uranium hexafluoride, UF6. ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... Relative formula mass UF6 = .................................... g (1) (ii) Calculate the mass of uranium that would be produced from 134.5 g of uranium hexafluoride. ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... Mass of uranium = .................................. g (2) (Total 12 marks) Page 21 of 71 Q26. The chemical equation for the formation of iron is: Fe2O3(s) + 3CO(g) → 2Fe(s) + 3CO2(g) Calculate the relative formula mass of iron oxide, Fe2O3. Relative atomic masses: O 16; Fe 56. ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... Relative formula mass Fe2O3 = ................................ (Total 2 marks) Q27. Petrol is a mixture of hydrocarbons such as octane, C8H18 When petrol is burned in a car engine, a large amount of carbon dioxide is produced. This car uses 114 g of petrol to travel one mile. Calculate the mass of carbon dioxide produced when this car travels one mile. Assume that petrol is octane and that combustion is complete. (Relative atomic masses: H = 1; C = 12; O = 16) Page 22 of 71 The combustion of octane can be represented by this equation. C8H18 + 12 O2 → 8CO2 + 9H2O ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... Mass of carbon dioxide = ........................ g (Total 3 marks) Q28. Silicon is an important element used in the electronics industry. (a) Silicon can be made by heating a mixture of sand (silicon dioxide) with magnesium powder. The equation for this reaction is shown below. SiO2 (s)+ 2Mg (s) → 2MgO (s) + Si (s) Calculate the mass of silicon dioxide needed to make 1 g of silicon. Relative atomic masses: O = 16; Si = 28 ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... Mass = ........................................................g (3) Page 23 of 71 (b) The resulting mixture of magnesium oxide and silicon is added to a beaker containing hydrochloric acid. The silicon is then filtered from the solution. (i) The magnesium oxide reacts with the hydrochloric acid and forms magnesium chloride (MgCl2) solution and water. magnesium oxide + hydrochloric acid → magnesium chloride solution + water Write a balanced symbol equation for this reaction, including state symbols. .......................................................................................................................... (2) (ii) The gases produced are a mixture of several silicon hydrides. One of the gases produced in the reaction is the silicon hydride with the formula SiH4. The structure of this molecule is similar to methane, CH4. Draw a diagram to show the bonding in a molecule of SiH4. Represent the electrons as dots and crosses and only show the outer shell (energy level) electrons. (1) Page 24 of 71 (iii) A sample of a different silicon hydride was found to contain 1.4 g of silicon and 0.15 g of hydrogen. Calculate the formula of this silicon hydride. You must show all your working to gain full marks. Relative atomic masses: H = 1; Si = 28 ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... (4) (iv) The silicon hydrides react immediately they come into contact with oxygen in the air. They burst into flames with a small explosion and give out energy. Which letter, A to H, best describes this reaction? Energy involved in breaking and forming bonds The energy released from forming new bonds is greater than the energy needed to break existing bonds Activation energy high low The energy needed to break existing bonds is greater than the energy released from forming new bonds high low Rate of reaction Letter fast A slow B fast C slow D fast E slow F fast G slow H Letter ................... (1) Page 25 of 71 (c) The structure of silicon is similar to the structure of diamond. Describe the structure of silicon and explain why it has a high melting point. You may draw a diagram if this helps. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (4) (Total 15 marks) Q29. Calcium carbonate tablets are used to treat people with calcium deficiency. (a) Calculate the relative formula mass (M r)of calcium carbonate. Relative atomic masses: C = 12; O = 16; Ca = 40. ..................................................................................................................................... ..................................................................................................................................... Relative formula mass = .............................. (2) (b) Calculate the percentage of calcium in calcium carbonate, CaCO3. ..................................................................................................................................... ..................................................................................................................................... Percentage of calcium = .......................... % (2) Page 26 of 71 (c) Calculate the mass of calcium in each tablet. ..................................................................................................................................... ..................................................................................................................................... Mass of calcium = .................................... g (2) (d) An unwanted side effect of this medicine is that it can cause the patient to have ‘wind’ (too much gas in the intestine). The equation below represents the reaction between calcium carbonate and hydrochloric acid (the acid present in the stomach). CaCO3 (s) + 2HCl (aq) →CaCl2 (aq) + H2O (l) + CO2 (g) Suggest why the patient may suffer from ‘wind’. ..................................................................................................................................... ..................................................................................................................................... (1) (Total 7 marks) Q30. Ammonium nitrate and potassium chloride are both salts. They can be made by neutralisation reactions. Choose substances from the box to complete the word equations for the formation of these two salts. ammonia potassium nitrate hydrochloric acid water nitric acid potassium hydroxide ammonia + ........................................ → ammonium nitrate + water .................................. + hydrochloric acid → potassium chloride + .......................... (Total 3 marks) Q31. This cake recipe is taken from a cookery book. • • • • Soda Cake Mix the flour and butter and add the sugar, currants and flavouring. Then add the beaten egg. Add a little milk with a teaspoonful of baking soda (sodium hydrogencarbonate) and mix it in well. Bake in a moderate oven for about 30 minutes. Page 27 of 71 When sodium hydrogencarbonate is heated in an oven, it forms carbon dioxide gas. 2 NaHCO3 Na2CO3 + H2O + CO2 A teaspoonful of baking soda contains a mass of 11 g of sodium hydrogencarbonate. Calculate the mass of carbon dioxide that could be made from 11 g of sodium hydrogencarbonate. Show clearly how you work out your final answer. Relative atomic masses: H = 1; C = 12; O = 16; Na = 23. ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... ............................................................................................................................................... Mass of carbon dioxide = ............................................... g (Total 3 marks) Q32. Iron ore contains iron oxide. (i) Calculate the relative formula mass of iron oxide, Fe2O3. Relative atomic masses: O = 16; Fe = 56. ..................................................................................................................................... ..................................................................................................................................... Answer = ................................................ (2) (ii) Calculate the percentage by mass of iron in iron oxide. ..................................................................................................................................... Percentage of iron = .......................................... % (2) (iii) Calculate the mass of iron that could be extracted from 1000 kg of iron oxide. Use your answer to part (c) (ii) to help you with this calculation. ..................................................................................................................................... Mass of iron = ................................................... kg (1) (Total 5 marks) Page 28 of 71 Q33. Cosmetic powders were widely used in ancient Egypt. Cosmetic powders that may have been used in face paints have been analysed. These powders contained compounds that are rare in nature. The compounds must have been made by the ancient Egyptians using chemical reactions. Page 29 of 71 One of these compounds is called phosgenite. Analysis of this compound shows that it contains: 76.0% lead (Pb) 13.0% chlorine (Cl) 2.2% carbon (C) 8.8% oxygen (O) Calculate the empirical formula of this compound. To gain full marks you must show all your working. Relative atomic masses: C = 12 ; O = 16 ; Cl = 35.5 ; Pb = 207 ............................................................................................................................................. ............................................................................................................................................. ............................................................................................................................................. ............................................................................................................................................. ............................................................................................................................................. ............................................................................................................................................. ............................................................................................................................................. ............................................................................................................................................. (Total 4 marks) Q34. Toothpastes often contain fluoride ions to help protect teeth from attack by bacteria. Some toothpastes contain tin(II) fluoride. This compound has the formula SnF2 . Page 30 of 71 (a) Calculate the relative formula mass (M r) of SnF2. Relative atomic masses: F = 19; Sn = 119 .................................................................................................................................... .................................................................................................................................... .................................................................................................................................... .................................................................................................................................... Relative formula mass (M r) = .......................................... (2) (b) Calculate the percentage by mass of fluorine in SnF2. .................................................................................................................................... .................................................................................................................................... .................................................................................................................................... .................................................................................................................................... Percentage by mass of fluorine = .......................................... % (2) (c) A tube of toothpaste contains 1.2 g of SnF2. Calculate the mass of fluorine in this tube of toothpaste. .................................................................................................................................... .................................................................................................................................... .................................................................................................................................... .................................................................................................................................... Mass of fluorine = .......................................... g (1) Page 31 of 71 (d) The diagram represents the electron arrangement of a fluorine atom. Explain how a fluorine atom can change into a fluoride ion, F–. .................................................................................................................................... .................................................................................................................................... .................................................................................................................................... .................................................................................................................................... (2) (Total 7 marks) Q35. (a) A chemist was asked to identify a nitrogen compound. The chemist carried out an experiment to find the relative formula mass (M r) of the compound. The M r of the compound was 44. Relative atomic masses: N = 14, O = 16 Draw a ring around the formula of the compound. NO NO2 N 2O 4 N2O (1) (b) Potassium nitrate is another nitrogen compound. It is used in fertilisers. It has the formula KNO3. The M r of potassium nitrate is 101. Calculate the percentage of nitrogen by mass in potassium nitrate. Relative atomic mass: N = 14. ..................................................................................................................................... ..................................................................................................................................... Percentage of nitrogen = .............................. % (2) (Total 3 marks) Page 32 of 71 Q36. Perfumes contain a mixture of chemicals. The main ingredients of perfumes are a solvent and a mixture of fragrances. (a) A sample of the solvent used in one perfume contained 0.60 g of carbon, 0.15 g of hydrogen and 0.40 g of oxygen. Relative atomic masses: H = l; C = 12; O = 16. Calculate the empirical (simplest) formula of the solvent. You must show all of your working to gain full marks for this question. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (4) (b) Solvent molecules evaporate easily. Explain why substances made of simple molecules evaporate easily. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (2) Page 33 of 71 (c) Most companies claim that their perfumes have been tested on skin. A study was made of the tests they used. The study found that each company used different tests. The perfumes were tested in the companies’ own laboratories and not by independent scientists. Some companies did not give any information about the tests that they had used. (i) Suggest why companies test their perfumes on skin. ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... (1) (ii) Did the study show that the tests made by the different companies were valid and reliable? Explain your answer. ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... ........................................................................................................................... (2) (Total 9 marks) Q37. This question is about methods of treating water. (a) Chlorine is used to kill microorganisms in water. When chlorine is added to water a chemical reaction takes place. The equation for this reaction is shown below. Cl2(g) + H2O(l) 2H+(aq) + OCl–(aq) + Cl–(aq) An acidic solution is produced when chlorine reacts with water. Which ion, shown in the equation, makes the solution acidic? .................................. (1) (b) Calcium hypochlorite tablets are added to water in some swimming pools to kill microorganisms. The formula of calcium hypochlorite is CaCl2O2 Page 34 of 71 (i) Calculate the relative formula mass (M r) of calcium hypochlorite. Relative atomic masses: O = 16; Cl = 35.5; Ca = 40. ........................................................................................................................... ........................................................................................................................... Relative formula mass (M r) of calcium hypochlorite = ................................... (2) (ii) Calculate the percentage by mass of chlorine in calcium hypochlorite. ........................................................................................................................... ........................................................................................................................... Percentage by mass of chlorine in calcium hypochlorite = ......................... % (2) (iii) Calculate the mass of chlorine in a 20 g tablet of calcium hypochlorite. ........................................................................................................................... ........................................................................................................................... Mass of chlorine = ............................................. g (1) (c) Waste water from some industrial processes sometimes contains harmful metal ions, such as chromium ions. These ions must be removed from the water before it can be returned to a river. A method of removing chromium ions (Cr3+) from water is represented by this equation. Cr3+(aq) + 3OH–(aq) → Cr(OH)3(s) (i) What type of substance would be added to the water to provide the OH– ions? ........................................................................................................................... ........................................................................................................................... (1) (ii) A precipitate is formed in this reaction. What is a precipitate? ........................................................................................................................... ........................................................................................................................... (1) Page 35 of 71 (iii) What method could be used to separate the precipitate from the solution? ........................................................................................................................... ........................................................................................................................... (1) (Total 9 marks) Q38. Liquefied petroleum gases such as propane and butane are used as heating fuels for caravans, boats and barbecues. (a) Propane and butane have no smell, so for safety reasons a very small amount of thioethanol – the smelliest substance known – is added, even though it is toxic in large concentrations. Suggest one safety reason why thioethanol is added to propane and butane. ..................................................................................................................................... ..................................................................................................................................... (1) (b) Suggest how mass spectrometry could be used to distinguish between propane (C3H8) and butane (C4H10). ..................................................................................................................................... ..................................................................................................................................... (1) Page 36 of 71 (c) When 0.4 g of a hydrocarbon gas was completely burned in oxygen, 1.1 g of carbon dioxide and 0.9 g of water were the only products. Relative formula masses: CO2 = 44; H2O = 18. Use this information to calculate the number of moles of carbon dioxide and of water produced in this reaction. Use your answers to calculate the empirical formula of this hydrocarbon. You must show all your working to gain full marks. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... Empirical formula is .............................. (4) (Total 6 marks) Q39. Iron is an essential part of the human diet. Iron(II) sulfate is sometimes added to white bread flour to provide some of the iron in a person’s diet. Page 37 of 71 (a) The formula of iron(II) sulfate is FeSO4 Calculate the relative formula mass (M r) of FeSO4 Relative atomic masses: O = 16; S = 32; Fe = 56. ..................................................................................................................................... ..................................................................................................................................... The relative formula mass (M r) = .............................. (2) (b) What is the mass of one mole of iron(II) sulfate? Remember to give the unit. .............................. (1) (c) What mass of iron(II) sulfate would be needed to provide 28 grams of iron? Remember to give the unit. .............................. (1) (Total 4 marks) Q40. Aspirin tablets have important medical uses. A student carried out an experiment to make aspirin. The method is given below. 1. 2. 3. 4. 5. 6. 7. 8. Weigh 2.00 g of salicylic acid. Add 4 cm3 of ethanoic anhydride (an excess). Add 5 drops of concentrated sulfuric acid. Warm the mixture for 15 minutes. Add ice cold water to remove the excess ethanoic anhydride. Cool the mixture until a precipitate of aspirin is formed. Collect the precipitate and wash it with cold water. The precipitate of aspirin is dried and weighed. Page 38 of 71 (a) The equation for this reaction is shown below. C7H6O3 + salicylic acid C4H6O3 → C9H8O4 + CH3COOH aspirin Calculate the maximum mass of aspirin that could be made from 2.00 g of salicylic acid. The relative formula mass (M r) of salicylic acid, C7H6O3, is 138 The relative formula mass (M r) of aspirin, C9H8O4, is 180 ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... Maximum mass of aspirin = .............................. g (2) (b) The student made 1.10 g of aspirin from 2.00 g of salicylic acid. Calculate the percentage yield of aspirin for this experiment. (If you did not answer part (a), assume that the maximum mass of aspirin that can be made from 2.00 g of salicylic acid is 2.50 g. This is not the correct answer to part (a).) ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... Percentage yield of aspirin = .............................. % (2) (c) Suggest one possible reason why this method does not give the maximum amount of aspirin. ..................................................................................................................................... ..................................................................................................................................... (1) Page 39 of 71 (d) Concentrated sulfuric acid is a catalyst in this reaction. Suggest how the use of a catalyst might reduce costs in the industrial production of aspirin. ..................................................................................................................................... ..................................................................................................................................... (1) (Total 6 marks) Q41. Aspirin tablets have important medical uses. (a) Aspirin is made when salicylic acid reacts with ethanoic anhydride. The equation for this reaction is: C7H6O3 salicylic acid + C4H6O3 → C9H8O4 + CH3COOH aspirin Calculate the maximum mass of aspirin that could be made from 100 g of salicylic acid. Show clearly how you work out your answer. The relative formula mass (M r) of salicylic acid (C7H6O3) is 138. The relative formula mass (M r) of aspirin (C9H8O4) is 180. ........................................................................................................................ ........................................................................................................................ ........................................................................................................................ ........................................................................................................................ Maximum mass of aspirin = .................................... g (2) Page 40 of 71 (b) (i) In an experiment a chemist calculated that the maximum yield of aspirin is 400 g. The chemist did the experiment but only made 250 g of aspirin. Calculate the percentage yield of aspirin for this experiment. Show clearly how you work out your answer. ............................................................................................................... ............................................................................................................... ............................................................................................................... ............................................................................................................... Percentage yield of aspirin = ........................... % (2) (ii) Suggest one possible reason why the chemist did not have a percentage yield of 100%. ............................................................................................................... ............................................................................................................... (1) (c) The use of a catalyst might reduce costs in the industrial production of aspirin. Suggest how. ........................................................................................................................ ........................................................................................................................ (1) (Total 6 marks) Q42. The diagram shows the main parts of an instrumental method called gas chromatography linked to mass spectroscopy (GC-MS). This method separates a mixture of compounds and then helps to identify each of the compounds in the mixture. Page 41 of 71 (a) In which part of the apparatus: (i) is the mixture separated? ................................................................... (1) (ii) is the relative molecular mass of each of the compounds in the mixture measured? ............................................................................................................... (1) (b) (i) Athletes sometimes take drugs because the drugs improve their performance. One of these drugs is ephedrine. Ephedrine has the formula: C10H15NO What relative molecular mass (M r) would be recorded by GC-MS if ephedrine was present in a blood sample taken from an athlete? Show clearly how you work out your answer. Relative atomic masses: H = 1; C = 12; N = 14; O = 16. ............................................................................................................... ............................................................................................................... ............................................................................................................... ............................................................................................................... Relative molecular mass = ..................................... (2) (ii) Another drug is amphetamine which has the formula: C9H13N The relative molecular mass (M r) of amphetamine is 135. Calculate the percentage by mass of nitrogen in amphetamine. Relative atomic mass: N = 14 ............................................................................................................... ............................................................................................................... Percentage of nitrogen = ..................................... % (2) Page 42 of 71 (c) Athletes are regularly tested for drugs at international athletics events. An instrumental method such as GC-MS is better than methods such as titration. Suggest two reasons why. ........................................................................................................................ ........................................................................................................................ ........................................................................................................................ ........................................................................................................................ (2) (d) When a blood sample is taken from an athlete the sample is often split into two portions. Each portion is tested at a different laboratory. Suggest why. ........................................................................................................................ ........................................................................................................................ ........................................................................................................................ ........................................................................................................................ (2) (Total 10 marks) Page 43 of 71 M1. (a) Mg S O4 24 + 32 + 16 (×4) or 64 / evidence of all Ar’s gains 1 mark but (Mr) = 120 gains 2 marks 2 (b) evidence that 24(g) magnesium would produce 120(g) mapesiurn sulphate gains 1 mark or correct scaling by 1/6 but 20(g) magnesium sulphate gains 2 marks [credit error carried forward from (a) with full marks in (b)] 2 [4] M2. Mg S O4 24 + 32 + 16 (×4) or 64 / evidence of all Ar’s correct [so 24 + 32 + 16 1 mark] gains 1 mark but (Mr) = 120 No ECF gains 2 marks [2] M3. Ca = 40 (OH)2 = (16 + 1)2 or 34 gain 1 mark each but Mr = 74 gains 3 marks [3] Page 44 of 71 ## (a) Fe2 [56 × 2] or 112 O3 [16 × 3] or 48 each gain 1 mark but Mr = 160 gains 3 marks 3 (b) [Fe2 O3 + 2A1 → 2Fe + A12 O3] 160 → 112 (NB Credit if unworked (or value (or value but should be totalled) from (a)) from (a)) gains 1 mark but 32 g. of Fe2 O3 → 32/160 × 112 gains 2 marks but = 22.4 gains 3 marks 3 [6] M5. (a) reference to hydrogen (atoms) ) nitrogen (atoms) ) each for 1 mark but not molecules ratio of 1N to 3H atoms for 1 further mark or 1 nitrogen atom and 3 hydrogen atoms (ignore any incorrect statements about nature of bonding) 3 Page 45 of 71 (b) evidence of H=1 N = 14 O = 16 gains 1 mark but H=1 N = 14 O = 16 × 3 or 48 gains 2 marks but 63 gains 3 marks 3 [6] M6. (a) Mg + 2H+ → Mg2+ + H2 * reactants correct in every detail * products correct in every detail if the spectator ions are sown then (1) mark should be credited but only if they are shown correctly on both sides e.g. Mg + 2H+ + 2CI- → Mg2+ + 2CI- + H2 2 (b) 24 (parts) of magnesium → 2 (parts) 1 of hydrogen or equally clear working (so) 6 grams/g (are needed) 1 unit required (c) (i) two (and no more) atoms shown to be sharing their single electrons examples do not credit if anything which contradicts the impression that these are hydrogen atoms 1 (ii) (single) covalent (bond) 1 Page 46 of 71 (d) (×100) = 6 (just 6 is worth (1) mark) 1 × 100 = 6 or similar is (0) do not credit 5.8823529 and the like 1 [8] M7. 73 (seventy three) if answer is incorrect allow 1 mark for the correct proportion that H2:HCl is 1:2 and 1 mark for 36.5 [3] M8. (i) 4 and 1 both answers must be correct 1 (ii) 53.5 if incorrect relative formula mass allow 1 mark for correct working accept e.c.f. from c(i) for 2 marks 2 [3] M9. 36.8 / 37 correct answer, no workings = 3 if incorrect, allow 1 mark for rfm FeSO4 = 152 or if incorrect rfm, allow 1 mark for 56/Y × 100 where Y is incorrect formula mass allow 2 marks for × 100 [3] M10. (a) 1400 1 Page 47 of 71 (b) 980 correct answer gains full credit 160 tonnes Fe2O3 produces 112 tonnes Fe if incorrect allow one mark for relative formula mass iron oxide = 160 allow e.c.f. 1400 tonnes Fe2O3 will produce 1400 / 160 × 112 tonnes Fe use of 2000 tonnes Fe2O3 – deduct one mark only if working out is correct 4 [5] M11. (a) the answer yes or no does not gain a mark Yes – plants will grow faster do not accept grow better 1 more food available, greater yield 1 OR No – plants still grow without adding nitrates accept the idea that small amounts of nitrate could be used 1 (nitrates) can ‘kill’ babies / causes brain damage do not accept can stop respiration in babies 1 (b) (i) 2 accept two 1 Page 48 of 71 (ii) 2 × 14 + 4 × 1 + 3 × 16 1 = 80 1 % 1 allow 1 mark for correct working for percentage 28/Y × 100, where Y is an incorrect formula mass allow 2 marks for formula mass of 80 where no working or correct working is shown allow 3 marks for 35 where no working or correct working is shown [6] M12. (a) plot correct (2 segments) for 1 mark 1 (b) nitrogen + hydrogen or N2 H2 ammonia NH3 all correct for 1 mark 1 (c) largest area labelled nitrogen or shaded for 1 mark 1 (d) (i) nitrogen 1 oxygen hydrogen 1 three correct for 2 marks two correct for 1 mark (ii) potassium chloride for 1 mark 1 (e) (i) NH4NO3 = 14 + (4 × 1) + 14 + (3 × 16) = 80 for one mark 1 Page 49 of 71 (ii) ecf (error carried forward from part (i)) look for 28 / 80 for first mark gains 1 mark but 35% (% sign not needed) special case of (14 / 80 × 100 = 17.5%) gains 1 mark gains 2 marks 2 [9] M13. (a) sodium hydroxide / caustic soda / NAOH for 1 mark 1 (b) negative ions move to the positive electrode etc. /because it is negative /opposite charges attract for 1 mark 1 (c) loss of electrons for 1 mark 1 [3] M14. (i) neutralise (the acid) / reduce acidity / because it is alkaline /increases pH for 1 mark 1 (ii) CaO = 56 Ca(OH)2 = 74 each for 1 mark 2 (iii) mass = 1000 × 74/56 gains 1 mark = 1321 kg (1320 accepted) gains 2 marks (error carried forward from (ii)) 56g → 74g 1 g → 74/5 6 g (1 mark) 2 [5] Page 50 of 71 M15. 56 74 each for 1 mark [2] M16. (a) Gas A = Chlorine / Cl2 not Cl and Gas B = Hydrogen / H2 not H for 1 mark Solution C = sodium hydroxide/NaOH/spent brine for 1 mark 2 (b) (i) 2, 2 for 1 mark (ii) 2, 2 for 1 mark 2 (c) water/H2O/hydrogen oxide not hydrogen hydroxide for 1 mark 1 (d) ions/positive ions/negative ions/cations/anions not charged particles/positive particles/negative particles not H+ / Cl-/Na+ / OHAllow hydrogen ions etc. not sulphate ions for 1 mark 1 [6] M17. (a) 4 HCl / 2H2O, allow multiples or fractions if whole equation balances for 1 mark 1 (b) germanium tetrachloride + water = germanium oxide + hydrochloric acid If symbol equation given it must be correctly balanced Allow germanium for 1 mark 1 Page 51 of 71 (c) to purify the germanium oxide/remove impurities/give in pure product/to make pure germanium for 1 mark 1 ensure complete reaction/reaction does not give a good yield not to increase efficiency/to purify germanium for 1 mark 1 (d) (i) remove oxygen/addition of hydrogen/gain up electrons allow remove oxygen molecules (ii) GeO2 = 73 + (2 × 16) = 105 mass of germanium = 525 × (73/105) = 365 g (or alternative methods) apply consequential marking for 1 mark each 3 (e) (i) germanium is shiny/lustrous conducts a small amount of electricity * germanium oxide reacts with hydrochloric acid (and) metal oxides react with acid metal oxides are basic metal oxides are reduced by hydrogen Information must be taken from the passage. Apply the list principle if more than three answers are given. Assume the word ‘it’ refers to the metal. any 3 for 1 mark each 3 (ii) germanium is brittle germanium tetrachloride is a (volatile) liquid made of molecules germanium tetrachloride has covalent bonding or when two non-metals react they have covalent bonding GaC14/the salt of germanium undergiven hydrolysis/reacts with water germanium is not a good conductor of electricity* * conductivity mark can only be given once any 3 for 1 mark each 3 [13] M18. (a) 56g for 1 mark 1 Page 52 of 71 (b) 44 tonnes for 1 mark 1 [2] M19. (i) neutralisation/acid base reaction for 1 mark 1 (ii) 17 (tonnes) give 80 (tonnes) (even if only in working) for 1 mark each 320 (tonnes) or alternative method) 3 marks for correct answer (if 17 and 80 not given allow 1 mark for correct answer using their figures) 3 [4] M20. (a) (i) 45% for 1 mark 1 (ii) 126 000 (consequential on (i)) for 1 mark 1 (b) (i) Cl2 = 71 1 × 71/24 or correct mathematical attempt for 1 mark (If Cl2 wrong take figure given) for 1 mark = 2.96 kg gains 3 marks (or alternative methods) (if units not given - 3 marks. If units wrong - 2 marks) 3 Page 53 of 71 (ii) any sensible eg. bleach/disinfectant/antiseptics/kill bacteria/ sterilise water/solvents/refrigerents/CFCs/PVC (not water treatment or warfare) for 1 mark 1 [6] M21. (a) 40 + 12 + (3 × 16) = 100 each for 1 mark 2 (b) Mr of CaO = 56 for 1 mark mass required = 60 × 100/56 for 2 marks = 107.1 for 1 mark 4 (c) (i) calcium hydroxide 1 (ii) solid 1 [8] M22. 144 accept TiCl4 = 190 for 1 mark accept another correct step in calculation eg 570/190 = 3 for 1 mark [3] Page 54 of 71 M23. (i) 160 ignore units 1 (ii) 112 ignore units 1 (iii) 70 do not carry forward errors 1 [3] M24. (i) a reaction in which the products can be changed back to reactants accept a reaction that can go forwards or backwards 1 under certain conditions 1 (ii) Mr CaCO3 = 100 1 Mr CaO = 56 1 mass of CaO = 140 (tonnes) 1 mark consequentially [5] M25. (a) calcium atom loses two electrons accept diagrams with correct labelling 1 (each) fluorine atom gains one electron accept two electrons transfer from a calcium atom to the two fluorine atoms for these first two marks 1 Page 55 of 71 forming full (outer) shells of electrons accept forming full (outer) energy levels or noble gas electronic structures do not accept stable unless qualified 1 giving the ions Ca2+ and F− 1 attraction between ions of opposite charges accept electrostatic attraction between ions if candidate mentions sharing or pairing of electrons then no credit if explanation is entirely correct but they state this is called covalent bonding, the maximum mark is four 1 (b) atoms of the same element 1 atomic number is same accept each contains 92 or same number of protons 1 mass numbers differ or each has a different number of neutrons 1 one has 146 neutrons the other has 143 neutrons accept one has three more or less neutrons than the other 1 (c) (i) 349 1 (ii) 349g UF2 produces 235g U [1] first mark can be awarded if answer is incorrect answer = 117.5 1 [12] M26. 160 ignore units if answer incorrect then (2 × 56) + (3 × 16) or 112 + 48 for one mark [2] Page 56 of 71 M27. 352 g gains 3 marks (moles C8H18 = 114 / 114 = 1 mole) moles CO2 = 8 (1) mass CO2 = 8 × 44 (1) = 352 g (1) 1 mark for each point (ecf allowed between parts) or 114 → 8 (1) × 44 (1) 114 → 352 g (1) ecf allowed between parts [3] M28. (a) Mr (SiO2) = 60 if Mr incorrect ecf for max 2 1 60 g SiO2 → 28 g Si correct answer for 3 marks 1 2.14 g SiO2 → 1 g Si allow 2, 2.1, 2.14 (or anything rounding to 2.14), 2.16 or 2.2 a unit is not required but an incorrect unit loses the third mark OR Mr (SiO2) = 60 (1) moles if silicon needed = = 0.0357 mass of SiO2 needed = 0.0357 × 60 (1) = 2.14 g (1) allow 2, 2.1, 2.14 (or anything rounding to 2.14), 2.16 or 2.2 OR Mr (SiO2) = 60 (1) mass SiO2 = 1 × (1) = 2.14 g (1) allow 2, 2.1, 2.4 (or anything rounding to 2.14), 2.16 or 2.2 3 Page 57 of 71 (b) (i) MgO(s) + 2HCl(aq) → MgCl 2(aq) + H2O(l) penalise incorrect symbols correctly balanced equation for 1 mark state symbols for 1 mark allow correct multiples / fractions 2 (ii) or ignore inner shell electrons of silicon allow correct drawings without symbols must clearly indicate four shared pairs of electrons with one electron from each atom (iii) Si H 1 = 0.05 = 0.15 1 1 3 for whole number ratio can be implied 1 Si H3 accept H3 Si or any correct formula with 1:3 ratio if in step 1 they get either of ratios incorrect they lose first 2 marks but can be ecf for 3rd and 4th mark evidence of mass / Ar 1 mark proportions of each whole number ratio correct formula 1 mark 1 mark 1 mark 1 Page 58 of 71 (iv) C accept c 1 (c) any four from: • giant structure / macromolecule / lattice / giant molecule allow giant molecular / giant atomic structure • each silicon atom joined to four other atoms (or diagram) • covalent bonds • bonds are strong or large amount of energy needed to break bonds accept hard to break bonds • large number of bonds to be broken mention of giant ionic structure or intermolecular forces or intermolecular bonds max 1 mark diamond or carbon discussion max 3 marks unless clearly linked to silicon 4 [15] M29. (a) 100 ignore units 40 + 12 + (3 × 16) for 1 mark 1 (b) 40 (ecf from part (a) can get 2 marks) for 1 mark 1 (c) 0.5 (ecf from part (b) can get 2 marks) or other correct working for 1 mark 2 (d) gas produced or carbon dioxide / CO2 produced 1 [7] Page 59 of 71 M30. nitric acid 1 potassium hydroxide 1 water 1 [3] 168g → 44g M31. 1 1g → 1 11g → 2.88g (2.9g) care with rounding 1 or Mr values 84 and 44 (1) moles hydrogen carb = (1) mass of CO2 = = 2.9g answer 2.88 to 2.9 gets 3 marks answer of 3 gets 2 marks (1) [3] M32. (i) 160 ignore units (2 × 56) + (3 × 16) for 1 mark 2 (ii) 70 for 1 mark allow ecf from part (i) 2 Page 60 of 71 (iii) 700 allow ecf from part (ii) 1 [5] M33. Pb 76 207 Cl 13 35.5 C 2.2 12 O 8.8 16 1 mark for dividing one mass by Ar allow upside down ratio to lose this mark only 1 = 0.367 = 0.366 = 0.183 = 0.55 1 mark for one correct proportion – accept to one d.p. or rounded up to 1 d.p. 1 1 mark for all four correct proportions correctly rounded 1 2 2 1 3 or Pb2Cl2CO3 1 mark for correctly written formula or correct whole number ratio correct formula without working gets only 1 mark. e.c.f. can be allowed from incorrect proportions to formula or ratio 1 [4] M34. (a) 157 correct answer with or without working (2 × 19 + 119) for 1 mark only allow (119 + 19 =) 138 for 1 mark only ignore units 2 (b) 24.2 accept answers in the range 24 to 24.2038..... ignore incorrect rounding after correct answer 25 only without working gains 1 mark or 38/157 × 100 gains 1 mark or (19/157 × 100 =) 12 to 12.1 gains 1 mark allow error carried forward from part(a) 38/(a) × 100 gains 2 marks if calculated correctly (19/138 × 100 =) 13.8 gains 1 mark 2 Page 61 of 71 (c) 0.29 accept answers in the range 0.28 to 0.3 allow error carried forward from part (b) (b)/100 × 1.2 correctly calculated ignore units 1 (d) an electron allow electrons allow electron shared / lost for 1 mark apply list principle for additional particles 1 is gained owtte must be linked to electron accept can hold / take in if in correct context eg it can hold another electron (in its outer shell) = 2 marks it can take an electron (from another atom) = 2 marks ignore reference to fluoride ions incorrect number of electrons gained does not gain the second mark 1 [7] M35. (a) N2O 1 (b) 13.8 to 14 gains full marks without working if answer incorrect 13 gains 1 mark or 14/101 × 100 gains 1 mark 2 [3] Page 62 of 71 M36. (a) C 0.60 H 0.15 O 0.40 1 12 1 = 0.05 = 0.15 16 = 0.025 1 2 6 1 1 C2H6O 1 mark for dividing the correct amount or multiples of correct amount by Ar 1 mark for proportions 1 mark for whole number ratio – accept any multiple 1 mark for correctly written simplest formula correct formula without working gets only 2 marks correct formula gains full marks provided steps 1 and 2 are correct. ecf can be allowed from step 2 to 3 or step 3 to 4 formula can be in any order eg OH6C2 1 (b) intermolecular forces / bonds 1 are weak (covalent) bonds are weak = 0 or forces between molecules or bonds between molecules (1) (attractive) forces are weak = 1 are weak (1) if no marks awarded, allow low boiling point or small Mr for 1 mark 1 (c) (i) to check the safety of the perfume (owtte) accept references to possible harmful / dangerous effects of perfume or possible reactions on skin eg to show it does not damage skin / cause cancer etc. allow to see what it smells like on the skin allow so the company do not have to test on animals 1 Page 63 of 71 (ii) any two from: idea from text linked with an explanation • the company claim to have tested the product: but we cannot be certain they have or how thorough they are or how accurately reported • companies did not disclose how they did their tests: so they could not be checked or so they could not be shown to be reliable / valid or so they could not be repeated or converse eg companies should disclose how they did their tests so that results can be checked etc. • companies may not have repeated their tests: so they may not be reliable • companies do their own tests: so they may be biased or so they may not be truthful about their results or so they may not be reliable or converse eg independent tests should be done so as to ensure there is no bias etc. • the companies are using different tests: so the results cannot be compared or so results will be different or so results will not be fair / valid / reliable or converse eg companies should do the same tests so that the results will be fair etc. • companies would not give false information because of damage to reputation or it might lead to litigation 2 [9] M37. (a) hydrogen / H+ /2H+ / H3O+ allow H / 2H do not accept H2 apply list principle 1 (b) (i) 143 correct answer with or without working = 2 marks ignore units if answer is not correct 40 + (2 × 35.5) + (2 × 16) gains 1 mark 2 Page 64 of 71 (ii) 49.7% (49.6 to 50) correct answer with or without working = 2 marks answer 49 gains 1 mark if answer is not correct: (71 ÷ 143) × 100 gains 1 mark allow error carried forward from part (b)(i) ie. (71 or their (2 × 35.5) ÷ answer to (b)(i)) × 100 gains 2 marks if calculated correctly and 1 mark if not calculated correctly. Special case 35.5 ÷ 143 × 100 = 24.8 to 25% or 35.5 ÷ answer to (b)(i) × 100 correctly calculated for 1 mark 2 (iii) 9.9 to 10g allow ecf from (b)(i) or (b)(ii) 1 (c) (i) an alkali apply list principle accept named alkali accept hydroxide accept soluble base ignore base 1 (ii) a solid / insoluble substance (owtte) 1 (iii) filter / filtration allow decant / centrifuge accept filtration followed by evaporation or filtration and evaporation do not accept filtration or evaporation do not accept evaporation and filtration 1 [9] M38. (a) (smell) warns of a leak / gas escape accept leak / gas escape by implication ignore smell alone 1 (b) eg (mass spectrometry gives) different molecular ions / M r / formula mass or shows that one has mass 44 and the other 58 ‘mass of butane is more than mass of propane’ is insufficient accept different fragmentation / pattern do not accept Ar / RAM accept references to butane deflects less or converse 1 Page 65 of 71 (c) CO2 2 H2O 1.1 ––– 44 0.9 ––– 18 1 = 0.025 = 0.05 1 1 (mole) CO2 2 (moles) H2O 1 1 4 or CH4 1 or alternative method Mass of C = (1) Mass of H = (1) C:H proportions 0.025 : 0.1 (1) whole number 1 : 4 (1) or CH4 correct formula with no working is only 1 mark M3 can be awarded from the formula if steps one and two are clear correct formula from their incorrect ratio gets 1 mark if fraction is wrong way around e.g. Mr / mass, then lose M1 and M2 but accept ecf for M3 and M4 max 4 [6] M39. (a) 152 correct answer with or without working = 2 marks 56 + 32 + (4 ×16) gains 1 mark ignore any units 2 Page 66 of 71 (b) 152g(rams) ecf from the answer to (a) and g must have unit g / gram / gramme / grams etc accept g / mol or g per mole or g mole–1 or g/mol or g per mol or g mol–1 do not accept g m do not accept G 1 (c) 76(g) ecf from their answer to (a) or (b) divided by 2 ignore units 1 [4] M40. (a) 2.61 / range 2.5 to 2.7 correct answer with or without or with wrong working gains 2 marks (accept answers between 2.5 and 2.7) if answer incorrect moles of salicylic acid = 2/138 = 0.0145 moles ie 2/138 or 0.0145 gains 1 mark or (180/138) × 2 gains 1 mark or 1 g → 180/138 = (1.304 g) gains 1 mark (not 1.304g alone) 2 (b) 42.1 range 40.7 to 42.3 accept correct answer with or without or with wrong working for 2 marks ecf ie (1.1 / their answer from (a)) × 100 correctly calculated gains 2 marks if answer incorrect percentage yield = 1.1 / 2.61 × 100 gains 1 mark if they do not have an answer to part (a) or they choose not to use their answer then: • yield = (1.1 / 2.5) × 100 (1) • = 44 accept 44 for 2 marks with no working 2 Page 67 of 71 (c) any one from: • errors in weighing • some (of the aspirin) lost do not allow ‘lost as a gas’ • not all of the reactant may have been converted to product eg reaction didn’t go to completion allow loss of some reactants • the reaction is reversible accept other products / chemicals • side reactions ignore waste products • reactants impure • not heated for long enough • not hot enough for reaction to take place 1 (d) any one from: • use lower temperature • use less fuel / energy ignore references to use of catalyst • produce product faster or speed up reaction • more product produced in a given time (owtte) • increased productivity • lowers activation energy 1 [6] M41. (a) 130.4 accept 130 to 130.43478……… correct answer gains two marks with or without working an answer of 131 would gain one mark. if answer is not correct then: moles of salicylic acid = 0.7 .......... (1 mark) or mass of aspirin = moles of salicylic acid x 180 (1 mark) or 100 x (180/138) (1 mark) 2 Page 68 of 71 (b) (i) 62.5% accept 63% correct answer gains two marks with or without working if answer is not correct then: 250/400 x 100 (1 mark) 2 (ii) any one from: • reversible reaction accept not all of the reactant converted to product • some of product lost 1 (c) use lower temperatures or less energy needed allow product made faster or more product made in a given time 1 [6] M42. (a) (i) column 1 (ii) mass spectrometer 1 (b) (i) 165 if answer is not correct then evidence of correct working gains one mark. e.g. (10 × 12) + 15 + 14 + 16 2 (ii) 10.37% accept 10 / 10.4 / 10.37............... if answer is not correct then evidence of correct working gains one mark. e.g. minimum evidence would be 14/135 2 (c) any two from: • faster • more accurate • detects smaller amounts 2 Page 69 of 71 (d) to avoid bias accept to check / compare the result 1 to improve reliability 1 [10] Page 70 of 71 Page 71 of 71