* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download ME_U1_L5 - Heat and Work
Survey
Document related concepts
Transcript
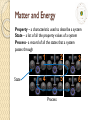
Matter and Energy Drill Identify which of the two descriptions of your class is an intensive property and which is extensive: The number of students in the class. The grade level of the students in class. Matter and Energy Now So farlet’s we learn have how learned the state a bit of about a system systems can and howthrough change we describe energythem transfer. using properties. Thermodynamics - study of systems and energy transfer System – what we choose to study; can be open or closed Boundary – separates system from surroundings Surroundings – everything outside of system Property – a characteristic used to describe a system • Extensive – “the whole is the sum of the parts,” Mass,Volume, Energy • Intensive – “the whole is the same as each part,” Density, Temperature Matter and Energy Property – a characteristic used to describe a system State – a list of all the property values of a system Process– a record of all the states that a system passes through State 1 2 3 4 5 6 Process Matter and Energy Thermodynamics - “the study of systems and energy transfer” Energy can go into or out of a system as either work or heat … Energy In (Weights) Energy Out (Ice Bath) Energy In (Match) Piston & Cylinder Matter and Energy Thermodynamics - “the study of systems and energy transfer” Work (W) = a force acting over some distance Force Block Distance Travelled If we pushed a block, we would be doing work on it… Mechanical Work = Force x Distance = F x d Matter and Energy Thermodynamics - “the study of systems and energy transfer” Mechanical work example: Block 40 N d=3m Remember…only Mechanical Work =the F xcomponent d of the force that is parallel W to the = (40 displacement N)(3 m) does work. W = 120 N-m W = 120 J Matter and Energy Thermodynamics - “the study of systems and energy transfer” Mechanical work example: 30O Mechanical Work = F x d O Remember…only W =the (40component N)(Cos 30of )(3the m)force that is parallel W to the displacement does work. = 103.9 N-m W ≈ 104 J Matter and Energy Thermodynamics - “the study of systems and energy transfer” Heat (Q) – energy transfer caused by a temperature difference •If we place a flame beneath a system, we would be adding heat to it. •We could also remove heat by surrounding the system with an ice bath. Matter and Energy Thermodynamics - “the study of systems and energy transfer” Potential Energy (PE) Block Height, h The block to the right will fall because a force equal to its mass times the acceleration of gravity is acting on it… PE = mgh g = 9.8 m/s2 Matter and Energy Thermodynamics - “the study of systems and energy transfer” Potential Energy Example: Block h=3m PE = mgh, where g = 9.8 m/s2 PE = (1.5 kg)(9.8 m/s2)(3 m) PE = 44.1 kg-m2/s2 Remember, PE = 44.1 N-m 1 N = kg-m/s2 PE = 44.1 J m = 1.5 kg Matter and Energy Thermodynamics - “the study of systems and energy transfer” Kinetic Energy (KE) Block If the block above moves with a velocity, V, then its kinetic energy is… 1 2 KE mV 2 Matter and Energy Thermodynamics - “the study of systems and energy transfer” Kinetic Energy Example: m = 2 kg V = 5 m/s Block If the block above moves with a velocity, V, then its kinetic energy is… 1 KE mV 2 2 2 1 1 m 2 KE (2kg)(5m / s ) (2kg)( 25 2 ) 25 J 2 2 s Matter and Energy Thermodynamics - “the study of systems and energy transfer” Expansion or Compression Work V2 W PdV V1 In a constant pressure (isobaric) process, W P(V2 V1 ) Matter and Energy Thermodynamics - “the study of systems and energy transfer” Expansion Work Example P = 120 kPa V2 4 m 3 V1 1.2 m 3 In a constant pressure (isobaric) process, W P (V2 V1 ) W 120 kPa(4 m 1.2 m ) W 336 J 3 3