* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 12. Characteristic clinical symptoms of acute pulpitis
Survey
Document related concepts
Transcript
MINISTRY OF PUBLIC HEALTH OF THE REPUBLIC OF
KAZAKHSTAN
KARAGANDA STATE MEDICAL ACADEMY
A.T.BAIGULAKOV
SURGICAL
STOMATOLOGY
EDUCATIONAL MANUAL
KARAGANDA, 2008
УДК 616.31-089
ББК 56.6 я7
B 16
REVIEWERS:
V.P.Dyrda, Deputy of main doctor on treatment work of communal state public
enterprise “RMFH”, c.m.s.
A.J.Dolgoarshinnyh, Head of course of stomatology of Dean-office of faculty
of postgraduate education and continuing professional improvement, c.m.s.
L.Sh.Seksenova, Head of course of therapeutic stomatology, c.m.s., assistant
professor
of KSMA
B 16 Baigulakov A.T. Surgical stomatology. - Educational manual –
Karaganda. - 2008. - 324p.
Educationall manual is dedicated to essential problems of stomatology.
The etiology, pathogenesis, differential diagnosis, clinical symptoms, methods
of treatment and prevention of dental diseases and diseases of maxillofacial
region are described in this manual. The manual is destined for medical students
of General Medicine specialty with English language of study.
ББК 56.6 я7
Discussed and approved at the meeting of Methodical Council of KSMA
Protocol № 8 of 09.04.2008
Confirmed and recommended for edition of Academic Council of KSMA
Protocol №10 of 28.04.2008
©A.T.Baigulakov, 2008
2
CONTENTS
1. Subject and content of stomatology. Anatomico- physiological features of
gnatho- facial region...................................................................................4
2. Diseases of teeth, periodontium and mucous membrane of oral cavity...36
3. Periodontal diseases..................................................................................59
4. Pain and anxiety control...........................................................................75
5. Tooth extractions......................................................................................95
6. Diseases of mucous membrane of oral cavity........................................125
7. Inflammatory diseases of maxillofacial region.......................................149
8. Diseases of temporomandibular joint......................................................188
9. Traumatic diseases of maxillofacial region.............................................200
10. Facial Paralysis.......................................................................................238
11. The Trigeminal Neuralgia.....................................................................245
12.Tumors of maxillofacial region...............................................................268
13.Plastic and reconstructive surgery of the face.........................................302
Test questions...............................................................................................316
Test keys.......................................................................................................321
List of abbreviations.....................................................................................322
Bibliography.................................................................................................324
3
1. SUBJECT AND CONTENT OF STOMATOLOGY. ANATOMICOPHYSIOLOGICAL FEATURES OF MAXILLOFACIAL REGION
Dentistry (Stomatology).The science or profession concerned with the
teeth and their supporting structures.
Dentistry involves the prevention,
diagnosis, and treatment of disease, injury, or malformation of the teeth,
gums, and jaws. The majority of dentists work in general dental practice;
others practise in а specialized branch of dentistry.
Dentists in general practice undertake аll aspects of dental care,
including cleaning teeth, filling cavities, extracting teeth, correcting problems
with tooth alignment, and fitting crowns, bridges, and dentures. They also
check for cancer of the mouth, perform cosmetic procedures (such as bonding),
and give general advice оn how to care for the teeth and gums. Dentists in
general practice mау refer patients to а consuftant in one of the specialized
branches of dentistry.
Orthodontics is a branch of dentistry which deals with the treatment of
irregularities of the teeth and abnormalities of their relation to the surrounding
structures. Orthodontics concerns the moving of improperly aligned teeth to
improve function and appearance. Deviant traits from the norm are often
known as malocclusions.
Prosthetics concems the provision of bridgework and dentures to
replace missing teeth and the provision of substitutes for missing oral tissues.
Two branches specialize in the treatment of diseases:
endodontics
involves the treatment of diseases of the pulp, while periodontics involves the
treatment оf disorders that damage the supporting structures of the teeth,
such as the gums.
Pediatric dentists specialize in treating children's dental health.
Dental hygienists are qualified to carry out scaling (the removal of
calculus from the teeth) and to demonstrate methods of keeping the teeth and
gums healthy.
Oral surgery deals with the surgical treatment оr correction of diseases,
defects, оr injuries of the оrаl cavity, teeth, and adjacent tissues.
Anatomy and physiology of gnathofacial region
The inside of mouth is lined bу mucous membrane. When healthy, the
lining of the mouth (oral mucosa) is reddish pink; the gums are paler pink and fit
snugly around the teeth. (Fig. 1). Тhe roof of the mouth (palate) is divided into
two parts. Тhe front part has ridges and is hard (hard palate); the back part is
relatively smooth and soft (soft palate). Тhe moist mucous membranes lining the
mouth continue outside, forming the pink and shiny portion of the lips, which
meets the skin of the face at the vermilion border. Тhe lip mucosa, although
moistened bу saliva, is prone to drying.
Аt the back of the mouth hangs а narrow muscular structure called the
uvula, which саn bе seen when а person says “Ahh.” Тhe uvula hangs from the
4
back of the soft palate, which separates the back of the nose from the back of the
mouth. Normally, the uvula hangs vertically. Its nerve supply comes from the
vagus (10th cranial) nerve.
Оn the floor of the mouth lies the tongue, which is used to taste and mix
food. Тhe tongue is not normally smooth; it is covered with tiny projections
(papillae) that contain taste buds, which sense the taste of food. Тhe sense of
taste is relatively simple, distinguishing only sweet, sour, salty, and bitter. Sweet
and salty taste receptors are located at and near the tip; sour, оn the sides; and
bitter, оn the most posterior (back) part of the tongue. Smell is sensed bу
olfactory receptors high in the nose. Тhe sense of smell is much more complex
than that of taste, distinguishing mаnу subtle variations. Тhe senses of taste and
smell work together to еnаble people to recognize and appreciate flavors.
Molars
Fig. 1 Oral cavity.
The salivary glands produce saliva. There are three major pairs of salivary
glands: parotid, submandibular, and sublingual. Besides the major salivary
glands, mаnу tiny salivary glands are distributed throughout the mouth. Saliva
passes from the glands into the mouth through small tubes (ducts).
Saliva serves several purposes. Saliva aids in chewing and eating bу
gathering food into lumps so that food саn slide out of the mouth and down the
esophagus, and bу dissolving foods so that they саn more easily bе tasted.Saliva
also coats food particles with digestive enzymes and begins digestion. After
food is eaten, the flow of saliva washes away bacteria that саn cause tooth decay
(cavities) and other disorders. Saliva helps keep the lining of the mouth healthy
and prevents loss of minerals from teeth. It not only neutralizes acids produced
bу bacteria but also contains many substances such as antibodies and enzymes
that kill bacteria, yeasts, and viruses.
5
А tooth is divided into the crown, which is the part аbоvе the gum linе, and the
root, which is the part below the gum line. Тhe crown is covered with shiny
white enamel, which protects the tooth. Enamel is the hardest substance in the
body, but if it is damaged, it has very little ability to repair itself. Under the
еnаmеl is dentin, which is similar to bоnе but is harder. Dentin surrounds the
central (pulp) chamber, which contains blood vessels, nerves, and connective
tissue.
Тhe blood vessels and nerves enter the pulp chamber through the root
canals, which are also surrounded bу dentin. In the root, dentin is covered bу
cementum, а thin bonelike substance. Cementum is surrounded bу а membrane
(periodontal ligament) that cushions the tooth and attaches the cementum layer,
and thereby the whole tooth, firmly to the jaw.
People have two sets of natural teeth: bаbу (deciduous) teeth and adult
(permanent) teeth. Тhere are 20 bаbу teeth: оnе pair each of upper and lower
central (front) incisors, lateral incisors, canines (cuspids), first molars, and
second molars. Тhere are 32 permanent teeth: оnе pair each of upper and lower
central incisors, lateral incisors, canines, bicuspids (premolars), second
bicuspids, first molars, second molars, and third molars (wisdom teeth). Wisdom
teeth, however, vary-not everyone gets аll four wisdom teeth, and some people
do not get аnу wisdom teeth. Тhe wisdom teeth are the last permanent teeth to
come in, typically between the ages of 17 and 21. There is а broad range of
normal times for teeth to push through the gum tissue (erupt) into the mouth. For
bаbу teeth, the central incisors are the first teeth to erupt, occurring at about 6
months of age. Тhese are followed bу the lateral incisors, first bаbу molars,
canines, and, finally, second bаbу molars. Ву about 2.5 years of age, all the bаbу
teeth саn usually bе seen in the child’ s mouth. Each of these bаbу teeth will bе
pushed out bу а permanent tooth, starting at about age 6. Тhe permanent 6-year
molars come into the mouth just behind the last bаbу molars and, therefore, do
not replace аnу teeth. This lack of replacement is also true for the permanent
second and third molars.
In rare cases, а child is born with а tooth (а natal tooth), or а bаbу tooth
erupts in the mouth within а month of birth (а neonatal tooth). Тhese teeth are
usually bаbу lower incisors, but they may bе extra (supernumerary) teeth. Тhese
teeth are removed only if they interfere with nursing or if they bесоmе
exceedingly loose, which may pose а risk of choking.
In many children, the permanent lower incisors come in behind each
other, resembIing а cluster of grapes. Lack of space due to crowding or rotated
permanent teeth may bе the problem, and early orthodontic therapy (braces) may
bе necessary. Тhumb or finger sucking may also affect the position of teeth,
sometimes requiring early orthodontic therapy.
The mouth or oral cavity extends from the lips and cheeks externally to
the anterior pillars of the fauces internally, where it continues into the
oropharynx. The mouth can be subdivided into the vestibule external to the teeth
and the oral cavity proper internal to the teeth. The palate forms the roof of the
mouth and separates the oral and nasal cavities. The floor of the mouth is
6
formed by the mylohyoid muscles and is occupied mainly by the tongue. The
lateral walls of the mouth are defined by the cheeks and retromolar regions.
Three pairs of major salivary glands (parotid, submandibular and sublingual)
and numerous minor salivary glands (labial, buccal, palatal, lingual) open into
the mouth. The muscles in the oral cavity are associated with the lips, cheeks,
floor of the mouth and tongue.
The mouth is concerned primarily with the ingestion and mastication of
food, which is mainly the function of the teeth. The mouth is also associated
with phonation and ventilation, but these are secondary functions.
Cheeks
Few structural landmarks are visible. The parotid duct drains into the
cheek opposite the maxillary second molar tooth at a small parotid papilla. A
hyperkeratinized line (the linea alba) may be seen at a position related to the
occlusal plane of the teeth. In the retromolar region, a fold of mucosa which
contains the pterygomandibular raphe extends from the upper to the lower
alveolus. The entrance to the pterygomandibular space (which contains the
lingual and inferior alveolar nerves) lies lateral to this fold and medial to the
ridge produced by the anterior border of the ramus of the mandible. This is the
site for injection for an inferior alveolar nerve block.
Vascular supply and innervation
The cheek receives its arterial blood supply principally from the buccal
branch of the maxillary artery, and is innervated by cutaneous branches of the
maxillary division of the trigeminal nerve, via the zygomaticofacial and
infraorbital nerves, and by the buccal branch of the mandibular division of the
trigeminal nerve.
Lips
The central part of the lips contain orbicularis oris. Internally, the labial
mucosa is smooth and shiny and shows small elevations caused by underlying
mucous glands.
The position and activity of the lips are important in controlling the
degree of protrusion of the incisors. With normal (competent) lips, the tips of the
maxillary incisors lie below the upper border of the lower lip, and this
arrangement helps to maintain the 'normal' inclination of the incisors. When the
lips are incompetent, the maxillary incisors may not be so controlled and the
lower lip may even lie behind them, thus producing an exaggerated proclination
of these teeth. A tight, or overactive, lip musculature may be associated with
retroclined maxillary incisors.
Vascular supply and innervation
The lips are mainly supplied by the superior and inferior labial branches
of the facial artery. The upper lip is innervated by superior labial branches of the
infraorbital nerve and the lower lip is innervated by the mental branch of the
mandibular division of the trigeminal.
7
Oral vestibule
The oral vestibule is a slit-like space between the lips or cheeks on one
side and the teeth on the other. When the teeth occlude, the vestibule is a closed
space that only communicates with the oral cavity proper in the retromolar
regions behind the last molar tooth on each side. Where the mucosa that covers
the alveolus of the jaw is reflected onto the lips and cheeks, a trough or sulcus is
formed which is called the fornix vestibuli. A variable number of sickle-shaped
folds containing loose connective tissue run across the fornix vestibuli. In the
midline these are the upper and lower labial frena (or frenula). Other folds may
traverse the fornix near the canines or premolars. The folds in the lower fornix
are said to be more pronounced than those in the upper fornix
The upper labial frenum is normally attached well below the alveolar
crest. A large frenum with an attachment near the crest may be associated with a
midline gap (diastema) between the maxillary first incisors. This can be corrected by simple surgical removal of the frenum, as it contains no structures of
clinical importance. Prominent frena may also influence the stability of dentures.
Oral mucosa
The oral mucosa is continuous with the skin at the labial margins and with
the pharyngeal mucosa at the oropharyngeal isthmus. It varies in structure,
function and appearance in different regions of the oral cavity and is
traditionally divided into lining, masticatory and specialized mucosae.
Lingual mucosa
The lining mucosa is red in colour, and covers the soft palate, ventral
surface of the tongue, floor of the mouth, alveolar processes excluding the
gingivae and the internal surfaces of the lips and cheeks. It has a non-keratinized
stratified squamous epithelium which overlies a loosely fibrous lamina propria,
and the submucosa contains some fat deposits and collections of minor mucous
salivary glands. The oral mucosa covering the alveolar bone - which supports
the roots of the teeth - and the necks (cervical region) of the teeth is divided into
two main components. That portion lining the lower part of the alveolus is
loosely attached to the periosteum via a diffuse submucosa and is termed the
alveolar mucosa. It is delineated from the masticatory gingival mucosa, which
covers the upper part of the alveolar bone and the necks of the teeth, by a welldefined junction, the mucogingival junction. The alveolar mucosa appears dark
red, the gingival appears pale pink. These colour differences relate to differences
in the type of keratinization and the proximity to the surface of underlying small
blood vessels which may sometimes be seen coursing beneath the alveolar
mucosa.
Masticatory mucosa and gingivae
Masticatory mucosa, i.e. mucosa that is subjected to masticatory stress, is
bound firmly to underlying bone or to the necks of the teeth, and forms a
mucoperiosteum in the gingivae and palatine raphe. Gingival, palatal and dorsal
lingual mucosae are keratinized or parakeratinized.
8
The gingivae may be further subdivided into the attached gingivae and the
free gingivae. Attached gingivae are firmly bound to the periosteum of the
alveolus and to the teeth, whereas free gingivae, which constitute c.1 mm
margin of the gingivae, lie unattached around the cervical region of each tooth.
The free gingival groove between the free and attached gingivae corresponds
roughly to the floor of the gingival sulcus which separates the inner surface of
the attached gingivae from the enamel. The interdental papilla is that part of the
gingivae which fills the space between adjacent teeth. The surface of the
attached gingivae is characteristically stippled, although there is considerable
inter-individual variation in the degree of stippling, and variation according to
age, sex and the health of the gingivae. The free gingivae are not stippled. A
mucogingival line delineates the attached gingivae on the lingual surface of the
lower jaw from the alveolar mucosa towards the floor of the mouth. There is no
corresponding obvious division between the attached gingivae and the
remainder of the palatal mucosa because this whole surface is orthokeratinized
masticatory mucosa, which is pink.
A submucosa is absent from the gingivae and the midline palatine raphe,
but is present over the rest of the hard palate. Posterolaterally it is thick where it
contains mucous salivary glands and the greater palatine nerves and vessels, and
it is anchored to the periosteum of the maxillae and palatine bones by
collagenous septa.
Vascular supply and lymphatic drainage
The gingival tissues derive their blood supply from the maxillary and
lingual arteries. The buccal gingivae around the maxillary cheek teeth are
supplied by gingival and perforating branches from the posterior superior
alveolar artery and by the buccal branch of the maxillary artery. The labial
gingivae of anterior teeth are supplied by labial branches of the infraorbital
artery and by perforating branches of the anterior superior alveolar artery. The
palatal gingivae are supplied primarily by branches of the greater palatine artery.
The buccal gingivae associated with the mandibular cheek teeth are
supplied by the buccal branch of the maxillary artery and by perforating
branches from the inferior alveolar artery. The labial gingivae around the
anterior teeth are supplied by the mental artery and by perforating branches of
the incisive artery. The lingual gingivae are supplied by perforating branches
from the inferior alveolar artery and by its lingual branch, and by the main
lingual artery, a branch of the external carotid artery.
No accurate description is available concerning the venous drainage of the
gingivae, although it may be assumed that buccal, lingual, greater palatine and
nasopalatine veins are involved. These veins run into the pterygoid plexuses
(apart from the lingual veins, which pass directly into the internal jugular veins).
The lymph vessels of the labial and buccal gingivae of the maxillary and
mandibular teeth unite to drain into the submandibular nodes, though in the
labial region of the mandibular incisors they may drain into the submental
9
lymph nodes. The lingual and palatal gingivae drain into the jugulodigastric
group of nodes, either directly or indirectly through the submandibular nodes.
Innervation
The nerves supplying the gingivae in the upper jaw come from the
maxillary nerve via its greater palatine, nasopalatine and anterior, middle and
posterior superior alveolar branches. Surgical division of the nasopalatine nerve
causes no obvious sensory deficit in the anterior part of the palate, which
suggests that the territory of the greater palatine nerve reaches as far forwards as
the gingivae lingual to the incisor teeth. The mandibular nerve innervates the
gingivae in the lower jaw by its inferior alveolar, lingual and buccal branches.
Floor of the mouth
The floor of the mouth is a small horseshoe-shaped region situated
beneath the movable part of the tongue and above the muscular diaphragm
formed by the mylohyoid muscles. A fold of tissue, the lingual frenum, extends
onto the inferior surface of the tongue from near the base of the tongue. It
occasionally extends across the floor of the mouth to be attached onto the
mandibular alveolus. The submandibular salivary ducts open into the mouth at
the sublingual papilla, which is a large centrally positioned protuberance at the
base of the tongue.
The sublingual folds lie on either side of the sublingual papilla and cover
the underlying submandibular ducts and sublingual salivary glands. The blood
supply of the floor of the mouth is described with the blood supply of the
tongue. The main muscle forming the floor of the mouth is mylohyoid.
Immediately above it is geniohyoid.
Mylohyoid
Mylohyoid lies superior to the anterior belly of digastric and, with its
contralateral fellow, forms a muscular floor for the oral cavity. It is a flat,
triangular sheet attached to the whole length of the mylohyoid line of the
mandible. The posterior fibres pass medially and slightly downwards to the front
of the body of the hyoid bone near its lower border. The middle and anterior
fibres from each side decussate in a median fibrous raphe that stretches from the
symphysis menti to the hyoid bone. The median raphe is sometimes absent, in
which case the two muscles form a continuous sheet, or it may be fused with the
anterior belly of digastric. In about one-third of subjects there is a hiatus in the
muscle through which a process of the sublingual gland protrudes.
Relations
The inferior (external) surface is related to platysma, anterior belly of
digastric, the superficial part of the submandibular gland, the facial and
submental vessels, and the mylohyoid vessels and nerve. The superior (internal)
surface is related to geniohyoid, part of hyoglossus and styloglossus, the
hypoglossal and lingual nerves, the submandibular ganglion, the sublingual
10
gland, the deep part of the submandibular gland and its duct, the lingual and
sublingual vessels and, posteriorly, the mucous membrane of the mouth.
Vascular supply. Mylohyoid receives its arterial supply from the
sublingual branch of the lingual artery, the maxillary artery, via the mylohyoid
branch of the inferior alveolar artery, and the submental branch of the facial
artery.
Innervation. Mylohyoid is supplied by the mylohyoid branch of the
inferior alveolar nerve.
Actions. Mylohyoid elevates the floor of the mouth in the first stage of
deglutition. It may also elevate the hyoid bone or depress the mandible.
Geniohyoid
Geniohyoid is a narrow muscle which lies above the medial part of
mylohyoid. It arises from the inferior mental spine (genial tubercle) on the back
of the symphysis menti, and runs backwards and slightly downwards to attach to
the anterior surface of the body of the hyoid bone. The paired muscles are
contiguous and may occasionally fuse with each other or with genioglossus.
Vascular supply. The blood supply to geniohyoid is derived from the
lingual artery (sublingual branch).
Innervation. Geniohyoid is supplied by the first cervical spinal nerve,
through the hypoglossal nerve.
Actions. Geniohyoid elevates the hyoid bone and draws it forwards, and
therefore acts partly as an antagonist to stylohyoid. When the hyoid bone is
fixed, geniohyoid depresses the mandible.
Palate
The palate forms the roof of the mouth and is divisible into two regions,
namely, the hard palate in front and soft palate behind.
Hard palate
The hard palate is formed by the palatine processes of the maxillae and
the horizontal plates of the palatine bones. The hard palate is bounded in front
and at the sides by the tooth-bearing alveolus of the upper jaw and is continuous
posteriorly with the soft palate. It is covered by a thick mucosa bound tightly to
the underlying periosteum. In its more lateral regions it also possesses a
submucosa containing the main neurovascular bundle. The mucosa is covered
by keratinized stratified squamous epithelium which shows regional variations
and may be ortho- or parakeratinized.
The periphery of the hard palate consists of gingivae. A narrow ridge, the
palatine raphe, devoid of submucosa, runs anteroposteriorly in the midline. An
oval prominence, the incisive papilla, lies at the anterior extremity of the raphe
and covers the incisive fossa at the oral opening of the incisive canal. It also
marks the position of the fetal nasopalatine canal. Irregular transverse ridges or
rugae, each containing a core of dense connective tissue, radiate outwards from
the palatine raphe in the anterior half of the hard palate: their pattern is unique.
11
The submucosa in the posterior half of the hard palate contains minor
salivary glands of the mucous type. These secrete through numerous small
ducts, although bilaterally a larger duct collecting from many of these glands
often opens at the paired palatine foveae. These depressions, sometimes a few
millimetres deep, flank the midline raphe at the posterior border of the hard
palate. They provide a useful landmark for the extent of an upper denture. The
upper surface of the hard palate is the floor of the nasal cavity and is covered by
ciliated respiratory epithelium.
Vascular supply and lymphatic drainage of the hard palate
The palate derives its blood supply principally from the greater palatine
artery, a branch of the third part of the maxillary artery. The greater palatine
artery descends with its accompanying nerve in the palatine canal, where it gives
off two or three lesser palatine arteries which are transmitted through lesser
palatine canals to supply the soft palate and tonsil, and anastomose with the
ascending palatine branch of the facial artery. The greater palatine artery
emerges on to the oral surface of the palate at the greater palatine foramen and
runs in a curved groove near the alveolar border of the hard palate to the incisive
canal. It ascends this canal and anastomoses with septal branches of the
nasopalatine artery to supply the gingivae, palatine glands and mucous
membrane.
The veins of the hard palate accompany the arteries and drain largely to
the pterygoid plexus.
Innervation of the hard palate
The sensory nerves of the hard palate are the greater palatine and
nasopalatine branches of the maxillary nerve, which all pass through the
pterygopalatine ganglion. The greater palatine nerve descends through the
greater palatine canal, emerges on the hard palate from the greater palatine
foramen, runs forwards in a groove on the inferior surface of the bony palate
almost to the incisor teeth and supplies the gums and the mucosa and glands of
the hard palate. It also communicates with the terminal filaments of the
nasopalatine nerve. As it leaves the greater palatine canal, it supplies palatine
branches to both surfaces of the soft palate. The lesser (middle and posterior)
palatine nerves, which are much smaller, descend through the greater palatine
canal and emerge through the lesser palatine foramina in the tubercle of the
palatine bone to supply the uvula, tonsil and soft palate. The nasopalatine nerves
enter the palate at the incisive foramen and are branches of the maxillary nerve
which pass through the pterygopalatine ganglion to supply the anterior part of
the hard palate behind the incisor teeth.
Fibres conveying taste impulses from the palate probably pass via the
palatine nerves to the pterygopalatine ganglion, and travel through it without
synapsing to join the nerve of the pterygoid canal and the greater petrosal nerve
to the facial ganglion, where their cell bodies are situated. The central processes
of these neurones traverse the sensory root of the facial nerve (nervus intermedius) to pass to the gustatory nucleus in the nucleus of the tractus solitarius.
12
Parasympathetic postganglionic secretomotor fibres from the pterygopalatine
ganglion run with the nerves to supply the palatine mucous glands.
Tongue
The tongue is a highly muscular organ of deglutition, taste and speech. It
is partly oral and partly pharyngeal in position, and is attached by its muscles to
the hyoid bone, mandible, styloid processes, soft palate and the pharyngeal wall.
It has a root, an apex, a curved dorsum and an inferior surface. Its mucosa is
normally pink and moist, and is attached closely to the underlying muscles. The
dorsal mucosa is covered by numerous papillae, some of which bear taste buds.
Intrinsic muscle fibres are arranged in a complex interlacing pattern of
longitudinal, transverse, vertical and horizontal fasciculi and this allows great
mobility. Fasciculi are separated by a variable amount of adipose tissue which
increases posteriorly. The root of the tongue is attached to the hyoid bone and
mandible, and between them it is in contact inferiorly with geniohyoid and
mylohyoid. The dorsum (posterosuperior surface) is generally convex in all
directions at rest. It is divided by a V-shaped sulcus terminalis into an anterior,
oral (presulcal) part which faces upwards, and a posterior, pharyngeal
(postsulcal) part which faces posteriorly. The anterior part forms about twothirds of the length of the tongue. The two limbs of the sulcus terminalis run
anterolaterally to the palatoglossal arches from a median depression, the
foramen caecum, which marks the site of the upper end of the embryonic thyroid
diverticulum. The oral and pharyngeal parts of the tongue differ in their mucosa,
innervation and developmental origins.
Oral (presulcal) part. The presulcal part of the tongue is located in the
floor of the oral cavity. It has an apex touching the incisor teeth, a margin in
contact with the gums and teeth, and a superior surface (dorsum) related to the
hard and soft palates. On each side, in front of the palatoglossal arch, there are
four or five vertical folds, the foliate papillae, which represent vestiges of larger
papillae found in many other mammals. The dorsal mucosa has a longitudinal
median sulcus and is covered by filiform, fungiform and circumvallate papillae.
The mucosa on the inferior (ventral) surface is smooth, purplish and reflected
onto the oral floor and gums: it is connected to the oral floor anteriorly by the
lingual frenulum. The deep lingual vein, which is visible, lies lateral to the
frenulum on either side. The plica fimbriata, a fringed mucosal ridge directed
anteromedially towards the apex of the tongue, lies lateral to the vein. This part
of the tongue develops from the lingual swellings of the mandibular arch and
from the tuberculum impar.
The postsulcal part of the tongue constitutes its base and lies posterior to
the palatoglossal arches. Although it forms the anterior wall of the oropharynx,
it is described here for convenience. Its mucosa is reflected laterally onto the
palatine tonsils and pharyngeal wall, and posteriorly onto the epiglottis by a
median and two lateral glossoepiglottic folds which surround two depressions or
valleculae. The pharyngeal part of the tongue is devoid of papillae, and exhibits
low elevations. There are underlying lymphoid nodules which are embedded in
the submucosa and collectively termed the lingual tonsil. The ducts of small
13
seromucous glands open on the apices of these elevations. The postsulcal part of
the tongue develops from the hypobranchial eminence. On the rare occasions
that the thyroid gland fails to migrate away from the tongue during development
it remains in the postsulcal part of the tongue as a functioning lingual thyroid
gland.
Muscles of the tongue. The tongue is divided by a median fibrous
septum, attached to the body of the hyoid bone. There are extrinsic and intrinsic
muscles in each half, the former extending outside the tongue and moving it
bodily, the latter wholly within it and altering its shape. The extrinsic
musculature consists of four pairs of muscles namely genioglossus, hyoglossus,
styloglossus (and chondroglossus) and palatoglossus. The intrinsic muscles are
the bilateral superior and inferior longitudinal, the transverse and the vertical.
Genioglossus. Genioglossus is triangular in sagittal section, lying near
and parallel to the midline. It arises from a short tendon attached to the superior
genial tubercle behind the mandibular symphysis, above the origin of
geniohyoid. From this point it fans out backwards and upwards. The inferior
fibres of genioglossus are attached by a thin aponeurosis to the upper anterior
surface of the hyoid body near the midline (a few fasciculi passing between
hyoglossus and chondroglossus to blend with the middle constrictor of the
pharynx). Intermediate fibres pass backwards into the posterior part of the
tongue, and superior fibres ascend forwards to enter the whole length of the
ventral surface of the tongue from root to apex, intermingling with the intrinsic
muscles. The muscles of opposite sides are separated posteriorly by the lingual
septum. Anteriorly they are variably blended by decussation of fasciculi across
the midline. The attachment of the genioglossi to the genial tubercles prevents
the tongue from sinking back and obstructing respiration, therefore anaesthetists
pull the mandible forward to obtain the full benefit of this connection.
Vascular supply. Genioglossus is supplied by the sublingual branch of the
lingual artery and the submental branch of the facial artery.
Innervation. Genioglossus is innervated by the hypoglossal nerve.
Actions. Genioglossus brings about the forward traction of the tongue to
protrude its apex from the mouth. Acting bilaterally, the two muscles depress the
central part of the tongue, making it concave from side to side. Acting
unilaterally, the tongue diverges to the opposite side.
Hyoglossus. Hyoglossus is thin and quadrilateral, and arises from the
whole length of the greater cornu and the front of the body of the hyoid bone. It
passes vertically up to enter the side of the tongue between styloglossus laterally
and the inferior longitudinal muscle medially. Fibres arising from the body of
the hyoid overlap those from the greater cornu.
Relations. Hyoglossus is related at its superficial surface to the digastric
tendon, stylohyoid, styloglossus and mylohyoid, the lingual nerve and
submandibular ganglion, the sublingual gland, the deep part of the
submandibular gland and duct, the hypoglossal nerve and the deep lingual vein.
By its deep surface it is related to the stylohyoid ligament, genioglossus, the
middle constrictor and the inferior longitudinal muscle of the tongue, and the
14
glossopharyngeal nerve. Posteroinferiorly it is separated from the middle
constrictor by the lingual artery. This part of the muscle is in the lateral wall of
the pharynx, below the palatine tonsil. Passing deep to the posterior border of
hyoglossus are, in descending order: the glossopharyngeal nerve, stylohyoid
ligament and lingual artery.
Vascular supply. Hyoglossus is supplied by the sublingual branch of the
lingual artery and the submental branch of the facial artery.
Innervation. Hyoglossus is innervated by the hypoglossal nerve.
Action. Hyoglossus depresses the tongue.
Chondroglossus
Sometimes described as a part of hyoglossus, this muscle is separated
from it by some fibres of genioglossus, which pass to the side of the pharynx. It
is c.2 cm long, arising from the medial side and base of the lesser cornu and the
adjoining part of the body of the hyoid. It ascends to merge into the intrinsic
musculature between the hyoglossus and genioglossus muscles. A small slip
occasionally springs from the cartilago triticea and enters the tongue with the
posterior fibres of the hyoglossus muscle.
Vascular supply, innervation and action. These are similar to those
described for hyoglossus.
Styloglossus. Styloglossus is the shortest and smallest of the three styloid
muscles. It arises from the anterolateral aspect of the styloid process near its
apex, and from the styloid end of the stylomandibular ligament. Passing
downwards and forwards, it divides at the side of the tongue into a longitudinal
part, which enters the tongue dorsolaterally to blend with the inferior
longitudinal muscle in front of hyoglossus, and an oblique part, overlapping
hyoglossus and decussating with it.
Vascular supply. Styloglossus is supplied by the sublingual branch of the
lingual artery.
Innervation. Styloglossus is innervated by the hypoglossal nerve.
Action. Styloglossus draws the tongue up and backwards.
Stylohyoid ligament. The stylohyoid ligament is a fibrous cord which
extends from the tip of the styloid process to the lesser cornu of the hyoid bone.
It gives attachment to some fibres of styloglossus and the middle constrictor of
the pharynx and is closely related to the lateral wall of the oropharynx. Below it
is overlapped by hyoglossus. The ligament is derived embryologically from the
second branchial arch. It may be partially calcified.
Palatoglossus
Palatoglossus is closely associated with the soft palate in function and
innervation.
Intrinsic muscles.
Superior longitudinal. The superior longitudinal
muscle constitutes a thin stratum of oblique and longitudinal fibres lying
beneath the mucosa of the dorsum of the tongue. It extends forwards from the
submucous fibrous tissue near the epiglottis and from the median lingual septum
to the lingual margins. Some fibres are inserted into the mucous membrane.
15
Inferior longitudinal. The inferior longitudinal muscle is a narrow band of
muscle close to the inferior lingual surface between genioglossus and
hyoglossus. It extends from the root of the tongue to the apex. Some of its
posterior fibres are connected to the body of the hyoid bone. Anteriorly it blends
with styloglossus.
Transverse. The transverse muscles pass laterally from the median fibrous
septum to the submucous fibrous tissue at the lingual margin, blending with
palatopharyngeus.
Vertical. The vertical muscles extend from the dorsal to the ventral
aspects of the tongue in the anterior borders.
Vascular supply. The intrinsic muscles are supplied by the lingual artery.
Innervation. All intrinsic lingual muscles are innervated by the
hypoglossal nerve.
The intrinsic muscles alter the shape of the tongue. Thus, contraction of
the superior and inferior longitudinal muscles tend to shorten the tongue, but the
former also turns the apex and sides upwards to make the dorsum concave,
while the latter pulls the apex down to make the dorsum convex. The transverse
muscle narrows and elongates the tongue while the vertical muscle makes it
flatter and wider. Acting alone or in pairs and in endless combination, the
intrinsic muscles give the tongue precise and highly varied mobility, important
not only in alimentary function but also in speech.
Vascular supply and lymphatic drainage of the tongue.
Lingual artery. The tongue and the floor of the mouth are supplied
chiefly by the lingual artery, which arises from the anterior surface of the
external carotid artery. It passes between hyoglossus and the middle constrictor
of the pharynx to reach the floor of the mouth accompanied by the lingual veins
and the glossopharyngeal nerve. At the anterior border of hyoglossus, the lingual
artery bends sharply upwards. It is covered by the mucosa of the tongue and lies
between genioglossus medially and the inferior longitudinal muscle laterally.
Near the tip of the tongue it anastomoses with its contralateral fellow. The
branches of the lingual artery form a rich anastomotic network, which supplies
the musculature of the tongue, and a very dense submucosal plexus. Named
branches of the lingual artery in the floor of the mouth are the dorsal lingual,
sublingual and deep lingual arteries.
Dorsal lingual arteries. The dorsal lingual arteries are usually two or
three small vessels. They arise medial to hyoglossus and ascend to the posterior
part of the dorsum of the tongue. The vessels supply its mucous membrane, and
the palatoglossal arch, tonsil, soft palate and epiglottis. They anastomose with
their contralateral fellows.
Sublingual artery. The sublingual artery arises at the anterior margin of
hyoglossus. It passes forward between genioglossus and mylohyoid to the
sublingual gland, and supplies the gland, mylohyoid and the buccal and gingival
mucous membranes. One branch pierces mylohyoid and joins the submental
branches of the facial artery. Another branch courses through the mandibular
gingivae to anastomose with its contralateral fellow. A single artery arises from
16
this anastomosis and enters a small foramen (lingual foramen) on the mandible,
situated in the midline on the posterior aspect of the symphysis immediately
above the genial tubercles.
Deep lingual artery. The deep lingual artery is the terminal part of the
lingual artery and is found on the inferior surface of the tongue near the lingual
frenum.
In addition to the lingual artery, the tonsillar and ascending palatine
branches of the facial and ascending pharyngeal arteries also supply tissue in the
root of the tongue. In the region of the valleculae, epiglottic branches of the
superior laryngeal artery anastomose with the inferior dorsal branches of the
lingual artery.
Lingual veins. The veins draining the tongue follow two routes. Dorsal
lingual veins drain the dorsum and sides of the tongue, join the lingual veins
accompanying the lingual artery between hyoglossus and genioglossus, and
empty into the internal jugular vein near the greater cornu of the hyoid bone.
The deep lingual vein begins near the tip of the tongue and runs back just
beneath the mucous membrane on the inferior surface of the tongue. It joins a
sublingual vein from the sublingual salivary gland near the anterior border of
hyoglossus and forms the vena comitans nervi hypoglossi, which run back with
the hypoglossal nerve between mylohyoid and hyoglossus to join the facial,
internal jugular or lingual vein.
Lymphatic drainage. The mucosa of the pharyngeal part of the dorsal
surface of the tongue contains many lymphoid follicles aggregated into domeshaped groups, the lingual tonsils. Each group is arranged around a central deep
crypt, or invagination, which opens onto the surface epithelium. The ducts of
mucous glands open into the bases of the crypts. Small isolated follicles also
occur beneath the lingual mucosa. The lymphatic drainage of the tongue can be
divided into three main regions, namely marginal, central and dorsal. The
anterior region of the tongue drains into marginal and central vessels, and the
posterior part of the tongue behind the circumvallate papillae drains into the
dorsal lymph vessels. The more central regions may drain bilaterally.
Marginal vessels. Marginal vessels from the apex of the tongue and the
lingual frenulum area descend under the mucosa to widely distributed nodes.
Some vessels pierce mylohyoid as it contacts the mandibular periosteum to enter
either the submental or anterior or middle submandibular nodes, or else to pass
anterior to the hyoid bone to the jugulo-omohyoid node. Vessels arising in the
plexus on one side may cross under the frenulum to end in contralateral nodes.
Efferent vessels of median submental nodes pass bilaterally. Some vessels pass
inferior to the sublingual gland and accompany the companion vein of the
hypoglossal nerve to end in jugulodigastric nodes. One vessel often descends
further to reach the jugulo-omohyoid node, and passes either superficial or deep
to the intermediate tendon of digastric.
Vessels from the lateral margin of the tongue cross the sublingual gland,
pierce mylohyoid and end in the submandibular nodes. Others end in the
17
jugulodigastric or jugulo-omohyoid nodes. Vessels from the posterior part of the
lingual margin traverse the pharyngeal wall to the jugulodigastric lymph nodes.
Central vessels. The regions of the lingual surface draining into the
marginal or central vessels are not distinct. Central lymphatic vessels ascend
between the fibres of the two genioglossi; most pass between the muscles and
diverge to the right or left to follow the lingual veins to the deep cervical nodes,
especially the jugulodigastric and jugulo-omohyoid nodes. Some pierce
mylohyoid to enter the submandibular nodes.
Dorsal vessels. Vessels draining the postsulcal region and the
circumvallate papillae run posteroinferiorly. Those near the median plane may
pass bilaterally. They turn laterally, join the marginal vessels and all pierce the
pharyngeal wall, passing around the external carotid arteries to reach the
jugulodigastric and jugulo-omohyoid lymph nodes. One vessel may descend
posterior to the hyoid bone, perforating the thyrohyoid membrane to end in the
jugulo-omohyoid node.
Innervation of the tongue. The muscles of the tongue, with the exception
of palatoglossus, are supplied by the hypoglossal nerve. Palatoglossus is
supplied via the pharyngeal plexus. The pathways for proprioception associated
with the tongue musculature are unknown, but presumably may involve the
lingual, glossopharyngeal or hypoglossal nerves, and the cervical spinal nerves
which communicate with the hypoglossal nerve.
The sensory innervation of the tongue reflects its embryological
development. The nerve of general sensation to the presulcal part is the lingual
nerve, which also carries taste sensation derived from the chorda tympani branch
of the facial nerve. The nerve supplying both general and taste sensation to the
postsulcal part is the glossopharyngeal nerve. An additional area in the region of
the valleculae is supplied by the internal laryngeal branch of the vagus nerve.
Lingual nerve. The lingual nerve is sensory to the mucosa of the floor of
the mouth, mandibular lingual gingivae and mucosa of the presulcal part of the
tongue (excluding the circumvallate papillae). It also carries postganglionic
parasympathetic fibres from the submandibular ganglion to the sublingual and
anterior lingual glands.
The lingual nerve arises from the posterior trunk of the mandibular nerve
in the infratemporal fossa where it is joined by the chorda tympani branch of the
facial nerve and often by a branch of the inferior alveolar nerve. It then passes
below the mandibular attachment of the superior pharyngeal constrictor and
pterygomandibular raphe, closely applied to the periosteum of the medial
surface of the mandible, until it lies opposite the distal (posterior) root of the
third molar tooth, where it is covered only by the gingival mucoperiosteum. At
this point it usually lies 2-3 mm below the alveolar crest and c.0.6 mm from the
bone, but it sometimes lies above the alveolar crest. It next passes medial to the
mandibular attachment of mylohyoid, which carries it progressively away from
the mandible, and separates it from the alveolar bone covering the mesial root of
the third molar tooth, and then passes downward and forward on the deep
surface of mylohyoid to cross the lingual sulcus beneath the mucosa. In this
18
position it lies on the deep portion of the submandibular gland. It passes below
the submandibular duct which crosses it from medial to lateral, and curves
upward, forward and medially to enter the tongue. Within the tongue the lingual
nerve lies first on styloglossus and then the lateral surface of hyoglossus and
genioglossus, before dividing into terminal branches that supply the overlying
lingual mucosa. The lingual nerve is connected to the submandibular ganglion
by two or three branches, and also forms connecting loops with twigs of the
hypoglossal nerve at the anterior margin of hyoglossus.
The lingual nerve is at risk during surgical removal of (impacted) lower
third molars, and after such operations up to 10% of patients may have symptoms of nerve damage, although these are usually temporary. The nerve is also
at risk during operations to remove the submandibular salivary gland, because
the duct must be dissected from the lingual nerve during these operations.
Glossopharyngeal nerve. The glossopharyngeal nerve is distributed to
the postsulcal part of the tongue and the circumvallate papillae. It communicates
with the lingual nerve.
Hypoglossal nerve. After crossing the loop of the lingual artery a little
above the tip of the greater cornu of the hyoid, it inclines upwards and forwards
on hyoglossus, passing deep to stylohyoid, the tendon of digastric and the
posterior border of mylohyoid. Between mylohyoid and hyoglossus the
hypoglossal nerve lies below the deep part of the submandibular gland, the
submandibular duct and the lingual nerve, with which it communicates. It then
passes onto the lateral aspect of genioglossus, continuing forwards in its
substance as far as the tip of the tongue. It distributes fibres to styloglossus,
hyoglossus and genioglossus and to the intrinsic muscles of the tongue.
The special sensory innervation of the tongue. The sense of taste is
dependent on scattered groups of sensory cells, the taste buds, which occur in
the oral cavity and pharynx and are particularly plentiful on the lingual papillae
of the dorsal lingual mucosa.
Dorsal lingual mucosa. The dorsal mucosa is somewhat thicker than the
ventral and lateral mucosae, is directly adherent to underlying muscular tissue
with no discernible submucosa, and covered by numerous papillae. The dorsal
epithelium consists of a superficial stratified squamous epithelium, which varies
from non-keratinized, stratified squamous epithelium posteriorly, to fully
keratinized epithelium overlying the filiform papillae more anteriorly. These
features probably reflect the fact that the apex of the tongue is subject to greater
dehydration than the posterior and ventral parts and is subject to more abrasion
during mastication. The underlying lamina propria is a dense fibrous connective
tissue, with numerous elastic fibres, and is continuous with similar tissue
extending between the lingual muscle fasciculi. It contains numerous vessels
and nerves from which the papillae are supplied, and also large lymph plexuses
and lingual glands.
Lingual papillae. Lingual papillae are projections of the mucosa covering
the dorsal surface of the tongue. They are limited to the presulcal part of the
tongue, produce its characteristic roughness and increase the area of contact
19
between the tongue and the contents of the mouth. There are four principal
types, named filiform, fungiform, foliate and circumvallate papillae, and all
except the filiform papillae bear taste buds. Papillae are more visible in the
living when the tongue is dry.
Filiform papillae. Filiform papillae are minute, conical or cylindrical
projections which cover most of the presulcal dorsal area, and are arranged in
diagonal rows that extend anterolaterally, parallel with the sulcus terminalis,
except at the lingual apex where they are transverse. They have irregular cores
of connective tissue and their epithelium, which is keratinized, may split into
whitish fine secondary processes. They appear to function to increase the
friction between the tongue and food, and facilitate the movement of particles by
the tongue within the oral cavity.
Fungiform papillae. Fungiform papillae occur mainly on the lingual
margin but also irregularly on the dorsal surface, where they may occasionally
be numerous. They differ from filiform papillae because they are larger, rounded
and deep red in colour, this last reflecting their thin, non-keratinized epithelium
and highly vascular connective tissue core. Each usually bears one or more taste
buds on its apical surface.
Foliate papillae. Foliate papillae lie bilaterally in two zones at the sides
of the tongue near the sulcus terminalis, each formed by a series of red, leaf-like
mucosal ridges, covered by a non-keratinized epithelium. They bear numerous
taste buds.
Circumvallate papillae. Circumvallate papillae are large cylindrical
structures, varying in number from 8 to 12, which form a V-shaped row
immediately in front of the sulcus terminalis on the dorsal surface of the tongue.
Each papilla, 1-2 mm in diameter, is surrounded by a slight circular mucosal
elevation (vallum or wall) which is separated from the papilla by a circular
sulcus. The papilla is narrower at its base than its apex and the entire structure is
generally covered with non-keratinized stratified squamous epithelium.
Numerous taste buds are scattered in both walls of the sulcus, and small serous
glands (of von Ebner) open into the sulcal base.
Taste buds. Taste buds are microscopic barrel-shaped epithelial structures
which contain chemosensory cells in synaptic contact with the terminals of gustatory nerves. They are numerous on all types of lingual papillae (except filiform
papillae) particularly on their lateral aspects. Taste buds are not restricted to the
papillae, and are scattered over almost the entire dorsal and lateral surfaces of
the tongue and, rarely, on the epiglottis and lingual aspect of the soft palate.
Each taste bud is linked by synapses at its base to one of three cranial nerves
which carry taste, i.e. the facial, glossopharyngeal or vagus. They share some
physiological features with neurones, for example action potential generation
and synaptic transmission, and are therefore often referred to as paraneurones.
There is considerable individual variation in the distribution of taste buds
in humans. They are most abundant on the posterior parts of the tongue,
especially around the walls of the circumvallate papillae and their surrounding
sulci, where there is an average of c.250 taste buds for each of the 8-12 papillae.
20
Over 1000 taste buds are distributed over the sides of the tongue, particularly
over the more posterior folds of the two foliate papillae, whereas they are rare,
and sometimes even absent, on fungiform papillae (c.3 per papilla). Taste buds
have been described on the fetal epiglottis and soft palate but most disappear
from these sites during postnatal development.
Microstructure of taste buds. Each taste bud is a barrel-shaped cluster of
50-150 fusiform cells which lies within an oval cavity in the epithelium and
converges apically on a gustatory pore, a 2 μm wide opening on the mucosal
surface. The whole structure is about 70 μm in height by 40 μm across and is
separated by a basal lamina from the underlying lamina propria. A small
fasciculus of afferent nerve fibres penetrates the basal lamina and spirals around
the sensory cells. Chemical substances dissolved in the oral saliva diffuse
through the gustatory pores of the taste buds to reach the taste receptor cell
membranes, where they cause membrane depolarization.
Innervation of taste buds. Individual nerve fibres branch to give a complex distribution of taste bud innervation. Each fibre may have many terminals,
which may spread to innervate widely separated taste buds or may innervate
more than one sensory cell in each bud. Conversely, individual buds may
receive the terminals of several different nerve fibres. These convergent and
divergent patterns of innervation may be of considerable functional importance.
The gustatory nerve for the anterior part of the tongue, excluding the
circumvallate papillae, is the chorda tympani, which travels via the lingual
nerve. In most individuals, taste fibres run in the chorda tympani to cell bodies
in the facial ganglion, but occasionally they diverge to the otic ganglion, which
they reach via the greater petrosal nerve. Taste buds in the inferior surface of the
soft palate are supplied mainly by the facial nerve, through the greater petrosal
nerve, pterygopalatine ganglion and lesser palatine nerve: they may also be
supplied by the glossopharyngeal nerve. Taste buds in the circumvallate
papillae, postsulcal part of the tongue and in the palatoglossal arches and the
oropharynx are innervated by the glossopharyngeal nerve, and those in the
extreme pharyngeal part of the tongue and epiglottis receive fibres from the
internal laryngeal branch of the vagus.
Each taste bud receives two distinct classes of fibre: one branches in the
periphery of the bud to form a perigemmal plexus, the other forms an
intragemmal plexus within the bud itself which innervates the bases of the
receptor cells. The perigemmal fibres contain various neuropeptides including
calcitonin gene-related peptide (CGRP) and substance P, and appear to represent
free sensory endings. Intragemmal fibres branch within the taste bud and each
forms a series of synapses.
Taste discrimination. Gustatory receptors detect four main categories of
taste sensation, classified as salty, sweet, sour and bitter; other taste qualities
have been suggested, including metallic, and umami (Japanese: taste typified by
monosodium glutamate). Although it is commonly stated that particular areas of
the tongue are specialized to detect these different tastes, evidence indicates that
all areas of the tongue are responsive to all taste stimuli. Each afferent nerve
21
fibre is connected to widely separated taste buds and may respond to several
different chemical stimuli. Some respond to all four classic categories, others to
fewer or only one. Within a particular class of tastes, receptors are also
differentially sensitive to a wide range of similar chemicals. Moreover, taste
buds alone are able to detect only a rather restricted range of chemical
substances in aqueous solution. It is difficult to separate the perceptions of taste
and smell, because the oral and nasal cavities are continuous. Indeed, much of
what is perceived as taste is the result of airborne odorants from the oral cavity
which pass through the nasopharynx to the olfactory area above it.
Perceived sensations of taste are the results of the processing (presumably
central) of a complex pattern of responses from particular areas of the tongue.
Autonomic innervation of the tongue. The parasympathetic innervation
of the various glands of the tongue is from the chorda tympani branch of the
facial nerve which synapses in the submandibular ganglion: postganglionic
branches are distributed to the lingual mucosa via the lingual nerve. The
postganglionic sympathetic supply to lingual glands and vessels arises from the
carotid plexus and enters the tongue through plexuses around the lingual
arteries. Isolated nerve cells, perhaps postganglionic parasympathetic neurones,
have been reported in the postsulcal region: presumably they innervate glandular
tissue and vascular smooth muscle.
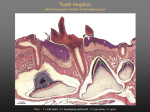
Teeth
Introduction and terminology . Humans have two generations of teeth:
the deciduous (primary) dentition and the permanent (secondary) dentition.
Teeth first erupt into the mouth at about 6 months after birth and all the
deciduous teeth have erupted by 3 years of age. The first permanent teeth appear
by 6 years, and thence the deciduous teeth are exfoliated one by one to be
replaced by their permanent successors. A complete permanent dentition is
present when the third molars erupt at or around the age of 18-21 years. In the
complete deciduous dentition there are 20 teeth, 5 in each jaw quadrant. In the
complete permanent dentition there are 32 teeth, 8 in each jaw quadrant.
There are three basic tooth forms in both dentitions: incisiform,
caniniform and molariform. Incisiform teeth (incisors) are cutting teeth, and
have thin, blade-like crowns. Caniniform teeth (canines) are piercing or tearing
teeth, and have a single, stout, pointed, cone-shaped crown. Molariform teeth
(molars and premolars) are grinding teeth and possess a number of cusps on an
otherwise flattened biting surface. Premolars are bicuspid teeth that are
restricted to the permanent dentition and replace the deciduous molars.
The tooth-bearing region of the jaws can be divided into four quadrants,
the right and left maxillary and mandibular quadrants. A tooth may thus be
identified according to the quadrant in which it is located (e.g. a right maxillary
tooth or a left mandibular tooth). In both the deciduous and permanent
dentitions, the incisors may be distinguished according to their relationship to
the midline. Thus, the incisor nearest the midline is the central (first) incisor and
the incisor that is more laterally positioned is termed the lateral (second) incisor.
The permanent premolars and the permanent and deciduous molars can also be
22
distinguished according to their mesiodistal relationships. The molar most
mesially positioned is designated the first molar, and the one behind it is the
second molar. In the permanent dentition, the tooth most distally positioned is
the third molar. The mesial premolar is the first premolar, and the premolar
behind it is the second premolar.
The terminology used to indicate tooth surfaces is shown in. The aspect of
teeth adjacent to the lips or cheeks is termed labial or buccal, that adjacent to the
tongue being lingual (or palatal in the maxilla). Labial and lingual surfaces of an
incisor meet medially at a mesial surface and laterally at a distal surface, terms
which are also used to describe the equivalent surfaces of premolar and molar
(postcanine) teeth. On account of the curvature of the dental arch, mesial
surfaces of postcanine teeth are directed anteriorly and distal surfaces are
directed posteriorly. Thus, the point of contact between the central incisors is the
datum point for mesial and distal. The biting or occlusal surfaces of postcanine
teeth are tuberculated by cusps which are separated by fissures forming a pattern
characteristic of each tooth. The biting surface of an incisor is the incisal edge.
Tooth morphology. There are two incisors, a central and a lateral, in each
half jaw or quadrant. In labial view, the crowns are trapezoid, the maxillary
incisors (particularly the central) are larger than the mandibular. The biting or
incisal edges initially have three tubercles or mamelons, which are rapidly
removed by wear. In mesial or distal view their labial profiles are convex while
their lingual surfaces are concavo-convex (the convexity near the cervical
margin is caused by a low ridge or cingulum, which is prominent only on upper
incisors). The roots of incisors are single and rounded in maxillary teeth, but
flattened mesiodistally in mandibular teeth. The upper lateral incisor may be
congenitally absent or may have a reduced form (peg-shaped lateral incisor).
Behind each lateral incisor is a canine tooth with a single cusp (hence the
American term cuspid) instead of an incisal edge. The maxillary canine is
stouter and more pointed than the mandibular canine. The canine root, which is
the longest of any tooth, produces a bulge (canine eminence) on the alveolar
bone externally, particularly in the upper jaw. Although canines usually have
single roots, that of the lower may sometimes be bifid.
Distal to the canines are two premolars, each with a buccal and lingual
cusp (hence the term bicuspid). The occlusal surfaces of the maxillary premolars
are oval (the long axis is buccopalatal) and a mesiodistal fissure separates the
two cusps. In buccal view, premolars resemble the canines but are smaller. The
maxillary first premolar usually has two roots (one buccal, one palatal) but may
have one, and very rarely three, roots (two buccal and one palatal). The
maxillary second premolar usually has one root. The occlusal surfaces of the
mandibular premolars are more circular or more square than those of the upper
premolars. The buccal cusp of the mandibular first premolar towers above the
lingual cusp to which it is connected by a ridge separating the mesial and distal
occlusal pits. In the mandibular second premolar a mesiodistal fissure usually
separates a buccal from two smaller lingual cusps. Each lower premolar has one
23
root, but very rarely the root of the first is bifid. Lower second premolars fail to
develop in about 2% of individuals.
Posterior to the premolars are three molars whose size decreases distally.
Each has a large rhomboid (upper jaw) or rectangular (lower jaw) occlusal
surface with four or five cusps. The maxillary first molar has a cusp at each
corner of its occlusal surface and the mesiopalatal cusp is connected to the
distobuccal by an oblique ridge. A smaller cusplet or tubercle (cusplet of
Carabelli) usually appears on the mesiopalatal cusp (most commonly in
Caucasian races). The tooth has three widely separated roots, two buccal and
one palatal. The smaller maxillary second molar has a reduced or occasionally
absent distopalatal cusp. Its three roots show varying degrees of fusion. The
maxillary third molar, the smallest, is very variable in form. It usually has three
cusps (the distopalatal being absent) and commonly the three roots are fused.
The mandibular first molar has three buccal and two lingual cusps on its
rectangular occlusal surface, the smallest cusp being distal. The cusps of this
tooth are all separated by fissures. It has two widely separated roots, one mesial
and one distal. The smaller mandibular second molar is like the first, but has
only four cusps (it lacks the distal cusp of the first molar) and its two roots are
closer together. The mandibular third molar is smaller still and, like the upper
third molar, is variable in form. Its crown may resemble that of the lower first or
second molar and its roots are frequently fused. As it erupts anterosuperiorly, the
third molar is often impacted against the second molar, which produces food
packing and inflammation, both indications for surgical removal. The maxillary
third molar erupts posteroinferiorly and is rarely impacted. One or more third
molars (upper or lower) fail to develop in up to 30% of individuals.
Impacted mandibular third molars.
In many subjects there is a
disproportion between the size of the teeth and the size of the jaws such that
there is insufficient space for all the teeth to erupt. As the third mandibular
molar teeth (the wisdom teeth) are the last to erupt they are often impeded in
their eruption and either become impacted against the distal aspect of the second
molar or remain unerupted deeply within the jaw bone. If the tooth is completely
covered by bone and mucosa it is very unlikely to cause any symptoms, and the
subject remains unaware of their presence unless the teeth are seen on a routine
dental radiograph. Very rarely the surrounding dental follicle may undergo
cystic degeneration which can 'hollow out' the jaw, usually the mandible, to a
considerable degree. The developing cyst may displace the tooth as it expands
and the tooth may end up as far away as the condylar neck or coronoid process.
More commonly, the erupting wisdom tooth erupts partially before
impacting against the distal aspect of the second molar. When this occurs,
symptoms are common due to recurrent soft tissue infection around the partially
erupted tooth. This condition is known as pericoronitis and if the infecting
organism is virulent, the infection may rapidly spread into the adjacent tissue
spaces as described elsewhere. It is for this reason that so many wisdom teeth
are removed in adolescents and young adults. The surgery itself requires
considerable skill as the lingual nerve passes across the surface of the
24
periosteum lingually, separated from the tooth only by a cortical plate of bone
no thicker than an egg shell. Damage to this nerve results in altered sensation to
the ipsilateral side of the tongue. The root apices of the impacted tooth often lie
immediately above the inferior alveolar canal, and removal of the tooth can
result in damage to the underlying nerve and artery. Maxillary third molars are
only rarely impacted.
The incisors, canine and premolars of the permanent dentition replace two
deciduous incisors, a deciduous canine and two deciduous molars in each jaw
quadrant. The deciduous incisors and canine are shaped like their successors but
are smaller and whiter and become extremely worn in older children. The
deciduous second molars resemble permanent molars rather than their
successors, the premolars. Each second deciduous molar has a crown which is
almost identical to that of the posteriorly adjacent first permanent molar. The
upper first deciduous molar has a triangular occlusal surface (its rounded 'apex'
is palatal) and a fissure separates a double buccal cusp from the palatal cusp.
The lower first deciduous molar is long and narrow, and its two buccal cusps are
separated from its two lingual cusps by a zigzagging mesiodistal fissure. Like
permanent molars, upper deciduous molars have three roots and lower
deciduous molars have two roots. These roots diverge more than those of
permanent teeth because each developing premolar tooth crown is
accommodated directly under the crown of its deciduous predecessor. The roots
of deciduous teeth are progressively resorbed by osteoclast-like cells
(odontoclasts) prior to being shed.
Eruption of teeth.
Information on the sequence of development and
eruption of teeth into the oral cavity is important in clinical practice and also in
forensic medicine and archaeology. The tabulated data provided in are largely
based on European-derived populations and there is evidence of ethnic variation.
When a permanent tooth erupts, about two-thirds of the root is formed and it
takes about another three years for the root to be completed. For deciduous
teeth, root completion is more rapid. The developmental stages of initial
calcification and crown completion are less affected by environmental
influences than eruption, the timing of which may be modified by several factors
such as early tooth loss and severe malnutrition.
Dental alignment and occlusion. It is possible to bring the jaws together
so that the teeth meet or occlude in many positions. When opposing occlusal
surfaces meet with maximal 'intercuspation' (i.e. maximum contact), the teeth
are said to be in centric occlusion. In this position the lower teeth are normally
opposed symmetrically and lingually with respect to the upper. Some important
features of centric occlusion in a normal (idealized) dentition may be noted.
Each lower postcanine tooth is slightly in front of its upper equivalent and the
lower canine occludes in front of the upper. Buccal cusps of the lower
postcanine teeth lie between the buccal and palatal cusps of the upper teeth.
Thus, the lower postcanine teeth are slightly lingual and mesial to their upper
equivalents. Lower incisors bite against the palatal surfaces of upper incisors,
the latter normally obscuring about one-third of the crowns of the lower. This
25
vertical overlap of incisors in centric occlusion is the overbite. The extent to
which upper incisors are anterior to lowers is termed the overjet. In the most
habitual jaw position, the resting posture, the teeth are slightly apart, the gap
between them being the free-way space or interocclusal clearance. During
mastication, especially with lateral jaw movements, the food is comminuted,
which facilitates the early stages of digestion.
The ideal occlusion is a rather subjective concept. If there is an ideal
occlusion, it can only presently be defined in broad functional terms. Therefore,
the occlusion can be considered 'ideal' when the teeth are aligned such that the
masticatory loads are within physiological range and act through the long axes
of as many teeth in the arch as possible; mastication involves alternating
bilateral jaw movements (and not habitual, unilateral biting preferences as a
result of adaptation to occlusal interference); lateral jaw movements occur
without undue mechanical interference; in the rest position of the jaw, the gap
between teeth (the freeway space) is correct for the individual concerned; the
tooth alignment is aesthetically pleasing to its possessor.
Variations from the ideal occlusion may be termed malocclusions (althoughugh these could be regarded as normal for they are more commonly found in
the population: c.75% of the population in the USA have some degree of occlusal 'disharmony'). However, the majority of malocclusions should be regarded as
anatomical variations rather than abnormalities for they are rarely involved in
masticatory dysfunction or pain, although they may be aesthetically displeasing.
The incidence of variation in number and form, which is often related to
race, is rare in deciduous teeth but not uncommon in the permanent dentition.
One or more teeth may fail to develop, a condition known as hypodontia.
Conversely, additional or supernumerary teeth may form, producing
hyperdontia. The third permanent molar is the most frequently missing tooth: in
one study one or more third molars failed to form in 32% of Chinese, 24% of
English Caucasians and 2.5% of West Africans. In declining order of incidence,
other missing teeth are maxillary lateral incisors, maxillary or mandibular
second premolars, mandibular central incisors and maxillary first premolars.
Hyperdontia affects the maxillary arch much more commonly than the
mandibular dentition. The extra teeth are usually situated on the palatal aspect of
the permanent incisors or distal to the molars. More rarely, additional premolars
develop. Although supernumerary teeth in the incisor region are often small with
simple conical crowns, they may impede the eruption of the permanent incisors.
A supernumerary tooth situated between the central incisors is known as a
mesiodens. Teeth may be unusually large (macrodontia) or small (microdontia).
For example, the crowns of maxillary central incisors may be abnormally wide
mesiodistally; in contrast, a common variant of the maxillary lateral incisor has
a small, peg-shaped crown. Epidemiological studies reveal that hyperdontia
tends to be associated with macrodontia and hypodontia with microdontia, the
most severely affected individuals representing the extremes of a continuum of
variation. Together with family studies, this indicates that the causation is
multifactorial, combining polygenic and environmental influences.
26
Some variations in the form of teeth, being characteristic of race, are of
anthropological and forensic interest. Mongoloid dentitions tend to have shovelshaped maxillary incisors with enlarged palatal marginal ridges. The additional
cusp of Carabelli is commonly found on the mesiopalatal aspect of maxillary
first permanent or second deciduous molars in Caucasian but rarely in
Mongoloid dentitions. In African races the mandibular second permanent molar
often has five rather than four cusps.
General arrangement of dental tissues. A tooth consists of a crown,
covered by very hard translucent enamel and a root covered by yellowish bonelike cementum. These meet at the neck or cervical margin. A longitudinal
ground section reveals that the body of a tooth is mostly dentine (ivory) with an
enamel covering up to about 2 mm thick, while the cementum is much thinner.
The dentine surrounds a central pulp cavity, expanded at its coronal end into a
pulp chamber and narrowed in the root as a pulp canal, opening at or near its tip
by an apical foramen, occasionally multiple. The pulp is a connective tissue,
continuous with the peridontal ligament via the apical foramen. It contains
vessels for the support of the dentine and sensory nerves.
The root is surrounded by alveolar bone, its cementum separated from the
osseous socket (alveolus) by the connective tissue of the periodontal ligament,
c.0.2 mm thick. Coarse bundles of collagen fibres, embedded at one end in
cementum, cross the periodontal ligament to enter the osseous alveolar wall.
Near the cervical margin, the tooth, periodontal ligament and adjacent bone are
covered by the gingiva. On its internal surface the gingiva is attached to the
tooth surface by the junctional epithelium, a zone of profound clinical
importance because just above it is a slight recess, the gingival sulcus. As the
sulcus is not necessarily self-cleansing, dental plaque may accumulate in it and
this predisposes to periodontal disease.
Enamel. Enamel is an extremely hard and rigid material which covers the
crowns of teeth. It is a heavily mineralized cell secretion, containing 95-96% by
weight crystalline apatites (88% by volume) and less than 1% organic matrix.
The organic matrix comprises mainly unique enamel proteins, amelogenins and
non-amelogenins such as enamelins, tuftelins. Although comprising a very small
percentage of the weight and volume of enamel, the organic matrix permeates
the whole of enamel. As its formative cells are lost from the surface during tooth
eruption, enamel is incapable of further growth. Repair is limited to the
remineralization of minute carious lesions.
Enamel reaches a maximum thickness of 2.5 mm over cusps and thins at
the cervical margins. It is composed of closely packed enamel prisms or rods. In
longitudinal section, enamel prisms extend from close to the enamel-dentine
junction to within c.20 μm of the surface, where they are generally replaced by
prismless (non-prismatic, aprismatic) enamel. In cross-section the prisms are
mainly horse-shoe shaped and are arranged in rows that are staggered such that
the tails of the prisms in one row lie between the heads of the prism in the row
above (prism pattern 3) and the tails are directed rootwards. The appearance of
prism boundaries results from sudden changes in crystallite orientation. Prisms
27
have a diameter of c.5 μm, and are packed with flattened hexagonal
hydroxyapatite crystals, far larger than those found in the other collagenousbased mineralized tissues.
Two types of incremental lines are visible in enamel, short-term and longterm. At intervals of c.4 μm along its length, each prism is crossed by a line,
probably reflecting diurnal swelling and shrinking in diameter during its growth.
This short-term daily growth line is known as a cross striation. The longer term
incremental lines pass from the enamel-dentine junction obliquely to the surface,
where they end in shallow furrows, perikymata, visible on newly erupted teeth.
Each line, known as an enamel stria, represents a period of 7-8 days enamel
growth. A prominent striation, the neonatal line, is formed in teeth whose
mineralization spans birth (see above). Neonatal lines are present in the enamel
and dentine of teeth mineralizing at the time of birth (all the deciduous teeth and
the first permanent molars and are therefore of forensic importance, indicating
that an infant has survived for a few days after birth. They reflect a disturbance
in mineralization during the first few days after birth.
Dentine. Dentine is a yellowish avascular tissue which forms the bulk of a
tooth. It is a tough and compliant composite material, with a mineral content of
c.70% dry weight (largely crystalline hydroxyapatite with some calcium
carbonate ) and 20% organic matrix (type I collagen, glycosaminoglycans and
phosphoproteins). Its conspicuous feature is the regular pattern of microscopic
dentinal tubules, c.3 μm in diameter, which extend from the pulpal surface to the
enamel-dentine junction. The tubules show lateral and terminal branching near
the enamel-dentine junction and may project a short distance into the enamel
(enamel spindles). Each tubule encloses a single cytoplasmic process of an
odontoblast whose cell body lies in a pseudostratified layer which lines the
pulpal surface. Processes are believed to extend the full thickness of dentine in
newly erupted teeth, but in older teeth they may be partly withdrawn and occupy
only the pulpal third, while the outer regions contain probably only extracellular
fluid. The diameter of the dentine tubule is narrowed by deposition of
peritubular dentine. This is different from normal dentine (intertubular dentine)
because it is more highly mineralized and lacks a collagenous matrix.
Peritubular dentine can therefore be identified by microradiography. In time, it
may completely fill the tubule, a process which gives rise to translucent dentine
and which commences in the apical region of the root.
The outermost zone (10-20 μm) of dentine differs in the crown and the root. In the crown it is referred to as mantle dentine and differs in the orientation
of its collagen fibres. In the root, the peripheral zone presents a granular layer with less overall mineral - beyond which is a hyaline layer which lacks a tubular
structure and may function to produce a good bond between the cementum and
dentine.
Dentine is formed slowly throughout life, and so there is always an
unmineralized zone of predentine at the surface of the mineralized dentine,
adjacent to the odontoblast layer at the periphery of the pulp. Biochemical
changes within the mineralizing matrix mean that predentine stains differently to
28
the matrix of the mineralized dentine. The predentine-dentine border is generally
scalloped, because dentine mineralizes both linearly and as microscopic
spherical aggregates of crystals (calcospherites). Dentine, like enamel, is
deposited incrementally, and exhibits both short- and long-term incremental
lines. Long-term lines are known as Andresen lines and are c.20 μm apart: they
represent increments of about 6-10 days. Daily incremental lines (von Ebner
lines) are c.4 μm apart. Where mineralization spans birth (i.e. all deciduous teeth
and usually the first permanent molars) a neonatal line is formed in dentine
similar to that which is seen in enamel, and it signals the abrupt change in both
environment and nutrition which occurs at birth.
Primary dentine formation proceeds at a steady but declining rate as first
the crown and then the root is completed. This slow and intermittent deposition
of dentine (regular secondary dentine) continues throughout life and further
reduces the size of the pulp chamber. The presence of the odontoblast process
means that dentine is a vital tissue. It responds to adverse external stimuli - such
as rapidly advancing caries, excessive wear or tooth breakage - by forming
poorly mineralized dead tracts, in which the odontoblasts of the affected region
die and the tubules remain empty (tertiary dentine). A dead tract may be sealed
from the pulp by a thin zone of sclerosed dentine and the deposition of irregular
(tertiary) dentine by newly differentiated pulp cells.
Dental pulp. Dental pulp provides the nutritive support for the synthetic
activity of the odontoblast layer. It is a well-vascularized, loose connective
tissue, enclosed by dentine and continuous with the periodontal ligament via
apical and accessory foramina. Several thin-walled arterioles enter by the apical
foramen and run longitudinally within the pulp to an extensive subodontoblastic
plexus. Blood flow rate per unit volume of tissue is greater in the pulp than in
other oral tissues, and tissue fluid pressures within the pulp appear to be
unusually high.
As well as typical connective tissue cells, pulp uniquely contains the cell
bodies of odontoblasts whose long processes occupy the dentinal tubules. Pulp
also has dendritic antigen-presenting cells. Approximately 60% of pulpal
collagen is type I, and the bulk of the remainder is type III. As dentine
deposition increases with age, the pulp recedes until the whole of the crown may
be removed without accessing the pulp.
Dental pulp is extensively innervated by unmyelinated postganglionic
vasoconstrictor sympathetic nerve fibres from the superior cervical ganglion,
which enter with the arterioles, and by myelinated (Aδ) and unmyelinated (C)
sensory nerve fibres from the trigeminal ganglion, which traverse the pulp
longitudinally and ramify as a plexus (Raschkow's plexus) beneath the
odontoblast layer. Here, any myelinated nerve fibres lose their myelin sheaths
and continue into the odontoblast layer, and some enter the dentinal tubules,
especially the region beneath the cusps. Stimulation of dentine, whether by
thermal, mechanical or osmotic means, evokes a pain response. Pulp nerves
release numerous neuropeptides.
29
Cementum. Cementum is a bone-like tissue which covers the dental roots, and is c.50% by weight mineralized (mainly hydroxyapatite crystals). However, unlike bone, cementum is avascular and lacks nerves. The cementum
generally overlaps the enamel slightly, although it may meet it end on. Occasionally, the two tissues may fail to meet, in which case dentine is exposed in the
mouth. If the exposed dentinal tubules remain patent then the teeth may be sensitive to stimuli such as cold water. In older teeth, the root may become exposed
in the mouth as a consequence of occlusal drift and gingival recession, and cementum is often abraded away by incorrect tooth brushing and dentine exposed.
Like bone, cementum is perforated by Sharpey's fibres, which represent
the attachment bundles of collagen fibres in the periodontal ligament (extrinsic
fibres). New layers of cementum, which are deposited incrementally throughout
life to compensate for tooth movements, incorporate new Sharpey's fibres.
Incremental lines are irregularly spaced. The first cement to be formed is thin
(up to 200 μm), acellular and contains only extrinsic fibres. Cementum formed
later towards the root apex is produced more rapidly and contains cementocytes
lying in lacunae joined by canaliculi. The latter are mainly directed towards their
source of nutrients from the periodontal ligament. This cementum contains both
extrinsic fibres derived from the periodontal ligament and intrinsic fibres of
cementoblastic origin which lie parallel to the surface. Varying arrangements of
layering between cellular and acellular cement occur. With increasing age,
cellular cement may reach a thickness of a millimetre or more around the apices
and at the branching of the roots, where it compensates for the loss of enamel by
attrition. Cementum is not remodelled but small areas of resorption with
evidence of repair may be seen.
Periodontal ligament. The principal functions of the periodontal
ligament are to support the teeth, generate the force of tooth eruption and
provide sensory information about tooth position and forces to facilitate reflex
jaw activity. The periodontal ligament is a dense fibrous connective tissue c.0.2
mm wide which contains cells associated with the development and maintenance
of alveolar bone (osteoblasts and osteoclasts) and of cementum (cementoblasts
and odontoclasts). It also contains a network of epithelial cells (epithelial cell
rests) which are embryological remnants of an epithelial root sheath. They have
no evident function but may give rise to dental cysts.
The majority of collagen fibres of the periodontal ligament are arranged as
variously oriented dense fibre bundles that connect alveolar bone and cementum
and which may help to resist movement in specific directions. About 80% of the
collagen in the periodontal ligament is type I, most of the remainder is type III.
The rate of turnover of collagen is probably the highest of any site in the body,
for reasons which are as yet unclear. A very small volume of fibres are oxytalan
fibres.
The periodontal ligament has a rich nerve and blood supply. The nerves
are both autonomic (for the vasculature) and sensory (for pain and
proprioception). The majority of proprioceptive nerve endings appear to be
Ruffini-like endings. The blood vessels tend to lie towards the bone side of the
30
periodontal ligament and the capillaries are fenestrated. Tissue fluid pressures
appear to be high.
Alveolar bone. That part of the maxilla or mandible which supports and
protects the teeth is known as alveolar bone. An arbitrary boundary at the level
of the root apices of the teeth separates the alveolar processes from the body of
the mandible or the maxilla. Like bone in other sites, alveolar bone functions as
a mineralized supporting tissue, gives attachment to muscles, provides a
framework for bone marrow and acts as a reservoir for ions, especially calcium.
It is dependent on the presence of teeth for its development and maintenance,
and requires functional stimuli to maintain bone mass. Where teeth are
congenitally absent, as for example in anodontia, it is poorly developed, and it
atrophies after tooth extraction.
The alveolar tooth-bearing portion of the jaws consists of outer and inner
alveolar plates. The individual sockets are separated by plates of bone termed
the interdental septa, while the roots of multi-rooted teeth are divided by
interradicular septa septas. The compact layer of bone which lines the tooth
socket has been called either the cribriform plate, on account of its content of
vascular (Volkmann's) canals which pass from the alveolar bone into the
periodontal ligament, or bundle bone, because numerous bundles of Sharpey's
fibres pass into it from the periodontal ligament.
In clinical radiographs, the bone lining the alveolus commonly appears as
a continuous dense white line about 0.5-1 mm thick, the lamina dura. However,
this appearance gives a misleading impression of the density of alveolar bone:
the X-ray beam passes tangentially through the socket wall, and so the radioopacity of the lamina dura is an indication of the quantity of bone the beam has
passed through, rather than the degree of mineralization of the bone.
Superimposition also obscures the Volkmann's canals. Chronic infections of the
dental pulp spread into the periodontal ligament, which leads to resorption of the
lamina dura around the root apex. The presence of a continuous lamina dura
around the apex of a tooth therefore usually indicates a healthy apical region
(except in acute infections where resorption of bone has not yet begun).
On the labial and buccal aspects of upper teeth, the two cortical plates
usually fuse, and there is very little trabecular bone between them, except where
the buccal bone thickens over the molar teeth near the root of the zygomatic
arch. It is easier and more convenient to extract upper teeth by fracturing the
buccal than the palatal plate. Anteriorly in the lower jaw, labial and lingual
plates are thin, but in the molar region the buccal plate is thickened as the
external oblique line. Near the lower third molar, the lingual bone is much
thinner than the buccal and it is mechanically easier to remove this tooth, when
impacted, via the lingual plate, although it is important to realize that the lingual
nerve is here exposed to damage.
31
Vascular supply and lymphatic drainage of the teeth and supporting
structures
Arterial supply of the teeth. The main arteries to the teeth and their
supporting structures are derived from the maxillary artery, a terminal branch of
the external carotid artery. The upper teeth are supplied by branches from the
superior alveolar arteries and the lower teeth by branches from the inferior
alveolar arteries.
Superior alveolar arteries. The upper jaw is supplied by posterior,
middle and anterior superior alveolar (dental) arteries. The posterior superior
alveolar artery usually arises from the third part of the maxillary artery in the
pterygopalatine fossa. It descends on the infratemporal surface of the maxilla,
and divides to give branches that enter the alveolar canals to supply molar and
premolar teeth, adjacent bone and the maxillary sinus, and other branches that
continue over the alveolar process to supply the gingivae. The middle and
anterior superior alveolar arteries are branches from the infraorbital artery.
The infraorbital artery often arises with the posterior superior alveolar
artery. It enters the orbit posteriorly through the inferior orbital fissure and runs
in the infraorbital groove and canal with the infraorbital nerve. When the small
middle superior alveolar artery is present it runs down the lateral wall of the
maxillary sinus and forms anastomotic arcades with the anterior and posterior
vessels, terminating near the canine tooth. The anterior superior alveolar artery
curves through the canalis sinuosus to supply the upper incisor and canine teeth
and the mucous membrane in the maxillary sinus. The canalis sinuosus swerves
laterally from the infraorbital canal and inferomedially below it in the wall of
the maxillary sinus, following the rim of the anterior nasal aperture, between the
alveoli of canine and incisor teeth and the nasal cavity. It ends near the nasal
septum where its terminal branch emerges. The canal may be up to 55 mm long.
Inferior alveolar artery. The inferior alveolar (dental) artery, a branch of
the maxillary artery, descends in the infratemporal fossa posterior to the inferior
alveolar nerve. Here, it lies between bone laterally and the sphenomandibular
ligament medially. Before entering the mandibular foramen it gives off a
mylohyoid branch, which pierces the sphenomandibular ligament to descend
with the mylohyoid nerve in its groove on the inner surface of the ramus of the
mandible. The mylohyoid artery ramifies superficially on the muscle and
anastomoses with the submental branch of the facial artery. The inferior alveolar
artery then traverses the mandibular canal with the inferior alveolar nerve to
supply the mandibular molars and premolars and divides into the incisive and
mental branches near the first premolar.
The incisive branch continues below the incisor teeth (which it supplies)
to the midline, where it anastomoses with its fellow, although few anastomotic
vessels cross the midline. In the canal the arteries supply the mandible, tooth
sockets and teeth via branches which enter the minute hole at the apex of each
root to supply the pulp. The mental artery leaves the mental foramen and
supplies the chin and anastomoses with the submental and inferior labial
arteries. Near its origin the inferior alveolar artery has a lingual branch, which
32
descends with the lingual nerve to supply the lingual mucous membrane. The
pattern of branching of the inferior alveolar artery reflects that of the nerves of
the same name.
Arterial supply of periodontal ligaments. The periodontal ligaments
supporting the teeth are supplied by dental branches of alveolar arteries. One
branch enters the alveolus apically and sends two or three small rami into the
dental pulp through the apical foramen, and other rami into the periodontal
ligament. Interdental arteries ascend in the interdental septa, sending branches at
right angles into the periodontal ligament, and terminate by communicating with
gingival vessels that also supply the cervical part of the ligament. The
periodontal ligament therefore receives its blood from three sources: from the
apical region; ascending interdental arteries; descending vessels from the
gingivae. These vessels anastomose with each other, which means that when the
pulp of a tooth is removed during endodontic treatment, the attachment tissues
of the tooth remain vital.
Venous drainage of the teeth. Veins accompanying the superior alveolar
arteries drain the upper jaw and teeth anteriorly into the facial vein, or
posteriorly into the pterygoid venous plexus. Veins from the lower jaw and teeth
collect either into larger vessels in the interdental septa or into plexuses around
the root apices and thence into several inferior alveolar veins. Some of these
veins drain through the mental foramen to the facial vein, others drain via the
mandibular foramen to the pterygoid venous plexus.
Lymphatic drainage of the teeth. The lymph vessels from the teeth
usually run directly into the ipsilateral submandibular lymph nodes. Lymph
from the mandibular incisors, however, drains into the submental lymph nodes.
Occasionally, lymph from the molars may pass directly into the jugulodigastric
group of nodes.
Innervation of the teeth
The teeth in the upper jaw are supplied by the superior alveolar nerves
while those in the lower jaw are supplied by the inferior alveolar nerve (Fig. 2).
Superior alveolar nerves. The teeth in the upper jaw are supplied by the
three superior alveolar (dental) nerves. These arise from the maxillary nerve in
the pterygopalatine fossa or in the infraorbital groove and canal. The posterior
superior alveolar (dental) nerve leaves the maxillary nerve in the pterygopalatine
fossa and runs anteroinferiorly to pierce the infratemporal surface of the maxilla,
descending under the mucosa of the maxillary sinus. After supplying the lining
of the sinus the nerve divides into small branches which link up as the molar
part of the superior alveolar plexus, supplying twigs to the molar teeth. It also
supplies a branch to the upper gingivae and the adjoining part of the cheek.
The middle superior alveolar (dental) nerve arises from the infraorbital
nerve as it runs in the infraorbital groove, and runs downwards and forwards in
the lateral wall of the maxillary sinus. It ends in small branches which link up
with the superior dental plexus, supplying small rami to the upper premolar
teeth. This nerve is variable, and it may be duplicated or triplicated or absent.
33
Fig. 2 Sensory innervation of the oral cavity is principally from
the trigeminal nerve (V) while the glossopharyngeal nerve (IX)
supplies the posterior third of the tongue. NB Taste sensation
in the anterior two-thirds of the tongue is provided by fibres of
VII nerve origin passing through the lingual nerve
The anterior superior alveolar (dental) nerve leaves the lateral side of the
infraorbital nerve near the midpoint of its canal and traverses the canalis
sinuosus in the anterior wall of the maxillary sinus. It curves first under the
infraorbital foramen, then passes medially towards the nose and finally turns
downwards and divides into branches to supply the incisor and canine teeth. It
assists in the formation of the superior dental plexus and it gives off a nasal
branch, which passes through a minute canal in the lateral wall of the inferior
meatus to supply the mucous membrane of the anterior area of the lateral wall as
high as the opening of the maxillary sinus, and the floor of the nasal cavity. It
communicates with the nasal branches of the pterygopalatine ganglion and
finally emerges near the root of the anterior nasal spine to supply the adjoining
part of the nasal septum.
Inferior alveolar (dental) nerve. Just before entering the mandibular
canal the inferior alveolar nerve gives off a small mylohyoid branch which
pierces the sphenomandibular ligament and enters a shallow groove on the
medial surface of the mandible following a course roughly parallel to its parent
nerve. It passes below the origin of mylohyoid to lie on the superficial surface of
the muscle, between it and the anterior belly of digastric, both of which it
supplies. It gives a few filaments to supply the skin over the point of the chin.
In the mandibular canal, the inferior alveolar nerve runs downward and
forward, generally below the apices of the teeth until below the first and second
34
premolars where it divides into terminal incisive and mental branches. The
incisive branch continues forward in a bony canal or in a plexiform
arrangement, giving off branches to the first premolar, canine and incisor teeth,
and the associated labial gingivae. The lower central incisor teeth receive a
bilateral innervation, fibres probably crossing the midline within the periosteum
to re-enter the bone via numerous canals in the labial cortical plate.
The mental nerve passes upward, backward and outward to emerge from
the mandible via the mental foramen between and just below the apices of the
premolar teeth. It immediately divides into three branches, two of which pass
upward and forward to form an incisor plexus labial to the teeth, supplying the
gingiva (and probably the periosteum). From this plexus and the dental
branches, fibres turn downwards and then lingually to emerge on the lingual
surface of the mandible on the posterior aspect of the symphysis or opposite the
premolar teeth, probably communicating with the lingual or mylohyoid nerve.
The third branch of the mental nerve passes through the intermingled fibres of
depressor anguli oris and platysma to supply the skin of the lower lip and chin.
Branches of the mental nerve also communicate with terminal filaments of the
mandibular branch of the facial nerve.
Variations in the fascicular organization of the inferior alveolar nerve are
clinically important when extracting impacted third molars. It may appear as a
single bundle lying a few millimetres below the roots of the teeth, or it may lie
much lower, and almost reach the lower border of the bone, so that it gives off a
variable number of large rami which pass anterosuperiorly towards the roots
before dividing to supply the teeth and interdental septa. Only rarely is it
plexiform. The nerve may lie on the lingual or buccal side of the mandible
(slightly more commonly on the buccal side). Even when the third molar tooth is
in a normal position, the nerve may be so intimately related to it that it grooves
its root. Exceptionally the nerve may be similarly related to the second molar.
Nerves may pass from the substance of temporalis to enter the mandible
through the retromolar fossa, where they communicate with branches of the
inferior alveolar nerve. Foramina occur in c.10% of retromolar fossae and
infiltration in this region can abolish sensation which occasionally remains after
an inferior alveolar nerve block. Similarly, branches from the buccal, mylohyoid
and lingual nerves may enter the mandible and provide additional routes of
sensory transmission from the teeth. Thus, even when the inferior alveolar nerve
has been anaesthetized correctly, pain may still be experienced by a patient
when undergoing dental cavity preparation.
35
2. DISEASES OF TEETH
Dental diseases. Under normal conditions, the mouth always contains
many bacteria. These bacteria may be harmless bacteria called saprophytic flora,
or the potentially harmful bacteria called pathogenic bacteria. In normal
circumstances, the harmful bacteria are kept in check by the immune system and
the disinfectant action of saliva.
Toothache usually occurs when this balance is altered, whether it is by
poor oral hygiene, tooth decay (called caries), misalignment of the upper and
lower teeth, or ill-fitting false teeth. If infections occur, and they are left
untreated, they can affect the whole mouth.
Dental caries.
Today it is estimated that more than 90% of the world’s population is
affected by caries. Caries are the most often cause of toothache. However, you
may not know that you have tooth decay since it does not always lead to
toothache. In an extreme case, the nerve (pulp) of the tooth can be destroyed yet
no pain will have been felt by the person. Therefore, it is essential to visit the
dentist regularly.
Tooth decay.Tooth decay happens when bacteria present in the mouth destroy the enamel of the tooth. Enamel is the hardest substance in the body and
usually acts as a very tough barrier to bacteria. It is kept in working order by
daily oral hygiene. However, once defences have been broken down, the enamel
cannot prevent bacteria from entering the tooth. It is not replaceable so, as it is
worn away, more and more bacteria are able to enter the tooth, making the decay
worse.
When caries are small and have not eaten away at the enamel and dentine below
it to any depth, the person may not feel any discomfort. Hot, cold or sweet
tasting foods and drinks may trigger pain by affecting the nerve of the tooth
directly.
The word "caries" is Latin for "rot", and in Greek ("Ker") it means
"death". Tooth decay, or caries, is the progressive destruction of the teeth by
plaque bacterial acid. This acid is generated by bacteria on the tooth surface
metabolising dietary sugars. The plaque holds the acid in direct contact with the
surface of the tooth.
The first signs of decay are often chalky white patches on the surfaces of
the teeth. These may be near the gum margin but, are sometimes confused with
faults of the enamel. It is often difficult to detect decay on the back teeth, where
it is initially seen as a black line or later as a shadow in the fissures or grooves of
the biting surface.
Caries develop rapidly, especially in children, resulting in
demineralisation of the dental enamel surface and collapse, thus forming a
cavity. Pain when eating hot or cold is often the first sign, and if it becomes
more persistent, is a sign that the decay is approaching the pulp of the tooth. If
untreated the pulp dies, the infection spreads into the surrounding alveolar bone.
This is often accompanied by a facial swelling and abscess.
36
Dental caries, also described as tooth decay, is an infectious disease which
damages the structures of teeth. The disease can lead to pain, tooth loss,
infection, and, in severe cases, death. There is a long history of dental caries,
with evidence showing the disease was present in the Bronze, Iron, and
Medieval ages but also prior to the neolithic period. The largest increases in the
prevalence of caries have been associated with diet changes. Today, it remains
one of the most common diseases throughout the world.
There are numerous ways to classify dental caries. Although the
presentation may differ, the risk factors and development among distinct types
of caries remain largely similar. Initially, it may appear as a small chalky area
but eventually develop into a large, brown cavitation. Though sometimes caries
may be seen directly, radiographs are frequently needed to inspect less visible
areas of teeth and to judge the extent of destruction.
Tooth decay is caused by certain types of acid-producing bacteria which
cause damage in the presence of fermentable carbohydrates such as sucrose,
fructose, and glucose. The resulting acidic levels in the mouth affect teeth
because a tooth's special mineral content causes it to be sensitive to low pH.
Specifically, a tooth (which is primarily mineral in content) is in a constant state
of back-and-forth demineralization and remineralization between the tooth and
surrounding saliva. When the pH at the surface of the tooth drops below 5.5,
demineralization proceeds faster than remineralization (i.e. there is a net loss of
mineral structure on the tooth's surface). This results in the ensuing decay.
Depending on the extent of tooth destruction, various treatments can be used to
restore teeth to proper form, function, and aesthetics, but there is no known
method to regenerate large amounts of tooth structure. Instead, dental health
organizations advocate preventative and prophylactic measures, such as regular
oral hygiene and dietary modifications, to avoid dental caries.
Archaeological evidence shows that dental caries is an ancient disease.
Skulls dating from a million years ago through the neolithic period show signs
of caries, excepting those from the Paleolithic and Mesolithic ages. The increase
of caries during the neolithic period may be attributed to the increase of plant
foods containing carbohydrates. A wooden bow drill available in the neolithic
period would have been able to make a hole in a tooth to relieve an abscess in 5
minutes. The beginning of rice cultivation in South Asia is also believed to have
caused an increase in caries.
A Sumerian text from 5000 BC describes a "tooth worm" as the cause of
caries. Evidence of this belief has also been found in India, Egypt, Japan, and
China.
Unearthed ancient skulls show evidence of primitive dental work. In
Pakistan, teeth dating from around 5500 BC to 7000 BC show nearly perfect
holes from primitive dental drills. References to caries are found in the writings
of Homer and Guy de Chauliac. The Ebers Papyrus, an Egyptian text from 1550
BC, mentions diseases of teeth. During the Sargonid dynasty of Assyria during
668 to 626 BC, writings from the king's physician specify the need to extract a
tooth due to spreading inflammation. During the Roman occupation of Europe,
37
wider consumption of cooked foods led to a small increase in caries prevalence.
The Greco-Roman civilization, in addition to the Egyptian, had treatments for
pain resulting from caries.
The rate of caries remained low through the Bronze and Iron ages, but
sharply increased during the Medieval age. Periodic increases in caries
prevalence had been small in comparison to the 1000 AD increase, when sugar
cane became more accessible to the Western world. Treatment consisted mainly
of herbal remedies and charms, but sometimes also included bloodletting. The
barber surgeons of the time provided services that included tooth extractions.
Learning their training from apprenticeships, these health providers were quite
successful in ending tooth pain and likely prevented systemic spread of
infections in many cases. Among Roman Catholics, prayers to Saint Apollonia,
the patroness of dentistry, were meant to heal pain derived from tooth infection.
There is also evidence of caries increase in North American Indians after
contact with colonizing Europeans. Before colonization, North American Indians subsisted on hunter-gatherer diets, but afterwards there was a greater reliance on maize agriculture, which made these groups more susceptible to caries.
By the Enlightenment, the belief that a "tooth worm" caused caries was no
longer accepted in the medical community. Pierre Fauchard, known as the father
of modern dentistry, was one of the first to reject the idea worms caused cavities
in teeth and noted that sugar was detrimental to the teeth and gingiva. In 1850,
another sharp increase in the prevalence of caries occurred and is believed to be
a result of widespread diet changes. Prior to this time, cervical caries was the
most frequent type of caries, but increased availability of sugar cane, refined
flour, bread, and sweetened tea corresponded with a greater number of pit and
fissure caries.
In the 1890s, W.D. Miller conducted a series of studies that led him to
propose an explanation for dental caries that was influential for current theories.
He found that bacteria inhabited the mouth and that they produced acids which
dissolved tooth structures when in the presence of fermentable carbohydrates.
This explanation is known as the chemoparasitic caries theory. Miller's contribution, along with the research on plaque by G.V. Black and J.L. Williams,
served as the foundation for the current explanation of the etiology of caries.
Epidemiology. An estimated 90% of schoolchildren worldwide and most
adults have experienced caries, with the disease being most prevalent in Asian
and Latin American countries and least prevalent in African countries. In the
United States, dental caries is the most common chronic childhood disease,
being at least five times more common than asthma. It is the primary
pathological cause of tooth loss in children. Between 29% and 59% of adults
over the age of fifty experience caries.
The number of cases has decreased in some developed countries, and this
decline is usually attributed to increasingly better oral hygiene practices and
preventive measures such as fluoride treatment. Nonetheless, countries that have
experienced an overall decrease in cases of tooth decay continue to have a
disparity in the distribution of the disease. Among children in the United States
38
and Europe, 60-80% of cases of dental caries occur in 20% of the population. A
similarly skewed distribution of the disease is found throughout the world with
some children having none or very few cavities and others having a high
number. Some countries, such as Australia, Nepal, and Sweden, have a low
incidence of cases of dental caries among children, whereas cases are more
numerous in Costa Rica and Slovakia.
Types. Caries can be classified by location, etiology, rate of progression,
and affected hard tissues. When used to characterize a particular case of tooth
decay, these descriptions more accurately represent the condition to others and
may also indicate the severity of tooth destruction.
Location. Generally, there are two types of caries when separated by
location: caries found on smooth surfaces and caries found in pits and fissures.
The location, development, and progression of smooth-surface caries differ from
those of pit and fissure caries.
The pits and fissures of teeth provide a location for caries formation.
Fig. 3 Pits & fissures on teeth.
Pits and fissures (Fig. 3) are anatomic landmarks on a tooth where tooth
enamel infolds creating such an appearance. Fissures are the grooves located on
the occlusal (chewing) surfaces of posterior teeth and lingual surfaces of
maxillary anterior teeth. Pits are small, pinpoint depressions that are found at the
ends or cross-sections of grooves. In particular, buccal pits are found on the
facial surface of molars. For all types of pits and fissures, the deep infolding of
enamel makes oral hygiene along these surfaces difficult, allowing dental caries
to be common in these areas.
The occlusal surfaces of teeth represent 12.5% of all tooth surfaces but are
the location of over 50% of all dental caries. Among children, pit and fissure
caries represent 90% of all dental caries. Pit and fissure caries can sometimes be
difficult to detect. As the decay progresses, caries in enamel nearest the surface
of the tooth spreads gradually deeper. Once the caries reaches the dentin at the
dentino-enamel junction, the decay quickly spreads laterally. The decay follows
a triangle pattern, which points to the tooth's pulp. This pattern of decay is
typically described as two triangles with their bases overlapping each other at
the dentino-enamel junction.
39
Smooth-surface caries. There are three types of smooth-surface caries.
Proximal caries, also called interproximal caries, form on the smooth surfaces
between adjacent teeth. Root caries form on the root surfaces of teeth. The third
type of smooth-surface caries occur on any other smooth tooth surface.
Fig. 4 Dental explorer
Proximal caries are the most difficult type to detect. Frequently, this type
of caries cannot be detected visually or manually with a dental explorer (Fig. 4).
Proximal caries form cervically (toward the roots of a tooth) just under the
contact between two teeth. As a result, radiographs are needed for early
discovery of proximal caries (Fig. 5).
Fig. 5 Interproximal decay on X-ray.
Root caries, (Fig. 6) which are sometimes described as a category of
smooth-surfaces caries, are the third most common type of caries and usually
occur when the root surfaces have been exposed due to gingival recession.
When the gingiva is healthy, root caries is unlikely to develop because the root
surfaces are not as accessible to bacterial plaque. The root surface is more
vulnerable to the demineralization process than enamel because cementum
begins to demineralize at 6.7 pH, which is higher than enamel's critical pH.
Regardless, it is easier to arrest the progression of root caries than enamel caries
because roots have a greater reuptake of fluoride than enamel. Root caries are
most likely to be found on facial surfaces, then interproximal surfaces, then
lingual surfaces. Mandibular molars are the most common location to find root
40
caries, followed by mandibular premolars, maxillary anteriors, maxillary
posteriors, and mandibular anteriors.
Fig. 6 Cervical Toothdecay
Lesions on other smooth surfaces of teeth are also possible. Since these
occur in all smooth surface areas of enamel except for interproximal areas, these
types of caries are easily detected and are associated with high levels of plaque
and diets promoting caries formation.
Other general descriptions. Besides the two previously mentioned
categories, carious lesions can be described further by their location on a
particular surface of a tooth. Caries on a tooth's surface that are nearest the
cheeks or lips are called "facial caries", and caries on surfaces facing the tongue
are known as "lingual caries". Facial caries can be subdivided into buccal (when
found on the surfaces of posterior teeth nearest the cheeks) and labial (when
found on the surfaces of anterior teeth nearest the lips). Lingual caries can also
be described as palatal when found on the lingual surfaces of maxillary teeth
because they are located beside the hard palate.
Caries near a tooth's cervix—the location where the crown of a tooth and
its roots meet—are referred to as cervical caries. Occlusal caries are found on
the chewing surfaces of posterior teeth. Incisal caries are caries found on the
chewing surfaces of anterior teeth. Caries can also be described as "mesial" or
"distal." Mesial signifies a location on a tooth closer to the median line of the
face, which is located on a vertical axis between the eyes, down the nose, and
between the contact of the central incisors. Locations on a tooth further away
from the median line are described as distal.
Etiology.In some instances, caries are described in other ways that might
indicate the cause. "Baby bottle caries", "early childhood caries", or "baby bottle
tooth decay" is a pattern of decay found in young children with their deciduous
(baby) teeth. The teeth most likely affected are the maxillary anterior teeth, but
41
all teeth can be affected. The name for this type of caries comes from the fact
that the decay usually is a result of allowing children to fall asleep with
sweetened liquids in their bottles or feeding children sweetened liquids multiple
times during the day. Another pattern of decay is "rampant caries" (Fig. 7),
which signifies advanced or severe decay on multiple surfaces of many teeth.
Rampant caries may be seen in individuals with xerostomia, poor oral hygiene,
methamphetamine use (due to drug-induced dry mouth, and/or large sugar
intake. If rampant caries is a result from previous radiation to the head and neck,
it may be described as radiation-induced caries.
Rate of progression.Temporal descriptions can be applied to caries to
indicate the progression rate and previous history. "Acute" signifies a quickly
developing condition, whereas "chronic" describes a condition which has taken
an extended time to develop. Recurrent caries is caries that appear at a location
with a previous history of caries. This is frequently found on the margins of
fillings and other dental restorations. On the other hand, incipient caries
describes decay at a location that has not experienced previous decay. Arrested
caries describes a lesion on a tooth which was previously demineralized but was
remineralized before causing a cavitation.
Fig. 7 Rampant caries
Affected hard tissue. Depending on which hard tissues are affected, it is
possible to describe caries as involving enamel, dentin, or cementum. Early in
its development, caries may affect only enamel. Once the extent of decay
reaches the deeper layer of dentin, "dentinal caries" is used. Since cementum is
the hard tissue that covers the roots of teeth, it is not often affected by decay
unless the roots of teeth are exposed to the mouth. Although the term
"cementum caries" may be used to describe the decay on roots of teeth, very
rarely does caries affect the cementum alone. Roots have a very thin layer of
cementum over a large layer of dentin, and thus most caries affecting cementum
also affects dentin.
42
Signs and symptoms. Until caries progresses, a person may not be aware
of it. The earliest sign of a new carious lesion, referred as incipient decay, is the
appearance of a chalky white spot on the surface of the tooth, indicating an area
of demineralization of enamel. As the lesion continues to demineralize, it can
turn brown but will eventually turn into a cavitation, a "cavity". The process
before this point is reversible, but once a cavitation forms, the lost tooth
structure cannot be regenerated. A lesion which appears brown and shiny
suggests dental caries was once present but the demineralization process has
stopped, leaving a stain. A brown spot which is dull in appearance is probably a
sign of active caries.
As the enamel and dentin are destroyed further, the cavitation becomes
more noticeable. The affected areas of the tooth change color and become soft to
the touch. Once the decay passes through enamel, the dentinal tubules, which
have passages to the nerve of the tooth, become exposed and cause the tooth to
hurt. The pain can be worsened by heat, cold, or sweet foods and drinks. Dental
caries can also cause bad breath and foul tastes. In highly progressed cases,
infection can spread from the tooth to the surrounding soft tissues which may
become life-threatening, as in the case with Ludwig's angina.
Diagnosis of caries. Primary diagnosis involves inspection of all visible
tooth surfaces using a good light source, dental mirror and explorer. Dental
radiographs, produced when X-rays are passed through the jaw and picked up on
film or digital sensor, may show dental caries before it is otherwise visible,
particularly in the case of caries on interproximal (between the teeth) surfaces.
Large dental caries are often apparent to the naked eye, but smaller lesions can
be difficult to identify. Unextensive dental caries was formerly found by
searching for soft areas of tooth structure with a dental explorer. Visual and
tactile inspection along with radiographs are still employed frequently among
dentists, particularly for pit and fissure caries.
Some dental researchers have cautioned against the use of dental
explorers to find caries. In cases where a small area of tooth has begun
demineralizing but has not yet cavitated, the pressure from the dental explorer
could cause a cavitation. Since the carious process is reversible before a
cavitation is present, it may be possible to arrest the caries with fluoride to
remineralize the tooth surface. When a cavitation is present, a restoration will be
needed to replace the lost tooth structure. A common technique used for the
diagnosis of early (uncavitated) caries is the use of air blown across the suspect
surface, which removes moisture, changing the optical properties of the
unmineralized enamel. This produces a white 'halo' effect detectable to the
naked eye. Fiberoptic transillumination, lasers and disclosing dyes have been
recommended for use as an adjunct when diagnosing smaller carious lesions in
pits and fissures of teeth.
Causes. There are four main criteria required for caries formation: a tooth
surface (enamel or dentin); cariogenic (or potentially caries-causing) bacteria;
fermentable carbohydrates (such as sucrose); and time. The caries process does
not have an inevitable outcome, and different individuals will be susceptible to
43
different degrees depending on the shape of their teeth, oral hygiene habits, and
the buffering capacity of their saliva. Dental caries can occur on any surface of a
tooth that is exposed to the oral cavity, but not the structures which are retained
within the bone.
Teeth. Having "soft teeth" is usually not the cause of caries, despite
commonly held belief to the contrary. There are certain diseases and disorders,
however, that affect teeth that can leave an individual at a greater risk for caries.
Amelogenesis imperfecta, which occurs between 1 in 718 and 1 in 14,000
individuals, is a disease in which the enamel does not form fully or in
insufficient amounts and can fall off a tooth. Dentinogenesis imperfecta is a
similar disease. In both cases, teeth may be left more vulnerable to decay
because the enamel is not as able to protect the tooth as it would in health.
In most people, disorders or diseases affecting teeth are not the primary
cause of dental caries. Ninety-six percent of tooth enamel is composed of
minerals. These minerals, especially hydroxyapatite, will become soluble when
exposed to acidic environments. Enamel begins to demineralize at a pH of 5.5.
Dentin and cementum are more susceptible to caries than enamel because they
have lower mineral content. Thus, when root surfaces of teeth are exposed from
gingival recession or periodontal disease, caries can develop more readily. Even
in a healthy oral environment, the tooth is susceptible to dental caries.
The anatomy of teeth may affect the likelihood of caries formation. In cases
where the deep grooves of teeth are more numerous and exaggerated, pit and
fissure caries are more likely to develop. Also, caries are more likely to develop
when food is trapped between teeth.
Bacteria. The mouth contains a wide variety of bacteria, but only a few
specific species of bacteria are believed to cause dental caries: Streptococcus
mutans and Lactobacilli among them. Particularly for root caries, the most
closely associated bacteria frequently identified are Lactobacillus acidophilus,
Actinomyces viscosus, Nocardia spp., and Streptococcus mutans. Bacteria
collect around the teeth and gums in a sticky, creamy-coloured mass called
plaque. Some sites collect plaque more commonly than others. The grooves on
the biting surfaces of molar and premolar teeth provide microscopic retention, as
does the point of contact between teeth. Plaque may also collect along the
gingiva. In addition, the edges of fillings or crowns can provide protection for
bacteria, as can intraoral appliances such as orthodontic braces or removable
partial dentures.
Fermentable carbohydrates. Bacteria in a person's mouth convert sugars
(glucose and fructose, and most commonly sucrose - or table sugar) into acids
such as lactic acid through a glycolytic process called fermentation. If left in
contact with the tooth, these acids may cause demineralization, which is the
dissolution of its mineral content. The process is dynamic, however, as
remineralization can also occur if the acid is neutralized; suitable minerals are
available in the mouth from saliva and also from preventative aids such as
fluoride toothpaste, dental varnish or mouthwash. Caries advance may be
arrested at this stage. If sufficient acid is produced over a period of time to the
44
favor of demineralization, caries will progress and may then result in so much
mineral content being lost that the soft organic material left behind would
disintegrate, forming a cavity or hole.
Time. The frequency of which teeth are exposed to cariogenic (acidic)
environments affects the likelihood of caries development. After meals or
snacks containing sugars, the bacteria in the mouth metabolize them resulting in
acids as by-products which decreases pH. As time progresses, the pH returns to
normal due to the buffering capacity of saliva and the dissolved mineral content
from tooth surfaces. During every exposure to the acidic environment, portions
of the inorganic mineral content at the surface of teeth dissolves and can remain
dissolved for 2 hours. Since teeth are vulnerable during these periods of acidic
environments, the development of dental caries relies greatly on the frequency
of these occurrences. For example, when sugars are eaten continuously
throughout the day, the tooth is more vulnerable to caries for a longer period of
time, and caries are more likely to develop than if teeth are exposed less
frequently to these environments and proper oral hygiene is maintained. This is
because the pH never returns to normal levels, thus the tooth surfaces cannot
remineralize, or regain lost mineral content.
The carious process can begin within days of a tooth erupting into the
mouth if the diet is sufficiently rich in suitable carbohydrates, but may begin at
any other time thereafter. The speed of the process is dependent on the interplay
of the various factors described above but is believed to be slower since the
introduction of fluoride. Compared to coronal smooth surface caries, proximal
caries progress quicker and take an average of 4 years to pass through enamel in
permanent teeth. Because the cementum enveloping the root surface is not
nearly as durable as the enamel encasing the crown, root caries tends to progress
much more rapidly than decay on other surfaces. The progression and loss of
mineralization on the root surface is 2.5 times faster than caries in enamel. In
very severe cases where oral hygiene is very poor and where the diet is very rich
in fermentable carbohydrates, caries may cause cavitation within months of
tooth eruption. This can occur, for example, when children continuously drink
sugary drinks from baby bottles. On the other hand, it may take years before the
process results in a cavity being formed, if at all.
Other risk factors. In addition to the four main requirements for caries
formation, reduced saliva is also associated with increased caries rate since the
buffering capability of saliva is not present to counterbalance the acidic
environment created by certain foods. As a result, medical conditions that
reduce the amount of saliva produced by salivary glands, particularly the parotid
gland, are likely to cause widespread tooth decay. Some examples include
Sjцgren's syndrome, diabetes mellitus, diabetes insipidus, and sarcoidosis.
Medications, such as antihistamines and antidepressants, can also impair
salivary flow. Moreover, 63% of the most commonly prescribed medications in
the United States list dry mouth as a known side effect. Radiation therapy to the
head and neck may also damage the cells in salivary glands, increasing the
likelihood for caries formation.
45
The use of tobacco may also increase the risk for caries formation.
Smokeless tobacco frequently contains high sugar content in some brands,
possibly increasing the susceptibility to caries. Tobacco use is a significant risk
factor for periodontal disease, which can allow the gingiva to recede. As the
gingiva loses attachment to the teeth, the root surface becomes more visible in
the mouth. If this occurs, root caries is a concern since the cementum covering
the roots of teeth is more easily demineralized by acids in comparison to enamel.
Currently, there is not enough evidence to support a causal relationship between
smoking and coronal caries, but there is suggestive evidence of a causal
relationship between smoking and root-surface caries.
Treatment. Destroyed tooth structure does not fully regenerate, although
remineralization of very small carious lesions may occur if dental hygiene is
kept at optimal level. For the small lesions, topical fluoride is sometimes used to
encourage remineralization. For larger lesions, the progression of dental caries
can be stopped by treatment. The goal of treatment is to preserve tooth structures
and prevent further destruction of the tooth.
Generally, early treatment is less painful and less expensive than
treatment of extensive decay. Anesthetics — local, nitrous oxide ("laughing
gas"), or other prescription medications — may be required in some cases to
relieve pain during or following treatment or to relieve anxiety during treatment.
A dental handpiece is used to remove large portions of decayed material from a
tooth. A spoon is a dental instrument used to remove decay carefully and is
sometimes employed when the decay in dentin reaches near the pulp. Once the
decay is removed, the missing tooth structure requires a dental restoration of
some sort to return the tooth to functionality and aesthetic condition.
Restorative materials include dental amalgam (Fig. 8), composite resin,
porcelain, and gold. Composite resin and porcelain can be made to match the
color of a patient's natural teeth and are thus used more frequently when
esthetics are a concern. Composite restorations are not as strong as dental
amalgam and gold; some dentists consider the latter as the only advisable
restoration for posterior areas where chewing forces are great. When the decay
is too extensive, there may not be enough tooth structure remaining to allow a
restorative material to be placed within the tooth. Thus, a crown may be needed.
This restoration appears similar to a cap and is fitted over the remainder of the
natural crown of the tooth. Crowns are often made of gold, porcelain, or
porcelain fused to metal.
Fig. 8 Amalgam
46
In certain cases, root canal therapy may be necessary for the restoration of
a tooth. Root canal therapy, also called "endodontic therapy", is recommended if
the pulp in a tooth dies from infection by decay-causing bacteria or from trauma.
During a root canal, the pulp of the tooth, including the nerve and vascular
tissues, is removed along with decayed portions of the tooth. The canals are
instrumented with endodontic files to clean and shape them, and they are then
usually filled with a rubber-like material called gutta percha. The tooth is filled
and a crown can be placed. Upon completion of a root canal, the tooth is now
non-vital, as it is devoid of any living tissue.
An extraction can also serve as treatment for dental caries. The removal of
the decayed tooth is performed if the tooth is too far destroyed from the decay
process to effectively restore the tooth. Extractions are sometimes considered if
the tooth lacks an opposing tooth or will probably cause further problems in the
future, as may be the case for wisdom teeth. Extractions may also be preferred
by patients unable or unwilling to undergo the expense or difficulties in
restoring the tooth.
Prevention. Oral hygiene. Personal hygiene care consists of proper
brushing and flossing daily. The purpose of oral hygiene is to minimize any
etiologic agents of disease in the mouth. The primary focus of brushing and
flossing is to remove and prevent the formation of plaque. Plaque consists
mostly of bacteria. As the amount of bacterial plaque increases, the tooth is
more vulnerable to dental caries. A toothbrush (Fig. 9) can be used to remove
plaque on most surfaces of the teeth except for areas between teeth. When used
correctly, dental floss removes plaque from areas which could otherwise
develop proximal caries.
Fig. 9 Toothbrush
Professional hygiene care consists of regular dental examinations and
cleanings. Sometimes, complete plaque removal is difficult, and a dentist or
47
dental hygienist may be needed. Along with oral hygiene, radiographs may be
taken at dental visits to detect possible dental caries development in high risk
areas of the mouth.
Dietary modification. For dental health, the frequency of sugar intake is
more important than the amount of sugar consumed. In the presence of sugar
and other carbohydrates, bacteria in the mouth produce acids which can
demineralize enamel, dentin, and cementum. The more frequently teeth are
exposed to this environment, the more likely dental caries are to occur.
Therefore, minimizing snacking is recommended, since snacking creates a
continual supply of nutrition for acid-creating bacteria in the mouth. Also,
chewy and sticky foods (such as dried fruit or candy) tend to adhere to teeth
longer, and consequently are best eaten as part of a meal. Brushing the teeth
after meals is recommended. For children, the American Dental Association and
the European Academy of Paediatric Dentistry recommend limiting the
frequency of consumption of drinks with sugar, and not giving baby bottles to
infants during sleep. Mothers are also recommended to avoid sharing utensils
and cups with their infants to prevent transferring bacteria from the mother's
mouth.
It has been found that milk and certain kinds of cheese like cheddar can
help counter tooth decay if eaten soon after the consumption of foods potentially
harmful to teeth. Also, chewing gum containing xylitol (wood sugar) is widely
used to protect teeth in some countries, being especially popular in the Finnish
candy industry. Xylitol's effect on reducing plaque is probably due to bacteria's
inability to utilize it like other sugars. Chewing and stimulation of flavour
receptors on the tongue are also known to increase the production and release of
saliva, which contains natural buffers to prevent the lowering of pH in the mouth
to the point where enamel may become demineralised.
Other preventive measures. The use of dental sealants is a good means of
prevention. Sealants are thin plastic-like coating applied to the chewing surfaces
of the molars. This coating prevents the accumulation of plaque in the deep
grooves and thus prevents the formation of pit and fissure caries, the most
common form of dental caries. Sealants are usually applied on the teeth of
children, shortly after the molars erupt. Older people may also benefit from the
use of tooth sealants, but usually their dental history and likelihood of caries
formation are taken into consideration.
Fluoride therapy is often recommended to protect against dental caries. It
has been demonstrated that water fluoridation and fluoride supplements decrease
the incidence of dental caries. Fluoride helps prevent decay of a tooth by binding
to the hydroxyapatite crystals in enamel. The incorporated fluoride makes
enamel more resistant to demineralization and, thus, resistant to decay. Topical
fluoride is also recommended to protect the surface of the teeth. This may
include a fluoride toothpaste or mouthwash. Many dentists include application
of topical fluoride solutions as part of routine visits.
Furthermore, recent research shows that low intensity laser radiation of
argon ion lasers may prevent the susceptibility for enamel caries and white spot
48
lesions. Also, there is current active research to find a vaccine for dental caries,
but no effective vaccine has been created yet.
Dental fluorosis OR Fluoride Mottling
A condition of emamel hypoplasia characterized by white chalky spots or
brown staining and pitting of teeth due to an increased level of fluoride affecting
enamel matrix formation and calcification by impairment of ameloblastic
function.
Dental fluorosis (Fig. 10, 11) occurs because of the excessive intake of
fluoride either through naturally occurring fluoride in the water, water
fluoridation, toothpaste, or other sources. The damage in tooth development
occurs between the ages of 6 months to 5 years, from the overexposure to
fluoride. Teeth are generally composed of hydroxyapatite and carbonated
hydroxyapatite; when fluoride is present, fluorapatite is created. Excessive
fluoride can cause yellowing of teeth, white spots, and pitting or mottling of
enamel. Consequently, the teeth become unsightly. Fluorosis cannot occur once
the tooth has erupted into the oral cavity. At this point, fluorapatite is beneficial
because it is more resistant to dissolution by acids (demineralization).
Although it is usually the permanent teeth which are affected,
occasionally the primary teeth may be involved. In mild cases, there may be a
few white flecks or small pits on the enamel of the teeth. In more severe cases,
there may be brown stains. The differential diagnosis for this condition may
include Turner's hypoplasia (although this is usually more localized), some mild
forms of amelogenesis imperfecta, and other environmental enamel defects of
diffuse and demarcated opacities.
Fig. 10 Fluorosis-mild
Fig. 11 Fluorosis-severe
Dental fluorosis is an irreversible condition caused by excessive ingestion
of fluoride during the tooth forming years. It is the first visible sign that a child
has been overexposed to fluoride.
Fluoride causes dental fluorosis by damaging the enamel-forming cells,
called ameloblasts. The damage to these cells results in a mineralization
49
disorder of the teeth, whereby the porosity of the sub-surface enamel is
increased.
While the dental profession claims that dental fluorosis is solely a
'cosmetic' effect, and not a health effect, this statement is an assumption and not
a fact. Certainly, dental fluorosis represents a toxic effect on tooth cells. The
question is whether tooth cells are the only cells in the body that are impacted.
As noted by former proponent of fluoridation, Dr. John Colquhoun,
"Common sense should tell us that if a poison circulating in a child's body can
damage the tooth-forming cells, then other harm also is likely."
As noted by Dr. Hardy Limeback, former President of the Canadian
Association of Dental Research, "it is illogical to assume that tooth enamel is the
only tissue affected by low daily doses of fluoride ingestion."
Over the past 50 years, the prevalence of dental fluorosis has increased
quite dramatically in the United States and other fluoridated countries.
According to the Centers for Disease Control, dental fluorosis now
impacts 32% of American children. (In the 1940s, dental fluorosis rates in
fluoridated areas averaged 10%.)
Not only is the prevalence of fluorosis increasing, but so to is its
severity. As noted by Dr. Gary Whitford:
"There is a growing body of evidence which indicates that the prevalence
and, in some cases, the severity of dental fluorosis is increasing in both
fluoridated and non-fluoridated regions in the U.S... This trend is undesirable for
several reasons: (1) It increases the risk of esthetically objectionable enamel
defects; (2) in more severe cases, it increases the risk of harmful effects to
dental function; (3) it places dental professionals at an increased risk of
litigation; and (4) it jeopardizes the perception of the safety and, therefore, the
public acceptance of the use of fluorides."
According to recent estimates from the U.S. and British Governments, 2
to 12% of children living in fluoridated communities have dental fluorosis of
"esthetic concern" (Griffin 2002; York Review 2000).
Dental fluorosis, of esthetic concern, is an expensive condition to treat. If
left untreated, it can cause embarrassment for school-aged children, resulting in
psychological stress and damaged self-esteem.
There is also mounting evidence that dental fluorosis in its more advanced
stages can leave teeth more susceptible to cavities. As noted by pro-fluoridation
dental researcher, Dr. Steven Levy, "With more severe forms of fluorosis, caries
risk increases because of pitting and loss of the outer enamel" (Levy 2003).
Deans Index. H.T. Dean's fluorosis index was developed in 1942 and is
currently the most universally accepted classification system. An individual's
fluorosis score is based on the most severe form of fluorosis found on two or
more teeth (Table 1, Table 2).
The Center of Disease Control found a 9% higher prevalence of dental
fluorosis in American children than was found in a similar survey 20 years ago.
In addition, the survey provides further evidence that black Americans suffer
from higher rates of fluorosis than whites.
50
The condition is more prevalent in rural areas where drinking water is
derived from shallow wells or hand pumps. It is also more likely to occur in
areas where the drinking water has a fluoride content of more than 1ppm (part
per million), and in children who have a poor intake of calcium (Table 3).
Table 1
Deans Index
Classification Criteria – description of enamel
Smooth, glossy, pale creamy-white translucent surface
Normal
Questionable A few white flecks or white spots
Very Mild
Small opaque, paper white areas covering less than 25% of the
tooth surface
Mild
Opaque white areas covering less than 50% of the tooth surface
Moderate
All tooth surfaces affected; marked wear on biting surfaces; brown
stain may be present
Severe
All tooth surfaces affected; discrete or confluent pitting; brown
stain present
Table 2 Comparative data of Deans
Index in 1987 and 2002
Deans Index
1987 2002
Questionable fluorosis
Very mild fluorosis
17%
11.8%
19%
Mild fluorosis
4%
Moderate fluorosis
1%
Severe fluorosis
0.3%
Total
22.3% 37.2%
Table 3
0.59%
Dietary Reference Intakes for Fluoride
Age group
Infants
months
0-6
Infants
months
7-12
Children
5.83%
Reference
weight kg (lb)
Adequate intake Tolerable
upper
(mg/day)
intake (mg/day)
7 (16)
0.01
0.7
9 (20)
0.5
0.9
0.7
1.3
1-3 13 (29)
51
years
Children
years
4-8
Children
years
9-13
22 (48)
1.0
2.2
40 (88)
2.0
10
Boys 14-18 years 64 (142)
3.0
10
Girls 14-18 years 57 (125)
3.0
10
Males 19 years
76 (166)
and over
4.0
10
Females 19 years
61 (133)
and over
3.0
10
If the water supply is fluoridated at the rate of 1ppm, it is necessary to
consume one litre of water in order to take in 1 mg of fluoride. It is highly
improbable a person will receive more than the tolerable upper limit from
consuming optimally fluoridated water alone.
Fluoride consumption can exceed the tolerable upper limit when someone
drinks a lot of fluoride containing water in combination with other fluoride
sources, such as swallowing fluoridated toothpaste use, and consuming food
with a high fluoride content. Dental fluorosis can be prevented by lowering the
amount of fluoride intake to below the tolerable upper limit.
Treatment. Dental fluorosis can be cosmetically treated by a dentist. The
cost and success can vary significantly depending on the treatment. Tooth
bleaching, microabrasion, and conservative composite restorations or porcelain
veneers are commonly used treatment modalities. Generally speaking, bleaching
and microabrasion are used for superficial staining, whereas the conservative
restorations are used for more unaesthetic situations.
Erosion of teeth
Dental erosion is defined as irreversible loss of dental hard tissue by a
chemical process that does not involve bacteria. Dissolution of mineralized tooth
structure occurs upon contact with acids that are introduced into the oral cavity
from intrinsic (e.g., gastroesophageal reflux, vomiting) or extrinsic sources (e.g.,
acidic beverages, citrus fruits). This form of tooth surface loss is part of a larger
picture of tooth wear, which also consists of attrition, abrasion, and possibly,
abfraction.
Most common chemicals are those with pH levels in the acidic range.
Continuous contact of the enamel with the chemicals will lead to leaching of the
calcifying salts and a reduction in its hardness.
52
Typically an excessive diet of foods with an acid pH (citrus fruits and
carbonated drinks) will produce smooth saucer shaped cavitations of the labila
surfaces of the anterior teeth. Patients with regurgitation of acidic stomach
contents often develop erosions on the lingual surfaces of the teeth.
Tooth erosion is caused by acidic foods and drinks ‘dissolving’ away the
surface of the tooth. It is becoming increasingly more common, especially due to
greater consumption of fizzy drinks – including ‘diet’ brands.
Erosion caused by foods and drinks. Acids in the mouth can dissolve
away tooth surfaces. Given the chance, teeth will repair themselves, using
minerals from saliva. But if acid is in the mouth too often, teeth cannot repair
themselves and the hard tooth surface (the enamel) becomes thinner - this is
called 'erosion'.
The teeth can then become extra sensitive to hot and cold food and drink.
Eroded teeth can also be more likely to suffer decay.
The main cause of erosion is too frequent consumption of certain kinds of
food and drink. All fizzy drinks (including 'diet' brands and fizzy mineral water),
all 'sports' drinks, all squashes and all fruit juices are acidic to varying degrees.
Pickles and citrus fruits are examples of acidic types of food. Some medicines
are acidic and, therefore, erosive.
And people with some illnesses (such as eating disorders) may suffer from
erosion because of frequent vomiting, as stomach acids also erode teeth. For this
reason, dentists may ask about eating disorders if they see teeth that are very
badly eroded.
Here are some key tips to prevent erosion.
1. Try and avoid consuming acidic food and / or drink too often during the
day. Try to have them only at mealtimes.
2. Drink acidic drinks quickly - don't sip them. And don't swish them round
your mouth.
3. Between meals you should only have 'safe' drinks, which are not sugary
or acidic. Milk and water are 'safe' drinks. So are tea and coffee if you do
not add sugar to them (you can use non-sugar sweeteners).
4. You should try and avoid snacking between meals. If you do snack, only
have 'safe' snacks, which are not sugary or acidic. Fruits, vegetables and
products (such as sandwiches, toast, crumpets and pitta bread) are all 'safe'
snacks. You should try and avoid snacking between meals. Some fruits,
especially citrus fruits, are acidic and are known to cause erosion if they
are consumed in large quantities. This is not normally a problem for most
people; however, you could discuss with your dentist or hygienist the
safest way of enjoying these fruits.
5. Because acids temporarily soften the tooth surface, don't brush your teeth
immediately after eating or drinking something acidic.
6. You should brush your teeth twice a day, and always use a fluoride
toothpaste.
53
7. Your dentist can identify erosion, pinpoint the causes and advise you how
to prevent further damage.
Diagnosis of erosion due to bulemia and other acid sources
Teeth are a mineralized structure made primarily from calcium. Because
of this, they can be dissolved readily by acid. When this acid comes from
bacteria, decay occurs. However there are other sources which can also provide
enough acid to damage the teeth.
One common source of acid is the hydrochloric acid found in the stomach.
Any time stomach contents are in the mouth, acid can cause erosion of the teeth.
This becomes a potential problem for persons with bulemia, (habitual vomitting)
reflux esophogitis or hiatal hernia (medical conditions which allow stomach
contents to seep into the mouth when lying down).
Erosion of the teeth can easily be distinguished from wear of the teeth by
clenching or bruxing. The following images describe characteristics of erosion.
Erosion of maxillary lingual surfaces . The side of the upper (maxillary)
teeth facing the tongue (known as the lingual surface) usually has the greatest
amount of erosion (arrows). The yellow portions in the center of the teeth are the
areas where the enamel (the hard outer layer of the teeth) has been disolved and
worn away. The portion of the tooth thereby exposed is the softer dentin which
is even more prone to erosion by acid and wear from chewing and clenching.
Erosion of mandibular buccal surfaces. The side of the lower
(mandibular) teeth facing the cheek (known as the buccal surface) is also prone
to erosion from acid from stomach contents. Notice how the lower teeth seem to
slant toward the tongue due to the loss of tooth structure toward the chewing
surface of the teeth. Both the upper and lower teeth are reduced in height to
about half of their normal length. These patterns of erosion on the upper and
lower teeth occurs because of where the acid tends to pool in the mouth. Also
notice the shortness of the posterior (back) teeth. When the erosion is severe
enough that the enamel of the chewing surfaces is lost, the softer portion of the
tooth (dentin later) is exposed and wears rapidly. Since the teeth are shorter the
jaws close further before the teeth contact. This condition is called a "closed
bite" resulting in a loss of the vertical height of the face, wrinkles in the skin at
the corners of the mouth and can sometimes cause soreness of the facial muscles
or headaches.
Raised fillings. Since most filling materials are more resistant to
dissolution by acid than the teeth, it is common to find that parts of the tooth
have dissolved away leaving the filling at its original height. This gives the
appearance that the filling is "raised" (arrows) while in fact, the true cause is the
heightn of the tooth is lowered. This is a classic finding in erosion cases where
the fillings have been present for a long time. When teeth are shortened due to
wear of the teeth by clenching or bruxing the fillings are worn to the same level
as the surface of the tooth and do not appear raised.
Erosion is often not recognized in its early stages nor are risk factors
identified and addressed. Lack of awareness of the multifactorial nature of tooth
54
wear may lead to only partial treatment of the problem (e.g., an occlusal splint).
Partial treatment may eventually result in the necessity of complex and
expensive restorative care. Since early recognition and initiation of preventive
measures can prevent significant damage to the dentition, dental erosion
warrants the careful attention of the primary dental care team.
A review of the literature on dental erosion indicates a relatively recent,
growing interest in the topic, particularly in Europe. For the first time, England
included the evaluation of tooth erosion in its national dental health survey in
1993, indicating the importance of this dental problem. The aim of this article is
to review the etiologies of dental erosion and provide recommendations for
diagnosis and management of this problem.
Pulpitis
Inflammation of the dental pulp. Pulpal disease (pulpitis) and its local
sequelae - necrosis of the pulp, apical periodontitis, periapical abscess, cellulitis,
and osteomyelitis of the jaw - can occur when caries progresses deeply in the
dentin, when a tooth requires multiple invasive procedures, or when trauma
disrupts the lymphatic and blood supply to the pulp. Inflammation that would
easily subside in other parts of the body leads to necrosis in the rigidly encased
(by the dentin) pulp because edema cannot occur there without compromising
circulation.
If dental infection spreads from maxillary teeth, it may cause purulent
sinusitis, meningitis, brain abscess, orbital cellulitis, and cavernous sinus
thrombosis. Infection from the mandibular teeth may cause Ludwig's angina
(submaxillary cellulitis; a deep infection of the tissues of the floor of the mouth),
parapharyngeal abscess, mediastinitis, pericarditis, empyema, and jugular
thrombophlebitis.
Symptoms and Diagnosis. In reversible pulpitis, pain is felt when a
stimulus (usually cold or sweets) is applied to the tooth. When the stimulus is
removed, the pain ceases within a few seconds.
Irreversible pulpitis produces pain that lingers for minutes after the
stimulus is removed or that occurs spontaneously. A patient may have difficulty
locating the precise tooth that is the source of the pain, even confusing the
maxillary and mandibular arches (but not the left and right sides of the mouth)
because the pulp has no proprioceptive fibers. The pain may then cease for
several days because of pulpal necrosis. When bacteria or their metabolites exit
through the apical foramen, thereby causing inflammation in the adjacent
periodontal ligament, the tooth becomes exquisitely sensitive to pressure and
percussion. As a periapical (dentoalveolar) abscess forms, the tooth is elevated
from its socket and feels "high" when biting.
Pulpitis is an inflammation of the pulpal tissue that may be acute or
chronic with or without symptoms, and reversible or irreversible.
Causes of Pulpitis
Caries that penetrate though the tooth enamel, the dentin, and into the
pulp
55
Repeated dental procedures or tooth trauma
Regardless of the cause of pulpitis the inflammation can be associated with a bacterial infection. As in the case of a carie that penetrates the pulp cavi-ty
the tooth is no longer sealed to infectious pathogens, where as when the blo-od
supply is cut off to the pulp, bacteria have an opportunity to over take the pulp.
When the pulp becomes inflamed pressure begins to build up in the pulp
cavity exerting pressure on the nerve of the tooth and the surrounding tissues.
Pressure from inflammation can cause mild to extreme pain, depending upon the
severity of the inflammation. Often, pulpitis can create so much pressure on the
tooth nerve the individual will have trouble locating the source of the pain,
confusing it with neighbouring teeth. Inflammation in the tooth provides a
difficult environment for reducing the inflammation in the pulp cavity. Unlike
other parts of the body where pressure can dissipate through the surrounding soft
tissues and where lymph can reach, the pulp cavity is very different. The dentin
surrounding the pulp is hard and does not give under the pressure of the
inflammation so the pressure has very little chance of dissipating before pulp
necrosis occurs. The pulp cavity inherently provides the body with an immune
system response challenge, which makes it very unlikely that the bacterial
infection can be eliminated. The pain will usually stop once the pulp has died,
however the infection can spread to the ancillary anatomy.
Treatment. Once the pulp has become inflamed the tooth can be
diagnostically divided into two categories: reversible pulpitis and irreversible
pulpitis.
Reversible pulpitis. Can be determined by applying hot or cold to the
affected tooth and determining how long the pain continues after the stimulus is
removed. If the duration of the pain episode lasts for seconds the tooth pulp can
be saved. Usually this condition is caused by average caries.
Irreversible pulpitis. Can likewise be determined by applying a hot or
cold stimulus to the affected tooth and determining how long the pain lasts. If
the duration of the pain lasts for minutes, then the tooth pulp requires a root
canal. If the situation is bad enough a tooth extraction may need to be done.
Toothaches are caused by an inflammation of the pulp inside the tooth.
The pulp is part of the inside of the tooth that has tissue and nerves. A toothache
usually follows injury to the tooth. The most common form of injury to the tooth
is from dental caries, or a cavity. This is often a result of poor dental hygiene.
Most toothaches are a result of a cavity. Sugar and starch in foods are the
substances that cause damage to teeth. The bacteria in the mouth feed on sugar
and starch and produce an acid that can eat through the teeth, leading to tooth
decay. Different types of bacteria are involved in this process that can lead to an
infection in the inside of the tooth.
The following are the most common symptoms of a toothache. However,
each child may experience symptoms differently.
Symptoms may include:
1. Constant, throbbing pain in a tooth.
2. A tooth is painful to touch.
56
3. Pain in the tooth that worsens with hot or cold foods or liquids.
4. The jaw in the area of the tooth that is sore and tender to touch.
5. Fever.
6. Malaise (generally tired and feeling badly)
The symptoms of a toothache may resemble other medical conditions or
dental problems. Always consult your child’s physician or dentist for a
diagnosis. A toothache is usually diagnosed based on a complete history and
physical examination of your child and your child’s mouth. Your child’s
physician will probably refer your child to a dentist for complete evaluation and
treatment. At the dentist, x-rays (a diagnostic test which uses invisible
electromagnetic energy beams to produce images of internal tissues, bones, and
organs onto film) of the teeth may be taken to help in the diagnosis and
treatment of the problem.
Treatment for a toothache:
Specific treatment for a toothache will be determined by your child’s
physician based on:
- your child’s age, overall health, and medical history;
- extent of the condition;
- your child’s tolerance for specific medications, procedures, or therapies;
- expectations for the course of the condition;
- your opinion or preference.
Treatment may include:
- antibiotics by mouth may be prescribed;
- pain medications may be prescribed;
- warm salt water rinses to the mouth;
- tooth extraction (full or partial removal);
- draining of an abscess, if present
- root canal – a surgical procedure that involves the removal of the
damaged nerve and tissue from the middle of the tooth.
If the infection is severe, the child may need to be treated in the hospital
and receive antibiotics through an intravenous (IV) catheter.
Acute Pulpitis
A common condition affecting a tooth accompanied by severe, relentless
pain; the acute inflammation associated with it invariably causes pulp death
requiring pulp extirpation or extraction.
Etiology: Bacterial, Chemical, or Physical Irritation
Location(s): Pulp of carious or restored tooth.
Clinical Features: Severe relentless pain; may be exacerbated by heat
and relieved by cold.
Radiographic Features: None
Microscopic Features: Acute inflammation
Complications: Death of dental pulp and extension of infection into
periapical tissues.
Treatment: Pulp removal (extirpation) or tooth extraction.
57
Prognosis: Invariably leads to the death of dental pulp.
Pathogenesis: Acute inflammation of dental pulp.
Acute Pulpitis. Acute pulpitis is a severe inflammation of the tooth pulp.
Usually, it is the result of dental caries. It is the most frequent cause of severas
soon as possible.
Chronic Pulpitis
A common condition affecting a tooth accompanied by dull, bearable
pain; the chronic inflammation associated with it usually causes pulp death
requiring pulp extirpation or tooth extraction.
Etiology: Bacterial, Chemical, or Physical Irritation
Location(s): Pulp of carious or restored tooth.
Clinical Features: Dull, bearable pain; may be intermittent or continual.
Radiographic Features: None.
Microscopic Features: Chronic inflammation
Complications: Death of dental pulp and extension of infection into
periapical tissues.
Treatment: Pulp removal (extirpation) or tooth extraction.
Prognosis: Invariably leads to the death of dental pulp.
Pathogenesis: Chronic inflammation
Toothache. Pain that is localized to a particular tooth and that is provoked
by sweets or cold is usually caused by caries (tooth decay) that is approaching
the dental pulp, which contains the nerves. Such pain is usually fleeting. A
patient should avoid the provoking stimuli, use mild systemic analgesics, and
promptly seek dental treatment.
Lingering toothache that is usually intensified by heat or cold and is
occasionally relieved by cold usually indicates that the pulp is irreversibly
damaged. Often, this condition leads to periapical inflammation, which may be
diagnosed by tenderness to percussion with a tongue blade. If many or all
maxillary posterior teeth on one side are sensitive to such percussion, maxillary
sinusitis should be suspected.
Periapical infection, often accompanied by swelling of contiguous soft
tissues, characteristically develops if pulpitis is untreated. Emergency treatment
consists of analgesics and antibiotics if dental treatment is not immediately
available. A periapical abscess that has spread beyond the alveolar bone and is
causing swelling and fluctuance in adjacent soft tissue may require incision and
drainage in addition to dental treatment of the tooth. Antibiotics alone are
inadequate and generally not indicated. An intraoral incision is usually
appropriate, but a percutaneous incision may be necessary for dependent
drainage. Culture results guide choice of antibiotic, if needed.
Erupting or impacted molars, particularly third molars, can cause pain and
inflammation of adjacent soft tissue (pericoronitis) that can progress to serious
infection.
Less common causes of acute perioral swelling include periodontal
abscess, infected cysts, antritis, allergy, salivary gland obstruction or infection,
and peritonsillar infection.
58
PERIODONTAL DESEASES
Periodontal disease is often a result of poor dental hygiene. It is caused by
the release of toxins from the bacteria of the dental plaque. Prevention and
control is based on daily removal of plaque with a toothbrush.
The first sign is an inflammation of the gum margin (gingivitis), that is
reversible and often appears in childhood. If untreated, the condition may
progress to periodontitis, where the underlying bone supporting the teeth is
destroyed and the teeth may become loose and painful.
Inflammation of the gum margin (gingivitis) may often be more severe
during pregnancy, among the debilitated, the immonucompromised and patients
taking certain drugs.
Mouth odor. Mouth odor, better known as bad breath or halitosis is due to
enteric bacteria. The bacteria are found on the tongue, under the tongue and
hiding in crevices under the gums. Food particles lodge under the gums and in
between teeth where enteric bacteria digest the particles to release odors. The
food particles contain nitrogen and sulfur. That means that the food particles are
proteins, mostly animal and poultry products. The toxins are some form of
ammonia or hydrogen sulfide gas. The odor may be ammonia or hydrogen
sulfide from the stomach and gut. The gases have been sent back to the oral
cavity when the proteins rot in the stomach and gut. The same enteric bacteria
found in the gut digest the rotten proteins and send the gases back to the mouth.
Treatment is to use protein enzyme supplements that include vitamins and
minerals. Also use plant aromatic acids to make the environment more acid to
neutralize the toxic fumes. Another supplement is to use probiotics to use
bacteria to control the enteric bacteria and neutralize the toxic fumes by using
their own enzymes to digest the gases.
Gingivitis
Gingivitis is the inflammation of the gums (gingiva) around the teeth
(Fig. 12, 13). Gingivitis may be caused by a build up of plaque and "Calculus
(dental)" tartar due to improper cleaning of teeth, or by injury to the gums from
over-vigorous brushing and/or flossing. The condition is generally reversible.
Brushing teeth thoroughly, but gently, with toothpaste and flossing with dental
floss are the best ways to prevent gingivitis.
Fig. 12 Acute gingivitis.
Fig. 13 Chronic hypertrophic gingivitis.
59
Causes. Gingivitis is usually caused by bacterial plaque that accumulates
in the spaces between the gums and the teeth and in calculus (tartar) that forms
on the teeth. These accumulations may be tiny, even microscopic, but the
bacteria in them produce foreign chemicals and toxins that cause inflammation
of the gums around the teeth. This inflammation can, over the years, cause deep
pockets between the teeth and gums and loss of bone around teeth otherwise
known as periodontitis. Since the bone in the jaws holds the teeth into the jaws,
the loss of bone can cause teeth over the years to become loose and eventually to
fall out or need to be extracted due to acute infection. Regular cleanings
(correctly termed periodontal debridement, scaling or root planing) below the
gum line, best accomplished professionally by a dental hygienist or dentist,
disrupt this plaque biofilm and remove plaque retentive calculus (tartar) to help
prevent inflammation. Once cleaned, plaque will begin to grow on the teeth
within hours. However, it takes approximately 3 months for the pathogenic type
of bacteria (typically gram negative anaerobes and spirochetes) to grow back
into the deep pockets and restart the inflammatory process. Calculus may start to
reform within 24 hours. Ideally, scientific studies show that all people with deep
periodontal pockets (greater than 5mm) should have the pockets between their
teeth and gums cleaned by a dental hygienist or dentist every 3-4 months. People
with a healthy periodontium (gums, bone and ligament) or people with gingivitis
only require periodontal debridement every 6 months. However, many dental
professionals only recommend periodontal debridement (cleanings) every 6
months, because this has been the standard advice for decades, and because the
benefits of regular periodontal debridement (cleanings) are too subtle for many
patients to notice without regular education from the dental hygienist or dentist.
If the inflammation in the gums becomes especially well-developed, it can
invade the gums and allow tiny amounts of bacteria and bacterial toxins to enter
the bloodstream. The patient may not be able to notice this, but studies suggest
this can result in a generalized increase in inflammation in the body cause
possible long term heart problems. Periodontitis has also been linked to diabetes,
arteriosclerosis, osteoporosis, pancreatic cancer and pre-term low birth weight
babies.
Sometimes, the inflammation of the gingiva can suddenly amplify, such
as to cause a disease called Acute Necrotizing Ulcerative Gingitivitis (ANUG),
otherwise known as trench mouth." The aetiology of ANUG is the overgrowth
of a particular type of pathogenic bacteria (fusiform-spirochete variety) but risk
factors such as stress, poor nutrition and a compromised immune system can
exacerbate the infection. This results in the breath being extremely bad-smelling,
and the gums feeling considerable pain and degeration of the periodontium
rapidly occurs. Fortunately, this can be cured with a 1-week course of
Metronidazole antibiotic, followed by a deep cleaning of the gums by a dental
hygienist or dentist and reduction of risk factors such as stress.
When the teeth are not cleaned properly by regular brushing and flossing,
bacterial plaque accumulates, and becomes mineralized by calcium and other
minerals in the saliva transforming it into a hard material called calculus (tartar)
60
which harbors bacteria and irritates the gingiva (gums). Also, as the bacterial
plaque biofilm becomes thicker this creates an anoxygenic environment which
allows more pathogenic bacteria to flourish and release toxins and cause
gingival inflammation. Alternatively, excessive injury to the gums caused by
very vigorous brushing may lead to recession, inflammation and infection.
Pregnancy, uncontrolled diabetes mellitus and the onset of puberty increase the
risk of gingivitis, due to hormonal changes that may increase the susceptibility
of the gums or alter the composition of the dentogingival microflora. The risk of
gingivitis is increased by misaligned teeth, the rough edges of fillings, and ill
fitting or unclean dentures, bridges, and crowns. This is due to their plaque
retentive properties. The drug phenytoin, birth control pills, and ingestion of
heavy metals such as bismuth may also cause gingivitis.
The sudden onset of gingivitis in a normal, healthy person should be
considered an alert to the possibility of an underlying viral aetiology, although
most systemically healthy individuals have gingivitis in some area of their
mouth, usually due to inadequate brushing and flossing.
Gingivitis is considered to be a bacterial infection of the gums. The exact
reason why gingivitis develops has not been proven, but several theories exist.
- For gingivitis to develop, plaque must accumulate in the areas between
the teeth. This plaque contains large numbers of bacteria thought to be
responsible for gingivitis. But it is not simply plaque that causes
gingivitis. Almost everyone has plaque on their teeth, but only a few
develop gingivitis.
- It is usually necessary for the person to have an underlying illness or take
a particular medication that renders their immune system susceptible to
gingivitis. For example, people with " leukemia and Wegner disease have
changes in the blood vessels of their gums that allow gingivitis to
develop. Other people with diabetes, Addison disease, HIV, and other
immune system diseases lack the ability to fight bacteria invading the
gums.
- Sometimes hormonal changes in the body during pregnancy, puberty, and
steroid therapy leave the gums vulnerable to bacterial infection.
- A number of medications used for seizures, high blood pressure, and
organ transplants can suppress the immune system and change the
structure of the gums enough to permit bacterial infection.
The symptoms of gingivitis are as follows:
- Swollen gums
- Mouth sores
- Bright-red, or purple gums
- Shiny gums
- Gums that are painless, except when pressure is applied
- Gums that bleed easily, even with gentle brushing
- Gums that itch with varying degrees of severity
- Receding gumline
61
Prevention.
Gingivitis can be prevented through regular oral
hygiene that includes daily brushing and flossing.
Researchers analyzed government data on calcium consumption and
periodontal disease indicators in nearly 13,000 people representing U.S. adults.
They found that men and women who had calcium intakes of fewer than 500
milligrams, or about half the recommended dietary allowance, were almost
twice as likely to have gum disease, as measured by the loss of attachment of the
gums from the teeth. The association was particularly evident for people in their
20s and 30s.
Researcher says the relationship between calcium and gum disease is
likely due to calcium’s role in building density in the alveolar bone that
supports the teeth.
Diagnosis. It is recommended that a dental hygienist or dentist be seen
after the signs of gingivitis appear. A dental hygienist or dentist will check for
the symptoms of gingivitis, and may also examine the amount of plaque in the
oral cavity. A dental hygienist or dentist should also test for periodontitis using
X-rays or gingival probing as well as other methods.
For simple gingivitis, work with your dentist. A concerted effort between
good home dental hygiene and regular dental visits should be all that is required
to treat and prevent gingivitis. If gingivitis continues despite the effort to prevent
it, contact your doctor to investigate the possibility of an underlying illness.
Gingivitis can usually be managed at home with good dental hygiene. If
gingivitis turns into the most severe periodontal infection, acute necrotizing
ulcerative gingivitis (ANUG), commonly referred to as trench mouth, treatment
at a hospital may be required.
ANUG not only affects the gums but may spread to adjacent tissues of the
face, neck, and bone. Bleeding, loss of periodontal architecture, and pain all
characterize ANUG. The breath takes on a fetid odor, the teeth become loose,
and the lymph nodes of the neck are often swollen. People with ANUG often
have fever and complain of a generalized weakness reflecting widespread
infection.
Like gingivitis, ANUG usually affects people with underlying immune
system situations such as malnutrition, HIV, or cancer. Therapy involves
getting rid of the oral bacteria with antibacterial mouthwashes, oral antibiotics,
periodontal treatment, and treatment of the underlying illness.
Exams and Tests. Gingivitis is a clinical diagnosis. This means that the
physician or dentist can arrive at the diagnosis by listening to the person’s
medical and dental history and performing a good oral exam. Blood work, xrays, and tissue samples are checked for cases not responding to initial therapy.
The person should, however, be evaluated for underlying disease.
Gingivitis Treatment. Dentist or dental hygienist will perform a
thorough cleaning of the teeth and gums; following this, persistent oral hygiene
is necessary. The removal of plaque is usually not painful, and the inflammation
of the gums should be gone between one and two weeks. Oral hygiene including
proper brushing and flossing is required to prevent the recurrence of gingivitis.
62
Anti-bacterial rinses or mouthwash, in particular Chlorhexidine digluconate
0.2% solution, may reduce the swelling and local mouth gels which are usually
antiseptic and anaesthetic can also help.
Self-Care at Home. The best home care for gingivitis is prevention.
Regular dental visits to remove plaque build-up are necessary to combat
gingivitis.
Once a dentist removes plaque, regular brushing and flossing will
minimize plaque formation. Even with good dental hygiene, plaque will begin to
accumulate again.
Medical Treatment
- Removing the source of the infection is primarily how simple gingivitis is
treated.
- By brushing teeth regularly with a toothbrush and fluoride toothpaste
approved by dentists, plaque build-up can be kept to a minimum.
- Flossing is another means of removing plaque in between teeth and other
areas hard to reach.
- Regular check-ups with a dentist are also important. A dentist is able to
remove plaque that is too dense to be removed by a toothbrush or dental
floss.
- Severe gingivitis may require antibiotics and consultation with a
physician. Antibiotics are medications used to help the body's immune
system fight bacterial infection and have been shown to reduce plaque. By
reducing plaque, bacteria can be kept to a level manageable by the human
immune system. Taking antibiotics is not without risks and should only be
done after consultation with a dentist or doctor.
Prevention.
Good mouth and teeth care, regular dental follow-up,
and treatment of underlying illnesses are also necessary for preventing
gingivitis.
Outlook. Most cases of simple gingivitis can be managed simply with
good oral hygiene and regular dental appointments.
Complications
- Recurrence of gingivitis
- Periodontitis
- Infection or abscess of the gingiva or the jaw bones
- Trench mouth (bacterial infection and ulceration of the gums)
- Gingivitis is one of many periodontal diseases that affect the health of the
periodontium (those tissues that surround the teeth and include the gums,
soft tissues, and bone).
Periodontal diseases are often classified according to their severity. They
range from mild gingivitis, to more severe periodontitis, and finally acute
necrotizing ulcerative gingivitis, which can be life threatening.
Bacteria can cause inflammation of the gums. Although bacteria are
normally found in our bodies and provide protective effects most of the time,
bacteria can be harmful. The mouth is a great place for bacteria to live. The
warm, moist environment and constant food supply are everything bacteria need
63
to thrive. If not for a healthy immune system, bacteria in the mouth would
rapidly reproduce out of control, overwhelming the body's defense system.
Infection begins when the body's immune system is overwhelmed.
Gingivitis is an infection that occurs when bacteria invade soft tissues, bone, and
other places that bacteria should not be. At the moment of infection, bacteria no
longer help us, they begin to harm us. Infections, like other diseases, range from
mild to severe or life threatening.
Periodentitis
Periodentitis is an inflammation of the periodontium and the periodontal
membrane - called also pericementitis.
Gum disease is most widespread among middle-aged people although it
can develop at any age. It is characterised by inflamed, red gums (gingivitis) or
in more severe cases, the bone around the teeth becomes inflamed and swollen
(periodentitis). Latest research suggests that there are many factors which can
lead to the development of gum disease. Bacteria growing plaque and especially
their toxic products can enter the blood stream and sensitize some of the cells of
the immune system. These cells are then unable to function effectively and
localised inflammation of the gums develops. If untreated, the toxic products
produced by the bacteria can eventually eat away at the jawbone itself and the
gums start to recede. Other factors such as amalgam fillings and smoking can
also contribute to gum disease.
Diet and lifestyle. Although no specific diet has been developed for
sufferers, there seems merit in following a high fibre diet as this increases
salivary secretion. Sufferers should also aim to cut out sugar-laden foods for
sugar is known to add to plaque formation.
Useful supplements
- Vitamin C – 1-2 grams daily – vitamin C and bioflavonoids are important
for the production of collagen and for a healthy immune system.
Decreased levels result in delayed wound healing and increased
susceptibility to infection.
- Co Q10 – 30mg daily – co-enzyme Q10 is an essential factor in cellular
energy production and is essential for the health of tissues. In studies,
diseased gum tissue has been shown to be deficient in Co Q10.
Marginal periodontitis, which affects the tissues that support the teeth
including the gums accounts for most lost teeth in adulthood than any other
dental problem.
Marginal periodontitis can cause facial disfigurement, pain, an inability to
eat leading to malnutrition and the anti-social stigma of profound halitosis (bad
breath). Up to 9 out of 10 adults in the west will suffer from some form of
periodontal disease in their later lives as a result 1 in 4 of those will lose all their
teeth before the age of 60. As a group, men are more likely to suffer from
periodontal disease than women although it is not clear why this should be so. It
manifests itself most commonly as gingivitis or inflammation of the gums.
Causes
64
Bacterial factors. The most frequent single cause is poor hygiene
characterised by bacterial plaque which are microbial colonies that cling to the
surface of the gums and teeth.
Clearly bacteria are essential agents in the development of marginal
periodontitis but increasingly researchers point to host defence factors and their
important role in maintaining oral health. Bacteria are known to produce and
secrete numerous compounds that are damaging to our body’s own defence
mechanism. These include free radicals and enzymes which are capable of
destroying connective tissue.
An adequate and steady supply of nutrients to the oral mucosa and
salivary glands is therefore of vital importance if oral tissues are to fulfil their
functions.
Researchers have shown that inadequate protein during pregnancy or
lactation lends to atrophy of and reduction in the nucleic acid and protein
content of salivary glands. Salivary glands secrete various types of proteins,
some of which are known to have antibacterial properties. The host defence
properties of saliva largely stem from its buffering capacity and its antimicrobial
activity.
Immune system. Defective functioning of neutrophil (white blood cells
involved in the production of antibodies) can have a catastrophic effect on the
Periodontium, the gums and tissues that support the teeth. This is particularly
common in older people and in those with diabetes and Crohn’s disease.
The release of histamine is also a major factor in marginal periodontitis. It
can be provoked by the development of certain antibodies, by mechanical
trauma, or the presence of bacterial toxins and free radicals. Increased
concentrations of certain types of antibodies known as IgE in the gums of
patients with periodontal disease suggests that allergic reactions may be a factor
in the progression of the disease.
Dental fillings provide the ideal location for the accumulation of plaque
and bacteria. Much has been written about mercury fillings and the effect that
they can have on the important antioxidant enzymes glutathione peroxidase,
superoxide dismutase (S.O.D.) and catalase. Connective tissue is particularly
sensitive to free radical damage.
Other factors. There are of course numerous other factors which can
favour the progression of periodontal disease. These include the impacting of
food, teeth grinding, unreplaced missing teeth and toothbrush trauma. Smoking
is associated with free radical damage and this affects the surface cells of the
gums. Diabetes is a common cause of periodontal disease and it is seen also in
leukaemia. Treatment of the underlying complaint often relieves the gingivitis
(inflammation). Medicinal drugs like the anti-epileptic phenyton, immune
suppressive cyclosporin and calcium channel blockers can all induce gingivitis
Structure and integrity of connective tissue. The status of the collagen
structure of the periodontium determines the ability of bacteria and enzymes to
attack the gums and teeth.
65
Due to the high rate of protein turnover in periodontal collagen, the
collagen matrix in this area is extremely vulnerable to atrophy when the
necessary nutrients for collagen synthesis are absent or deficient.
Dietary considerations. As with so many other diseases the advice to
sufferers is to avoid simple sugars e.g., white sugar, honey, and refined
carbohydrates as well as foods known to cause an allergic reaction. Opt instead
for a diet high in bioflavonoid-rich foods such as onions citrus rind and fruits.
It is thought that a diet high in dietary fibre may have a protective effect
due to increased salivary secretion. Sugar is known to increase plaque
formation.
Natural Considerations CO-ENZYME Q10
A constant feature of periodontal disease is a deficiency of Co-enzyme
Q10 in the gum tissue cells. This finding led many researchers to study what
would happen if Co-enzyme Q10 were given to restore gum levels to normal.
The results were quite enlightening in that most of those treated responded
dramatically to the therapy combined with regular periodontal care.
Vitamin C plays a major role in preventing periodontal disease.
Decreased levels of this vitamin not only affects collagen integrity and immune
system function but it is also associated with increased vulnerability of the oral
mucous to toxins and impaired white cell function.
Vitamin A and beta carotene.
A deficiency of vitamin A is
associated with delayed wound healing and with compromised integrity of the
gums. Beta Carotene is believed to be equally important for its antioxidant
properties.
Zinc and copper. Zinc is essential to the treatment of periodontal disease.
It plays an important role in the synthesis of the inhibition of plaque growth and
the reduction of the release of histamines. It is also known for its wound healing
properties.
Bioflavonoids are an important component in the treatment of periodontal
disease as they decrease membrane permeability and prevent free radical
damage.
Nicotinamide A common symptom of mild nicotinamide deficiency is
gingivitis, bleeding and secondary infection. The vitamin is essential for
maintaining healthy gums.
Periapical inflammation
A necrotic pulp, with or without the presence of infection, will provoke an
inflammatory response in the periapical periodontal ligament. Diagnosis of
periapical inflammation is made by interpretation of a combination of symptoms
and clinical and radiological signs.
Acute periapical periodontitis
Clinical features. The classic symptom is of a dull throbbing ache,
usually well localised to a heavily restored or grossly diseased tooth. It may be
difficult for the patient to determine whether an upper or lower tooth is affected
as the pain is experienced particularly when the teeth are occluded. However the
66
affected tooth is painful to touch. The tooth should be non-vital to simple tests
(as the periapical inflammation is usually provoked by a dead and/or infected
pulp) although, particularly with multirooted teeth, some vital response may still
be elicited, as well as tenderness on percussion. Acute periapical periodontitis
may also occur after trauma or endodontic treatment to a tooth. In such cases,
the history should lead to the diagnosis.
Radiology. The basic radiological sign accompanying acute inflammation
around the apex of a tooth is localised bone destruction. Where there is little or
no previous chronic inflammation, this will appear as loss of the lamina dura
(Fig. 14). Where the periapical periodontal ligament was previously widened or
a granuloma was present, acute inflammation will appear as a poorly defined
radiolucency, termed a rarefying osteitis (Fig. 15).
Pathology. Acute periapical periodontitis may arise de novo or develop
against a background of pre-existing chronic periapical periodontitis. In the
former, the periodontal ligament is infiltrated by neutrophil leukocytes and
macrophages while in the latter they accumulate within a periapical granuloma.
In both cases, suppuration may occur, leading to the development of a periapical
abscess.
Management. Endodontic therapy or extraction of the affected tooth is
required. In cases of post-traumatic acute periapical periodontitis, the
inflammation may resolve with splinting and time.
Chronic periapical periodontitis (periapical granuloma)
Fig. 14 Radiograph of loss of lamina dura on the fractured central incisor.
67
Fig. 15 Radiograph of rarefying osteitis associated with the lower right central incisor.
Clinical features. There may be few or no symptoms.
Radiology. The initial sign is widening of the periodontal ligament space
with preservation of the radio-opaque lamina dura (Fig. 16). This naturally
progresses with time to form a rounded periapical radiolucency with a welldefined margin—a granuloma (Fig. 17). Ultimately, this may undergo cystic
change. Differentiation between a large granuloma and a small radicular cyst is
not possible on purely radiological grounds, but lesions greater than 1 cm
diameter are often assumed to be cysts until histopathological diagnosis is
established. A further radiological sign frequently seen in chronic periapical
periodontitis is sclerosing (or condensing) osteitis (Fig. 18). This appears as a
fairly diffuse radioopacity, usually around the periphery of a widened
periodontal ligament or a periapical granuloma.
Fig. 16 Radiograph of widened
periodontal ligament on the
68
lateral incisor with intact lamina dura.
Fig. 17 Radiograph of granuloma on the premolar
tooth
Fig. 18 Radiograph of condensing osteitis relating
to the grossly cavious second molar.
Pathology. Chronic periapical periodontitis is characterised by the
formation of granulation tissue derived from the periodontal ligament, the
periapical granuloma, surrounding the apex of a tooth. Chronic inflammatory
cells infiltrate the granuloma in variable numbers. Often plasma cells
predominate because of multiple antigenic stimulations from pulpal infection.
Foamy macrophages, cholesterol clefts often rimmed by mulrinucleate giant
cells, and deposits of haemosiderin are also frequent findings. Remnants of
Hertwig's root sheath, the cell rests of Malassez, may proliferate as a result of
release of inflammatory mediators. Neutrophil infiltration within this epithelium
may be one factor leading
to cavitation and formation of a radicular cyst.
Management. Endodontic therapy or extraction of the affected tooth is
required. Should the lesion persist following orthograde endodontic therapy,
apicectomy should be considered.
Apicectomy surgical procedure
The procedure is also known as apical surgery, resection of root apex or
surgical endodontics.
1. A mucoperiostal flap is raised (Fig. 19).
2. Bone is removed over the buccal aspect of the tooth root in the area of the
apex and associated pathology using an irrigated round surgical bur. The bone is
thin and careful superficial sweeping movements are necessary to avoid
removing tooth root tissue.
3. Pathological soft tissue about the root apex is removed with curettes and sent
for histopathological examination.
4. At least 3 mm of the root apex should be removed using an irrigated fissure
bur. The cut is made as close as possible to 90° to the long axis of the root to
reduce the number of exposed dentinal tubules (Fig. 20).
5. A cavity is prepared in the root end using a small round bur or, better, an
ultrasonically powered tip.
69
Fig. 19 Typical flap design for apicectomy. Fig. 20 Apicectomy of tooth and retrograde
restoration.
6. The cavity is isolated and packed with a biological compatible material such
as mineral trioxide aggregate, super EBA, glass ionomer, composite resin with a
dentine bonding agent or reinforced zinc oxide-eugenol. Any excess material is
removed and the area is irrigated to check this. Amalgam is no longer
recommended.
7. The soft tissues are closed with a suture material such as vicryl.
Pathoses associated with periapical inflammation
Hypercementosis. Hypercementosis is usually identified on radiography.
Affected roots of teeth become bulbous because of accretion of cementum (Fig.
21). The cause may be unidentifiable, but it is frequently associated with teeth
affected by periodontal disease or periapical inflammation (hence its inclusion
here). It is also seen in Paget's disease when multiple teeth are often affected. No
treatment is indicated for hypercementosis per se, but its recognition is
obviously important if extractions are planned.
Fig. 21 Radiograph of hypercementosis affecting the premolar tooth.
70
External resorption. Resorption of the root surface, particularly apically,
is occasionally seen on teeth with periapical inflammation, although there are a
number of other known causes (e.g. trauma, iatrogenic (orthodontic), reimplanted teeth, adjacent cysts/tumours). Successful treatment of the periapical
lesion by endodontic therapy often arrests the resorption.
Oral surgery function
Oral surgery provides surgical treatment or correction of diseases, defects,
оr injuries of the oral cavity and facial structures. А wide variety of surgical
procedures takes place in the oral-maxillofacial surgery аrea. Exodontics is the
term used to describe the extraction of teeth in oral surgery. General dentists are
trained in surgical procedures; however, they mау choose to refer the patient
with а more complicated case to аn oral surgeon who has specialized training in
the аrea. А maxillofacial surgeon is аn oral surgeon who specializes in the
reduction of bone fractures and reconstruction of the mахillа оr mandible, and
performs reconstructive surgery.
Indications and contraindications. Before а surgical procedure саn bе
done, the oral surgeon will evaluate each patient's record fоr indications and
contraindications to treatment. Some indications for oral surgery include:
1. Carious teeth unrestorable bу restorative procedures.
2. Nonvital teeth when endodontic treatment is not indicated or has little chance
of success.
3. Removal of teeth to provide space in the arch for orthodontic treatment.
4. Teeth without sufficient bоnе support.
5. Supenumerary or impacted teeth interfering with normal dentition.
6. Malpositioned teeth that cannot bе aligned.
7. Root fragments from prior extractions or surgery.
8. Removal of soft-tissue.
9. Removal of exostosis (overgrowth of bоnе), such as torus mandibularis and
torus palatinus.
10. Accidental fracture оr reconstruction of the mandible or mахillа.
Indication for splinting.
Оnе obvious indication for splinting is
when а patient presents with multiрlе teeth that have bесоmе mobile as а direct
result of gradual alveolar bоnе loss, а reduced periodontium.
А second indication for splinting is when the patient presents with
increased tooth mobility accompanied bу pain оr disсоmfort the affected teeth.
Splinting mау bе а way to gain stability, reduce оr eliminate the mobility, and
relieve the pain and disсоmfort.
Тhе oral surgeon will also evaluate the patient for possible
contraindications to surgical treatment.
Extractions should bе avoided when аn active infection is present because
local anesthesia is difficult to achieve and the infection саn spread to other parts
of the body. Patients suffering from аnу potentially serious disease, such as heart
disease, diabetes, and blood disorders, should first bе evaluated bу а physician
71
to dеtеrminе if they саn withstand the prescribed treatment. Pаtients in the early
stages of pregnancy should have the surgery postponed until they are in the
second trimеstеr.
Contraindindications. Splinting teeth is not recomniended if occlusal
stability and optimal periodontal conditions cannot bе obtained. Аnу tooth
mobility present before treatment must bе reduced bу means of occlusal
equilibration combined with реriоdоntаl therapy; otherwise if the tooth involved
does nоt respond, it must bе extracted prior to proceeding frоm provisional
restorations to definitive treatment
Examination and informed consent. Ехаminаtiоn and informed consent
are essential to determine what trеаtmеnt is required, and provide аll relevant
information to the patient to mаkе аn informed decision regarding proposed
treatment.
The oral surgeon ехаminеs the patient to confirm the findings of the
referring dentist and gather аnу other additional information to make treatment
rеcommеndаtiоns. Oral surgeons should order radiographs of the teeth,
mandible, mахillа, оr other facial areas to verify the trеаtmеnt recommendations
if not already taken. The radiographs mау include periapical, extraoral of the
skull or facial aspects, panoramic, tеmроromаndibulаr, and occlusal. А
соmрrеhеnsivе medical history review is essential for the surgical patient
because of the strain surgery places оn the body. If there are аnу questions
regarding the patient's health оr ability to withstand surgery, the surgeon should
consult with the patient's physician before surgery. During the examination, the
oral surgeon also discusses appropriate pain-control mеthоds for the surgical
trеаtmеnt rесоmmеndеd, and informed consent with the patient or legal
guardian.
Elimination of favoring factors. During the course of this therapeutic
procedure, dental calculus and caries, poor conservative fillings and prosthetic
works, causes of food impaction, bad habits and mucogingival anomalies are
eliminated and corrected.
Subgingival curettage is a basic procedure in healing the periodontal
pockets. Subgingival curettage includes also the procedure of eliminating
subgingival concrements and necrotic cement, changed epithelium of the
periodontal pocket, granular tissue and inflamed subepithelial connective tissue.
This procedure is indicated in periodontal pockets of 4-6mm.
Application of physical methods. Physical methods used in the therapy
of diseases of the periodontium are cathode galvanization, drug electrophoresis,
igni-punction, periodontal bandages and gingival massage. The usage of lasers
(soft and hard lasers) in increased is growing. Soft lasers are applied in treatment
of gingivitis, periodontal disease, periodontal abscesses etc, for their antiinflammatory, anti-edematous, analgesic and biostimulation effect. Hard lasers
are used in periodontal surgery.
Control of general factors – systemic factors. Systemic diseases
decrease general immunity, and thus change the local response of the
periodontal tissue to microorganism actions, therefore drug administration,
72
nutrition, smoking, intercurrent diseases and other factors should be controlled
constantly.
Occlusion correction. In this therapy phase, the correction of rough
occlusive disorders of some teeth, central occlusion, protrusive and lateral
occlusive movements. In this way the occlusion balance is achieved which
reduces the intensity of masticatory forces. Diagnosis of traumatic occlusion,
selective grinding, orthodontic treatment and temporary splints are made prior to
surgery. After surgery, prosthetic management of the patient is provided and
final splints for dental fixation are made.
Surgical therapy. Surgical therapy understands radical treatment of
diseases of the periodontium and it is preceded by the initial therapy.
Periodontal surgery includes all surgical interventions involving soft and hard
periodontal tissues. The aim of these interventions is:
- Better approach to deeper periodontal tissues (treatment of root and bone
surface)- Elimination of periodontal pockets- Reconstruction of the gingiva and
bone fun-ctional anatomy to provide optimal conditions for dental plaque
control.
Division of periodontal surgery
Gingival and periodontal surgery - Gingivetomy - Gingivoplasty in the
proliferation of connective tissue, gingival fibromatosis, suprabone periodontal
pockets, preprosthetic elongation of the clinical crown and gingival correction
procedures.
Flap surgery
With regard to depth, the flap can be-Semi-thick flap - mucosal
(epithelium and part of connective tissue)-Full-thickness flap mucosa + periost
According to position, the flap can be:1. repositioned (shifted) modified
Widman flap 2. positioned -after elevation, the flap is positioned into new,
different positions:- apically positioned flap- coronary repositioned flapbipedicle flap
Indications for flap surgery
1. Treatment of deep suprabone and infra-bone periodontal pockets.
2. Insertion of implants into infrabone perio-dontal pockets and other
transplants.
3. Hemisection, amputation and bisection of tooth root.
4. Treatment of periodontal abscesses.
5. Preprosthetic preparation of the patient.
Apically repositioned flap
(The flap is repositioned apically in relation to initial position of the flap.
Indications:
1. Elimination of periodontal pockets of suprabone and infrabone type reaching
mucogin-gival line, or surpassing it.
2. Extension of attached gingiva - Deepening on the vestibule - Repositioning
of frenulum and lateral papillae junction.
Coronary repositioned flap
73
The flap is moved in the coronary direction aiming at elimination of
periodontal pockets and accomplishment of attached gingiva to exposed root
surface.
Laterally repositioned flap
Technique of laterally dislocated flap is used in the case of exposed tooth
root,
especially
in
the
front
teeth
due
to
the
gingival
withdrawal/detachment/insulated. The exposed tooth root is covered by the flap
from the region of adjacent teeth.
Double papilla flap
It is used for covering the exposed tooth root surface. It is applied when
the periodontium tissue from neither distal nor mezial side of the exposed root is
suitable for lateral flap movement.
Free mucogingival autotransplantant
This method of mucosa repositioning from one place to another is
introduced by Bjorn in 1963.
Indications:
1. Formation or extension of attached gingiva
2. Elimination of coronary joining frenulum of lips, tongue and lateral mucosa
plicae
3. Prevention and treatment of recesion of the gingiva and covering the exposed
roots
4. Deepening of fornix
Frenectomy is a surgical intervention by which too coronary inserted
frenula of lips, tongue and lateral mucose plicae are fully eliminated. It is
indicated for orthodontic reasons, preventive and therapeutic reasons in diseases
of the periodontium and preprosthetic preparation for the purpose of better
prosthesis retention.
Edlan-Mejchar surgery. It is used for deepening the vestibule.
Nowadays it is used less frequently.In the clinical practice, some modifications
of this method are used.
Resectional bone surgery
The gingival profile is in direct relationship with deeper periodontal
tissues, that is with the bone structure.
Diseases of the periodontium lead to bone resorption changing the bone
architecture and posi-tioning the bone more apically to the tooth root.With
resectional surgery bone defects are eliminated, and so called physiological
architecture of the alveolar bone is created but at a more apical level.
Therefore, the indications are:
1. Bone remodeling.
2. Elimination on bone craters and bone angle defects.
3. Reconstruction of physiological profile
Osteoplasty. The aim of this technique is bone remodeling without
elimination of bone tissue for the purpose of achieving physiological form.
74
Osteoctomy. This term understands the elimination of supportive bone to
achieve physiological profile of the bone ridge. It is indicated in the presence of
bone craters or defects, and usually vestibular or oral part of a bone is removed.
Tooth resection. If the pathological process compromises the bone tissue
in furcations, the surgical treatment is complex. To eliminate the periodontal
pocket, it is usually necessary to modify the tooth anatomically, or to eliminate a
part of the tooth with one or more roots.
a)Tooth hemisection
It is conducted in the molars, mostly lower. This intervention includes the
separation of a tooth in the axial direction, and afterwards extraction of one half
of the tooth (crown partly and root) of which the periodontium is destroyed. On
the cut part of the tooth, prosthetic construction is made up after endodontic
treatment.
b) Tooth bisection
That is a surgical procedure where two pre-molars are made out of one.
Detachment of the tooth, the approach to the tooth furcation is pro-vided and
eradication of the periodontal pocket. It is indicated if the periodontium
surrounding both roots is preserved, that is if the destruction involved only the
coronary part of furcation. After endodontic treatment and bisection, cut tooth
halves also should be prosthetically managed.
c) Tooth amputation. Tooth root amputation understands cutting one of
the multiroot tooth surrounded with destroyed periodontium.
Indications:
1. Removal of the distal or mesial root of the lower molars.
2. Removal of the palatine root and preservation of the mesio and distovestibular
root of the upper molars.
3. Removal of the mesiovestibular root and preservation of distovestibular and
palatine root.
4. Removal of the distovestibular root and preservation of the mesiovestibular
and palatine root.
d) Tooth removal
Tooth removal is used in elimination of the periodontal pocket when the
periodontium is so destroyed that it cannot maintain in the jaw
Indications:
- Bone destruction with formation of infra-bone defects in the jaw bone
Cysts - Granulomas- Injuries
PAIN AND ANXIETY CONTROL
When dental surgery is indicated, whether oral or periodontal, there are
several pain and anxiety control methods available to make the surgery as
smooth as possible and put the patient at ease. The three basic levels of
anesthesia аrе lосаl, conscious sedation, and general.
Pain sensation in teeth. The teeth are supplied by nociceptors that
generate pain sensation of a very high order. The mechanism underlying this
sensitivity is of considerable clinical significance and is controversial. Currently,
75
the most widely accepted view is that fluid movements through the dentine
tubules stimulate nerve endings at the periphery of the dental pulp
(hydrodynamic hypothesis).
Local anesthesia
Thе primary effect of local anesthetic agents is to penetrate the nerve cell
membrane and block the conduction of nerve impulses from the point where the
lосаl anesthetic is active. This produces anesthesia in the lосаl area. Local
anesthesia, using infiltration, nerve block, or а combination of both techniques,
is used in surgery cases to numb the surgery area. Most dental surgery
procedures require two оr more injections of а lосаl anesthetic. For this reason,
it is а good practice to include two aspirating syringes with each instrument
setup. This will let уоu supply the dentist with а loaded anesthetic syringe for as
long as needed with minimum loss of time. Since anesthetic solutions are bitter
and there is leakage frоm the injection sites, yоu will need to irrigate and
aspirate the fluids from the patient's mouth after injection.
It is technically possible to achieve profound regional anaesthesia by
depositing local anaesthetic solution adjacent to the trigeminal nerve trunks or
their branches within the infratemporal fossa. These injections can either be
performed transorally - posterior superior alveolar nerve block, maxillary nerve
block, inferior alveolar nerve block, lingual nerve block and mandibular nerve
block - or more rarely by an external route through the skin of the face maxillary nerve block, inferior alveolar nerve block and mandibular nerve block.
Techniques of Mandibular Anesthesia. In the case of the mandible, the
anterior teeth can be anaesthetized by simple diffusion techniques as the bone is
relatively thin. However, this is not adequate for the cheek teeth due to the
increased thickness of the bone. In this case, the inferior alveolar nerve has to be
anaesthetized before it enters the inferior alveolar canal. The needle has to be
placed within the pterygomandibular space to achieve a successful inferior
alveolar nerve block. The lingual nerve is also usually blocked as it lies close to
the inferior alveolar nerve. Because of the other structures within the
infratemporal fossa it is vitally important that the operator has a detailed
knowledge of the anatomy in this region to understand, and therefore try to
avoid, the complications that may arise. Any damage to blood vessels in the
infratemporal fossa, generally the pterygoid venous plexus, can lead to
haematoma formation. In extreme cases, bleeding can track through the inferior
orbital fissure resulting in a retrobulbar haematoma, which can result in loss of
visual acuity or blindness. Intravascular injection of local anaesthetic solution
(which usually contains adrenaline (epinephrine )) can have profound systemic
effects and for this reason an aspirating syringe is always used to check that the
needle has not entered a vessel prior to injection. If the needle is placed too
medially it may enter medial pterygoid, while if directed too laterally it may
penetrate temporalis. In either case, there will be lack of anaesthesia followed
later by trismus. If the needle is placed too deeply, anaesthetic solution may
76
cause a temporary Bell's palsy due to loss of conduction from the facial nerve in
the region of the parotid gland. Finally, if the needle is not sterile, infection of
the pterygomandibular space may ensue, which could spread to other important
tissue spaces. Diffusion of anaesthetic solution through the inferior orbital
fissure could give temporary orbital symptoms such as paralysis of lateral rectus
due to anaesthesia of the abducens nerve.
Anesthesia of the upper jaw
The techniques most commonly employed in maxillary anesthesia include
supraperiosteal (local) infiltration, periodontal ligament (intraligamentary)
injection, posterior superior alveolar nerve block, middle superior alveolar nerve
block, anterior superior alveolar nerve block, greater palatine nerve block,
nasopalatine nerve block, local infiltration of the palate, and intrapulpal
injection. Of less clinical application are the maxillary nerve block and
intraseptal injection.
Supraperiosteal (Local) Infiltration.
The supraperiosteal or local
infiltration is the one of the simplest and most commonly employed techniques
for achieving anesthesia of the maxillary dentition. This technique is indicated
when any individual tooth or soft tissue in a localized area is to be treated.
Contraindications to this technique are the need to anesthetize multiple teeth
adjacent to one another (in which case a nerve block is the preferred technique),
acute inflammation and infection in the area to be anesthetized, and less
significantly, the density of bone overlying the apices of the teeth. A 25- or 27gauge short needle is preferred for this technique. Technique- Identify the tooth
to be anesthetized and the height of the mucobuccal fold over the tooth. This
will be the injection site. The right handed operator should stand at the nine
o’clock to ten o’clock position whereas the left handed operator should stand at
the two o’clock to three o’clock position. Retract the lip and orient the syringe
with the bevel towards bone. This will prevent discomfort from the needle
coming into contact with the bone and will minimize the risk of tearing the
periosteum with the needle tip. Insert the needle at the height of the mucobuccal
fold above the tooth to a depth of no more than a few millimeters and aspirate. If
aspiration is negative, inject one third to one half (0.6-1.2cc) of a cartridge of
anesthetic solution slowly, over the course of thirty seconds. Withdraw the
syringe and recap the needle. Successful administration will provide anesthesia
to the tooth and associated soft tissue within two to four minutes. If adequate
anesthesia has not been achieved repeat the procedure and deposit another one
third to one half of the cartridge of anesthetic solution.1
Periodontal Ligament (Intraligamentary Injection). The periodontal
ligament or intraligamentary injection is a useful adjunct to the supraperiosteal
injection or a nerve block. Often, it is used to supplement these techniques to
achieve profound anesthesia of the area to be treated. Indications for the use of
this technique are the need to anesthetize an individual tooth or teeth, need for
soft tissue anesthesia in the immediate vicinity of a tooth, and partial anesthesia
77
following a field block or nerve block. A 25- or 27-gauge short needle is
preferred for this technique.
Technique. Identify the tooth or area of soft tissue to be anesthetized. The
sulcus between the gingiva and the tooth is the injection site for the periodontal
ligament injection. Position the patient in the supine position. For the right
handed operator, retract the lip with a retraction instrument held in the left hand
and stand where the tooth and gingiva are clearly visible. The same applies for
the left handed operator except that the retraction instrument will be held in the
right hand. Hold the syringe parallel to the long axis of the tooth on the mesial
or distal aspect. Insert the needle (bevel facing the root), to the depth of the
gingival sulcus . Advance the needle until resistance is met. A small amount of
anesthetic (0.2cc) is then administered slowly over the course of twenty to thirty
seconds. It is normal to experience resistance to the flow of anesthetic.
Successful execution of this technique provides pulpal and soft tissue anesthesia
to the individual tooth or teeth to be treated.
Posterior Superior Alveolar Nerve Block. The posterior superior
alveolar (PSA) nerve block, otherwise known as the tuberosity block or the
zygomatic block, is used to achieve anesthesia of the maxillary molar teeth up to
the 1st molar with the exception of its mesiobuccal root in some cases. One of
the potential complications of this technique is the risk of hematoma formation
from injection of anesthetic into the pterygoid plexus of veins or accidental
puncture of the maxillary artery. Aspiration prior to injection is indicated when
the PSA block is given. The indications for this technique are the need to
anesthetize multiple molar teeth. Anesthesia can be achieved with fewer needle
penetrations providing greater comfort to the patient by preventing the need for
multiple injections by the supraperiosteal technique. The PSA can be given to
provide anesthesia of the maxillary molars when acute inflammation and
infection are present. If inadequate anesthesia is achieved via the supraperiosteal
technique, the PSA can be used to achieve more profound anesthesia of a longer
duration. The PSA block also provides anesthesia to the premolar region in a
certain percentage of cases where the MSA is absent. Contraindications to the
procedure are related to the risk of hematoma formation. In individuals with
coagulation disorders, care must be taken to avoid injection into the pterygoid
plexus or puncture of the maxillary artery. 25- or 27-gauge short needle is
preferred for this technique. Technique- Identify the height of the
mucobuccal fold over the 2nd molar. This will be the injection site. The right
handed operator should stand at the nine o’clock to ten o’clock position whereas
the left handed operator should stand at the two o’clock to three o’clock
position. Retract the lip with a retraction instrument. Hold the syringe with the
bevel toward the bone. Insert the needle at the height of the mucobuccal fold
above the maxillary 2nd molar at a 45 degree angle directed superiorly,
medially, and posteriorly (one continuous movement). Advance the needle to a
depth of three quarters of its total length. No resistance should be felt while
advancing the needle through the soft tissue. If bone is contacted, the medial
angulation is too great. Slowly retract the needle (without removing it) and bring
78
the syringe barrel toward the occlusal plane. This will allow the needle to be
angulated slightly more lateral to the posterior aspect of the maxilla. Advance
the needle, aspirate, and inject one cartridge of anesthetic solution slowly over
the course of one minute aspirating frequently during the administration. Prior to
injecting, one should aspirate in two planes to avoid accidental injection into the
pterygoid plexus. After the first aspiration, the needle should be rotated one
quarter turn. The operator should then reaspirate. If positive aspiration occurs,
slowly retract the needle one to two millimeters and reaspirate in two planes.
Successful injection technique will result in anesthesia of the maxillary molars
(with the exception of the mesiobuccal root of the first molar in some cases),
and associated soft tissue on the buccal aspect.
Middle Superior Alveolar Nerve Block. The middle superior alveolar
nerve block is useful for procedures where the maxillary premolar teeth or the
mesiobuccal root of the 1st molar require anesthesia. Although not always
present, it is useful if the posterior or anterior superior alveolar nerve blocks or
supraperiosteal infiltration fails to achieve adequate anesthesia. Individuals in
whom the MSA nerve is absent, the PSA and ASA nerves provide innervation to
the maxillary premolar teeth and the mesiobuccal root of the 1st molar.
Contraindications include acute inflammation and infection in the area of
injection or a procedure involving one tooth where local infiltration will be
sufficient. A 25- or 27-gauge short needle is preferred for this technique.
Technique. Identify the height of the mucobuccal fold above the
maxillary 2nd premolar. This will be the injection site. The right handed
operator should stand at the nine o’clock to ten o’clock position whereas the left
handed operator should stand at the two o’clock to three o’clock position.
Retract the lip with a retraction instrument and insert the needle until the tip is
above the apex of the 2nd premolar tooth. Aspirate and inject two thirds to one
cartridge of anesthetic solution slowly over the course of one minute. Successful
execution of this technique provides anesthesia to the pulp, surrounding soft
tissue and bone of the 1st and 2nd premolar teeth and mesiobuccal root of the
1st molar.
Anterior Superior Alveolar Nerve Block/Infraorbital Nerve Block.
The anterior superior alveolar (ASA) nerve block or infraorbital nerve block is a
useful technique for achieving anesthesia of the maxillary central and lateral
incisors and canine as well as the surrounding soft tissue on the buccal aspect. In
patients that do not have an MSA nerve, the ASA nerve may also innervate the
premolar teeth and mesiobuccal root of the 1st molar. Indications for the use of
this technique include procedures involving multiple teeth and inadequate
anesthesia from the supraperiosteal technique. A 25 gauge long needle is
preferred for this technique.
Technique. Place the patient in the supine position. Identify the height of
the mucobuccal fold above the maxillary 1st premolar. This will be the injection
site. The right handed operator should stand at the ten o’clock position whereas
the left handed operator should stand at the two o’clock position. Identify the
infraorbital notch on the inferior orbital rim. The infraorbital foramen lies just
79
inferior to the notch usually in line with the second premolar. Slight discomfort
is felt by the patient when digital pressure is placed on the foramen. It is helpful
but not necessary to mark the position of the infraorbital foramen. Retract the lip
with a retraction instrument while noting the location of the foramen. Orient the
bevel of the needle toward bone and insert the needle at the height of the
mucobuccal fold above the 1st premolar. The syringe should be angled toward
the infraorbital foramen and kept parallel with the long axis of the 1st premolar
to avoid hitting the maxillary bone prematurely. The needle is advanced into the
soft tissue until the bone over the roof of the foramen is contacted. This is
approximately half the length of the needle however, this will vary from
individual to individual. After aspiration, approximately one half to two thirds
(0.9-1.2cc) of the anesthetic cartridge is deposited slowly over the course of one
minute. It is recommended that pressure be kept over the site of injection to
facilitate the diffusion of anesthetic solution into the foramen. Successful
execution of this technique results in aesthesia of the lower eyelid, lateral aspect
of the nose, and the upper lip. Pulpal anesthesia of the maxillary central and
lateral incisors, canine, buccal soft tissue, and bone is also achieved. In a certain
percentage of people, the premolar teeth and the mesiobuccal root of the 1st
molar is also anesthetized.1
Greater Palatine Nerve Block. The greater palatine nerve block is useful
when treatment is necessary on the palatal aspect of the maxillary premolar and
molar dentition. This technique targets the area just anterior to the greater
palatine canal. The greater palatine nerve exits the canal and travels forward
between the bone and soft tissue of the palate. Contraindications to this
technique are acute inflammation and infection at the injection site. A 25- or 27gauge long needle is preferred for this technique.
Technique. The patient should be in the supine position with the chin
tilted upward for visibility of the area to be anesthetized. The right handed
operator should stand at the eight o’clock position whereas the left handed
operator should stand at the four o’clock position. Using a cotton swab, locate
the greater palatine foramen by placing it on the palatal tissue approximately one
centimeter medial to the junction of the 2nd and 3rd molar. While this is the
usual position for the foramen, it may be located slightly anterior or posterior to
this location. Gently press the swab into the tissue until the depression created
by the foramen is felt. Malamed and Trieger found that the foramen is found
medial to the anterior half of the 3rd molar approximately 50% of the time,
medial to the posterior half of the 2nd molar approximately 39% of the time and
medial to the posterior half of the 3rd molar approximately 9% of the time.6 The
area approximately one to two millimeters anterior to the foramen is the target
injection site. Using the cotton swab, apply pressure to the area of the foramen
until the tissue blanches. Aim the syringe perpendicular to the injection site
which is one to two millimeters anterior to the foramen. While keeping pressure
on the foramen, inject small volumes of anesthetic solution as the needle is
advanced through the tissue until bone is contacted. The tissue will blanch in the
area surrounding the injection site. Depth of penetration is usually no more than
80
a few millimeters. Once bone is contacted, aspirate and inject approximately one
fourth (0.45cc) of anesthetic solution. Resistance to deposition of anesthetic
solution is normally felt by the operator. This technique provides anesthesia to
the palatal mucosa and hard palate from the 1st premolar anteriorly to the
posterior aspect of the hard palate and to the midline medially.
Nasopalatine Nerve Block. The nasopalatine nerve block, otherwise
known as the incisive nerve block and sphenopalatine nerve block, anesthetizes
the nasopalatine nerves bilaterally. In this technique anesthetic solution is
deposited in the area of the incisive foramen. This technique is indicated when
treatment requires anesthesia of the lingual aspect of multiple anterior teeth. A
25- or 27-gauge short needle is preferred for this technique.
Technique. The patient should be in the supine position with the chin
tilted upward for visibility of the area to be anesthetized. The right handed
operator should be at the nine o’clock position whereas the left handed operator
should be at the three o’clock position. Identify the incisive papillae. The area
directly lateral to the incisive papilla is the injection site. With a cotton swab,
hold pressure over the incisive papilla. Insert the needle just lateral to the papilla
with the bevel against the tissue. Advance the needle slowly toward the incisive
foramen while depositing small volumes of anesthetic and maintaining pressure
on the papilla. Once bone is contacted, retract the needle approximately one
millimeter, aspirate, and inject one fourth (0.45cc) of a cartridge of anesthetic
solution over the course of thirty seconds. Blanching of surrounding tissues and
resistance to the deposition of anesthetic solution is normal. Anesthesia will be
provided to the soft and hard tissue of the lingual aspect of the anterior teeth
from the distal of the canine on one side to the distal of the canine on the
opposite side.
Local Palatal Infiltration. The administration of local anesthetic for the
palatal anesthesia of just one or two teeth is common in clinical practice. When
a block is undesirable, local infiltration provides effective palatal anesthesia of
the individual teeth to be treated. Contraindications include acute inflammation
and infection over the area to be anesthetized. A 25- or 27-gauge short needle is
preferred for this technique.
Technique. The patient should be in the supine position with the chin
tilted upward for visibility of the area to be anesthetized. Identify the area to be
anesthetized. The right handed operator should be at the ten o’clock position
whereas the left handed operator should be at the two o’clock position. The area
of needle penetration is five to ten millimeters palatal to the center of the crown.
Apply pressure directly behind the injection site with a cotton swab. Insert the
needle at a forty five degree angle to the injection site with the bevel angled
toward the soft tissue. While maintaining pressure behind the injection site,
advance the needle and slowly deposit anesthetic solution as the soft tissue is
penetrated. Advance the needle until bone is contacted. Depth of penetration is
usually no more than a few millimeters. The tissue is very firmly adherent to the
underlying periosteum in this region causing resistance to the deposition of local
anesthetic. No more than 0.2 to 0.4cc of anesthetic solution is necessary to
81
provide adequate palatal anesthesia. Blanching of the tissue at the injection site
immediately follows deposition of local anesthetic. Successful administration of
anesthetic using this technique results in hemostasis and anesthesia of the palatal
tissue in the area of injection.
Intrapulpal Injection. Intrapulpal injection involves anesthesia of the
nerve within the pulp canal of the individual tooth to be treated. When pain
control cannot be achieved by any of the aforementioned methods, the
intrapulpal method may be used once the pulp chamber is open. There are no
contraindications to the use of this technique as it is at times the only effective
method of pain control. A 25- or 27-gauge short needle is preferred for this
technique. Technique- The patient should be in the supine position with the
chin tilted upward for visibility of the area to be anesthetized. Identify the tooth
to be anesthetized. The right handed operator should be at the ten o’clock
position whereas the left handed operator should be at the two o’clock position.
Assuming that the pulp chamber has been opened by an experienced dental
professional, place the needle into the pulp chamber and deposit one drop of
anesthetic. Advance the needle into the pulp canal and deposit another 0.2cc of
local anesthetic solution. It may be necessary to bend the needle in order to gain
access to the chamber especially with posterior teeth. The patient usually
experiences a brief period of significant pain as the solution enters the canal
followed by immediate pain relief.
Maxillary Nerve Block. Less often used in clinical practice, the
maxillary nerve block (second division block) provides anesthesia of a
hemimaxilla. This technique is useful for procedures that require anesthesia of
multiple teeth and surrounding buccal and palatal soft tissue in one quadrant or
when acute inflammation and infection preclude successful administration of
anesthesia by the aforementioned methods. There are two techniques one can
use to achieve the maxillary nerve block: the high tuberosity approach and the
greater palatine canal approach. The high tuberosity approach carries with it the
risk of hematoma formation and is therefore contraindicated in patients with
coagulation disorders. The maxillary artery is the vessel of primary concern with
the high tuberosity approach. Both techniques are contraindicated when acute
inflammation and infection is present over the injection site.
High Tuberosity Approach. A 25 gauge long needle is preferred for this
technique. Technique- The patient should be in the supine position with the
chin tilted upward for visibility of the area to be anesthetized. Identify the area
to be anesthetized. The right handed operator should be at the ten o’clock
position whereas the left handed operator should be at the two o’clock position.
This technique anesthetizes the maxillary nerve as it travels through the
pterygopalatine fossa. Identify the height of the mucobuccal fold just distal to
the maxillary 2nd molar. This is the injection site. The needle should enter the
tissue at a forty five degree angle aimed posteriorly, superiorly and medially as
in the PSA nerve block. The bevel should be oriented toward the bone. The
needle is advanced to a depth of approximately 30mm or a few millimeters shy
of the hub. At this depth, the needle lies within the pterygopalatine fossa. The
82
operator should then aspirate, rotate the needle one quarter turn, and aspirate
again. After negative aspiration in two planes has been established, slowly inject
one cartridge of anesthetic solution over the course of one minute. The needle is
then slowly withdrawn and recapped. Successful administration of anesthetic
using this technique provides anesthesia to the entire hemimaxilla on the
ipsilateral side of the block. This includes pulpal anesthesia to the maxillary
teeth, buccal and palatal soft tissue as far medially as the midline, as well as the
skin of the upper lip, lateral aspect of the nose and lower eyelid.
Greater palatine canal approach. A 25 gauge long needle is preferred
for this technique.
Technique. Place the patient in the supine position. The right handed
operator should be at the ten o’clock position whereas the left handed operator
should be at the two o’clock position. Identify the greater palatine foramen as
described in the technique for the greater palatine nerve block. The tissue
directly over the greater palatine foramen is the target for injection. This
technique anesthetizes the maxillary nerve as it travels through the
pterygopalatine fossa via the greater palatine canal. Apply pressure to the area
over the greater palatine foramen with a cotton tipped applicator. Administer a
greater palatine nerve block using the aforementioned technique. Once adequate
palatal anesthesia is achieved, gently probe for the greater palatine foramen with
the tip of the needle. For this technique, the syringe should be held so that the
needle is aimed posteriorly. It may be necessary to change the angulation of the
needle in order to locate the foramen. In a case study performed by Malamed
and Trieger, the majority of canals were angled 45-50 degrees. Once the
foramen has been located, advance the needle to a depth of 30mm. If resistance
is met, withdraw the needle a few millimeters and reenter at a different angle.
Malamed and Trieger’s study indicates that bony obstructions preventing
passage of the needle were found in approximately 5% to 15% of canals. If
resistance is met early on and the operator is unable to advance the needle into
the canal more than a few millimeters, the procedure should be aborted and the
high tuberosity approach should be considered. If no resistance is met and
penetration of the canal is successful, aspirate in two planes as described in
previous sections and slowly deposit one cartridge of local anesthetic solution.
As with the high tuberosity approach, the hemimaxilla on the ipsilateral side as
the injection becomes anesthetized with successful execution of this
technique.1,6,7
Intraseptal Injection. The intraseptal technique is a useful adjunct to the
aforementioned techniques (supraperiosteal, PSA, MSA, ASA). Although not
used as often in clinical practice, the technique is very similar to the PDL
injection and offers the added advantage of hemostasis in the area of injection.
Terminal nerve endings in the surrounding hard and soft tissue of individual
teeth are anesthetized with this technique. Contraindications to the procedure
include acute inflammation and infection over the site of injection. A 27 gauge
short needle is preferred for this technique. Technique- Place the patient in the
supine position. The target area is the interdental palpillae 2-3mm apical to the
83
apex of the papillary triangle. The right handed operator should be at the ten
o’clock position whereas the left handed operator should be at the two o’clock
position. The operator may ask the patient to turn his or her head for optimum
visibility. The syringe is held at a 45 degree angle to the long axis of the tooth
with the bevel facing the apex of the root. The needle is inserted into the soft
tissue and is advanced until bone is contacted. A few drops of anesthetic should
be administered at this time. The needle is then advanced into the interdental
septum and 0.2cc of anesthetic solution is deposited. Resistance to the flow of
anesthetic solution is expected and ischemia of the soft tissue surrounding the
injection site will ensue shortly after anesthetic solution is administered.1
Area anesthetized
Maxillary
Posterior Superior Alveolar Nerve Block. Maxillary
molars
(with
exception of mesiobuccal root of maxillary 1st molar in some cases), hard and
soft tissue on buccal aspect
Middle Superior Alveolar Nerve Block. Mesiobuccal root of maxillary
1st molar (in some cases), premolars and surrounding hard and soft tissue on
buccal aspect
Anterior Superior Alveolar Nerve Block/Infraorbital Nerve Block.
Maxillary central and lateral incisors andcanine, surrounding hard and soft tissue
on buccal aspect, mesiobuccal root of maxillary 1st molar (in some cases)
Greater Palatine Nerve Block. Palatal mucosa and hard palate from 1st
premolar anteriorly to posterior aspect ofthe hard palate, and to midline medially
Nasopalatine Nerve Block. Hard and soft tissue of lingual aspect of
maxillary anterior teeth from distal ofcanine on one side to distal of canine on
the contralateral side
Maxillary Nerve Block. Hemimaxilla on side of injection (teeth, hard and
soft, buccal and lingual tissue)
Mandibular
Inferior Alveolar Nerve Block. Mandibular teeth on side of injection,
buccal and lingual hard and soft tissue, lower lip
Buccal Nerve Block. Buccal soft tissue of molar region
Gow-Gates Mandibular Nerve Block. Mandibular teeth to midline, hard
and soft tissue of buccal and lingual aspect, anterior 2/3 of tongue, FOM, skin
over zygoma, posterior aspect of cheek, and temporal region on side of injection
Vazirani-Akinosi Closed Mouth. Mandibular teeth to midline, hard and
soft tissue of buccal aspect, anterior 2/3 of tongue, FOM
Mental Nerve Block. Buccal soft tissue anterior to mental foramen, lower
lip, chin
Incisive Nerve Block. Premolars, canine and incisors, lower lip, skin over
the chin, buccal soft tissue anterior to the mental foramen.
Equipment.
Administration of regional anesthesia of the maxilla
and mandible is achieved via the use of a dental syringe, needle, and anesthetic
cartridge. Several types of dental syringes are available for use, however the
most common is the breech-loading, metallic, cartridge-type, aspirating syringe.
84
The syringe is comprised of a thumb ring, finger grip, barrel containing the
piston with a harpoon, and a needle adaptor. A needle is attached to the needle
adaptor which engages the rubber diaphragm of the dental cartridge. The
anesthetic cartridge is placed into the barrel of the syringe from the side (breech
loading). The barrel contains a piston with a harpoon that engages the rubber
stopper at the end of the anesthetic cartridge. After the needle and cartridge have
been attached, a brisk tap is given to the back of the thumb ring to ensure the
harpoon has engaged the rubber stopper at the end of the anesthetic cartridge.
Dental needles are referred to in terms of their gauge which corresponds
to the diameter of the lumen of the needle. Increasing gauge corresponds to
smaller lumen diameter. Twenty-five and twenty-seven gauge needles are most
commonly used for maxillary and mandibular regional anesthesia and are
available in long and short lengths. The length of the needle is measured from
the tip of the needle to the hub. The conventional long needle is approximately
40mm in length while the short needle is approximately 25mm in length.
Variations in needle length do exist depending upon the manufacturer.
Anesthetic cartridges are prefilled, 1.8cc glass cylinders with a rubber
stopper at one end and an aluminum cap with a diaphragm at the other end. The
contents of an anesthetic cartridge are the local anesthetic, vasoconstrictor
(anesthetic without vasoconstrictor is also available), preservative for the
vasoconstrictor (sodium bisulfite), sodium chloride, and distilled water. The
most common anesthetics used in clinical practice are the amide anesthetics
lidocaine and mepivacaine. Other amide anesthetics available for use are
prilocaine, articaine, bupivacaine, and etidocaine. Esther anesthetics are not as
commonly used however remain available. Procaine, procaine plus
propoxycaine, chlorprocaine, and tetracaine are some common esther
anesthetics.
Additional armamentarium includes dry gauze, topical antiseptic and
anesthetic. The site of injection should be made dry with gauze and a topical
antiseptic should be used to clean the area. Topical anesthetic is applied to the
area of injection to minimize discomfort during insertion of the needle into the
mucous membrane. Common topical preparations include benzocaine, butacaine
sulfate, cocaine hydrochloride, dyclonine hydrochloride, lidocaine, and
tetracaine hydrochloride.
Topical anesthesia: Prior to injection, topical anesthetic can be applied
on the mucosa in the area of an injection to minimize discomfort to the patient
Universal precautions should always be observed by the clinician which include
the use of protective gloves, mask and eye protection. After withdrawing the
needle once a block has been completed, the needle should always be carefully
recapped to avoid accidental needle stick injury to the operator.1
Retraction of the soft tissue for visualization of the injection site should be
performed with the use of a dental mirror or retraction instrument. This is
recommended for all maxillary and mandibular regional techniques discussed
below. Use of an instrument rather than one’s fingers will help prevent
accidental needle stick injury to the operator.
85
Conscious Sedation
Conscious sedation is а minimally depressed lеvеl of consciousness that
retains the patient's ability to independently and continuously maintain an
airway, and respond appropriately to verbal commands. Conscious sedation
involves using variolls drugs or а combination of drugs to achieve pain and
anxiety control while maintaining the patient in а conscious state at all times.
The common routes of administration of conscious sedation are oral
premeditation, inhalation, and intravenous. Local anesthesia is administrated
with аll types of conscious sedation.
Local anesthetic
A local anesthetic is a drug that reversibly inhibits the propagation of
signals along nerves. When it is used on specific nerve pathways (nerve block),
effects such as analgesia (loss of pain sensation) and paralysis (loss of muscle
power) can be achieved.
Clinical local anesthetics belong to one of two classes: aminoamide and
aminoester local anesthetics. synthetic local anesthetics are structurally related
to cocaine. They differ from cocaine mainly in that they have no abuse potential
and do not act on the sympathoadrenergic system, i.e. they do not produce
hypertension or local vasoconstriction, with the exception of Ropivacaine and
Mepivacaine that do produce weak vasoconstriction.
Local anesthetics vary in their pharmacological properties and they are
used in various techniques of local anesthesia such as:
1. Topical anesthesia (surface).
2. Infiltration.
3. Plexus block.
4. Epidural (extradural) block.
5. Spinal anesthesia (subarachnoid block).
The local anesthetic lidocaine (lignocaine) is also used as a Class Ib
antiarrhythmic drug.
Mechanism of action. All local anesthetics are membrane stabilizing
drugs, they reversibly decrease the rate of depolarization and repolarization of
excitable membranes (like neurons). Though many other drugs also have
membrane stabilizing properties, all are not used as local anesthetics, for
example propranolol. Local anesthetic drugs act mainly by inhibiting sodium
influx through sodium-specific ion channels in the neuronal cell membrane, in
particular the so-called voltage-gated sodium channels.
When the influx of sodium is interrupted, an action potential cannot arise
and signal conduction is inhibited. The receptor site is thought to be located at
the cytoplasmic (inner) portion of the sodium channel. Local anesthetic drugs
bind more readily to "open" sodium channels, thus onset of neuronal blockade is
faster in neurons that are rapidly firing. This is referred to as state dependent
blockade.
Local anesthetics are weak bases and are usually formulated as the
hydrochloride salt to render them water-soluble. At the chemical's pKa the
protonated (ionised) and unprotonated (unionised) forms of the molecule exist in
86
an equilibrium but only the unprotonated molecule diffuses readily across cell
membranes. Once inside the cell the local anesthetic will be in equilibrium, with
the formation of the protonated (ionised form), which does not readily pass back
out of the cell. This is referred to as "ion-trapping". In the protonated form, the
molecule binds to the local anaesthetic binding site on the inside of the ion
channel near the cytoplasmic end.
Acidosis such as caused by inflammation at a wound partly reduces the
action of local anesthetics. This is partly because most of the anaesthetic is
ionised and therefore unable to cross the cell membrane to reach its cytoplasmicfacing site of action on the sodium channel.
Undesired Effects
Localized Adverse Effects. The local adverse effects of anesthetic agents
include neurovascular manifestations such as prolonged anesthesia (numbness)
and paresthesia (tingling, feeling of "pins and needles", or strange sensations).
These are symptoms of localized nerve impairment or nerve damage.
Risks. The risk of temporary or permanent nerve damage varies between
different locations and types of nerve blocks.
Recovery. Permanent nerve damage after a peripheral nerve block is rare.
Symptoms are very likely to resolve within a few weeks. The vast majority of
those affected (92–97%), recover within four to six weeks. 99% of these people
have recovered within a year. It is estimated that between 1 in 5,000 and 1 in
30,000 nerve blocks result in some degree of permanent persistent nerve
damage.
It is suggested that symptoms may continue to improve for up to 18
months following injury.
Causes. Causes of localized symptoms include:
1.
neurotoxicity due to allergenic reaction;
2.
excessive fluid pressure in a confined space;
3.
severing of nerve fibers or support tissue with the syringe/catheter;
4.
injection-site [Hematoma] that puts pressure on the nerve, or;
5.
injection-site infection that produces inflammatory pressure on the
nerve and/or necrosis.
General Adverse Effects. General systemic adverse affects are due to the
pharmacological effects of the anesthetic agents used. The conduction of electric
87
impulses follows a similar mechanism in peripheral nerves, the central nervous
system, and the heart. The effects of local anesthetics are therefore not specific
for the signal conduction in peripheral nerves. Side effects on the central
nervous system and the heart may be severe and potentially fatal. However,
toxicity usually occurs only at plasma levels which are rarely reached if proper
anesthetic techniques are adhered to. Additionally, persons may exhibit
allergenic reactions to the anesthetic compounds and may also exhibit cyanosis
due to methemoglobinemia.
Central nervous system. Depending on local tissue concentrations of
local anesthetics, there may be excitatory or depressant effects on the central
nervous system. At lower concentrations, a relatively selective depression of
inhibitory neurons results in cerebral excitation, which may lead to generalized
convulsions. A profound depression of brain functions occurs at higher
concentrations which may lead to coma, respiratory arrest and death. Such tissue
concentrations may be due to very high plasma levels after intravenous injection
of a large dose. Another possibility is direct exposure of the central nervous
system through the CSF, i.e. overdose in spinal anesthesia or accidental injection
into the subarachnoid space in epidural anesthesia.
Cardiovascular system. The conductive system of the heart is quite
sensitive to the action of local anesthetics. Lidocaine is often used as an
antiarrhythmic drug and has been studied extensively, but the effects of other
local anesthetics are probably similar to those of Lidocaine. Lidocaine acts by
blocking sodium channels, leading to slowed non-allergic reactions may
resemble allergy in their manifestations. In some cases, skin tests and
provocative challenge may be necessary to establish a diagnosis of allergy.
There are also cases of allergy to paraben derivatives, which are often added as
preservatives to local anesthetic solutions.
Allergic reactions during anaesthesia. Depending on local tissue
concentrations of local anesthetics, there may be excitatory or depressant effects
on the central nervous system. At lower concentrations, a relatively selective
depression of inhibitory neurons results in cerebral excitation, which may lead to
generalized convulsions. A profound depression of brain functions occurs at
higher concentrations which may lead to coma, respiratory arrest and death.
Such tissue concentrations may be due to very high plasma levels after
intravenous injection of a large dose. Another possibility is direct exposure of
the central nervous system through the CSF, i.e. overdose in spinal anesthesia or
accidental injection into the subarachnoid space in epidural anesthesia.
Methemoglobinemia. The systemic toxicity of prilocaine is
comparatively low, however its metabolite, o-toluidine, is known to cause
methemoglobinemia. As methemoglobinemia reduces the amount of
hemoglobin that is available for oxygen transport, this side effect is potentially
life-threatening. Therefore dose limits for prilocaine should be strictly observed.
Prilocaine is not recommended for use in infants.
88
Local anesthetics in clinical use
Chloroprocaine
Cocaine
Cyclomethycaine
Dimethocaine/Larocaine
Propoxycaine
Procaine/Novocaine
Proparacaine
Tetracaine/Amethocaine
Amino amides
Articaine
Bupivacaine
Carticaine
Cinchocaine/Dibucaine
Etidocaine
Levobupivacaine
Lidocaine/Lignocaine
Mepivacaine
Piperocaine
Prilocaine
Ropivacaine
Trimecaine
Esters are prone to producing allergic reactions, which may necessitate the
use of an Amide. The names of Amides contain an "i" somewhere before the aine. Esters do not.
Combinations
Lidocaine/prilocaine (EMLA)
Natural local anesthetics
89
Saxitoxin
Tetrodotoxin
Naturally occurring local anesthetics not derived from cocaine are usually
neurotoxins, and have the suffix -toxin in their names. Unlike cocaine produced
local anesthetics which are intracellular in effect, saxitoxin & tetrodotoxin bind
to the extracellular side of sodium channels.
For some dental procedures, your dentist will numb a part of your mouth
by injecting anesthetic drugs into your gum or inner cheek. This procedure,
called local anesthesia, numbs only the area near the injection.
The most common local anesthetic used in dental offices is lidocaine.
Others include prilocaine, mepivacaine, bupivacaine and other drugs with names
ending in "-caine." The newest local anesthetic released in the United States is
articaine. Procaine, the first synthetic anesthetic, which most people know as
Novocaine, is no longer used in dentistry because newer anesthetic drugs last
longer, are more effective and are less likely to cause allergic reactions.
The solution your dentist injects contains more than just an anesthetic. It
may also include:
A vasoconstrictor, such as epinephrine, which narrows your blood vessels
and helps the anesthetic's effect last longer
An antioxidant (which contains sulfites or methylparabens) to prevent
breakdown of the vasoconstrictor
Sodium hydroxide, which chemically adjusts the acidity (pH) of the
anesthetic solution to help it work properly
Sodium chloride, which helps the solution enter the bloodstream
Two types of local anesthesia injections are possible, depending on where
and how your dentist inserts the syringe. A block injection numbs an entire
region of your mouth, such as one side of the lower jaw. An infiltration injection
numbs a smaller area. In each case, the numbness will last from one to several
hours and may give you that "fat lip" feeling after you leave the dentist's office.
If you need local anesthesia, your dentist will dry the part of your mouth
where the injection will go by using a cotton swab or by blowing air on the area.
Many dentists then swab the area with an anesthetic gel to numb the injection
site.
Your dentist will slowly inject the anesthetic. The needle can sting. However,
most people don't feel the needle itself. Instead, the sting they feel is caused by
the anesthetic moving into the tissue.
After you leave the dentist's office, you may find it difficult to speak clearly or
eat, and drinking from a straw can be messy. Be careful not to bite your mouth
or lip while the area is still numb because you could cause significant damage
without realizing it.
90
Side Effects. Local anesthetics are the most common drugs used in the
dental office. Side effects are very rare.
One possible side effect is a hematoma, or blood-filled swelling, that can
form when the needle hits a blood vessel. The anesthetic sometimes causes numbness outside of the targeted area, and your eyelid or mouth can droop, but these
effects disappear when the anesthesia wears off. Sometimes, a medicine included in the injection — a vasoconstricter to narrow blood vessels — can cause
your heart to beat faster. If this happens, the effect lasts only a minute or two.
The needle can injure a nerve, causing numbness and pain after the anesthesia
wears off. The nerve usually heals over time, and the symptoms disappear.
General anesthesia
Sedation Dentistry (Sleep Dentistry). Sedation is a medical procedure
that involves the administration of drugs to calm the central nervous system
during an operation. Sedation is used in dentistry for reconstructive surgery,
removal of impacted wisdom teeth, or patients who experience high levels of
anxiety about visiting a dentist.
Sedation dentistry has been used for many years and has made previously
unbearable procedures completely comfortable for patients. There are a variety
of different sedation options depending on the needs of each patient. These
options range from basic local anesthesia to general anesthesia, when an
individual is unconscious during an operation.
Conscious Sedation. Conscious sedation induces an altered state of
consciousness that lowers pain and discomfort through the use of pain relievers
and sedatives. While under conscious sedation, patients are usually able to speak
and respond to verbal cues and communicate any discomfort they may
experience.
Conscious sedation is used in cosmetic dentistry during dental prosthetic
and reconstructive surgery procedures. Patients who undergo conscious sedation
are closely monitored by trained qualified providers. Some of the side effects of
conscious sedation can include headache, nausea, and a brief period of amnesia.
Conscious sedation is an extremely safe way for you to be alleviated of
any pain and discomfort you may experience during cosmetic dentistry
procedures. You deserve to have a perfect smile and to be cared for by the finest
dental specialists in the industry. Any questions or concerns you may have about
conscious sedation can be answered by your cosmetic dentist.
Sedation. IV sedation is an acronym for Intravenous Conscious Sedation,
the process of administering a sedative drug directly into the blood system.
Patients who undergo IV sedation remain conscious but often don't remember
much about what went on while they were sedated. While under IV sedation,
most people experience a state of deep relaxation and partial or full memory loss
from the time when the drug begins to take hold until it wears off.
IV sedation is administered through a tiny plastic tube attached to a vein
91
throughout the procedure. The patient's pulse and oxygen levels are closely
monitored by specially-trained personnel to ensure safety. As a result, IV
sedation is extremely safe and commonly practiced by a wide range of medical
professionals.
Oral Sedation. Usually oral sedation is used to relieve anxiety before a
dental appointment. The most common oral sedation technique is the use of antianxiety pills such as benzodiazepines. These pills are typically prescribed by a
dentist to be taken the night before an appointment to reduce stress and ensure
rest.
The benzodiazepine options for oral sedation are broken down into two
categories: Sedative hypnotics and anti-anxiety drugs. Different benzodiazepines
are designed to focus on specific areas of the brain. Sedative-hypnotic
benzodiazepines induce a calming effect, including drowsiness. Anti-anxiety
drugs have the primary purpose of keeping the patient calm.
Nitrous Oxide. Nitrous oxide is commonly known as laughing gas and is
used as an anesthetic in dentistry. When inhaled, nitrous oxide has exhilarating
effects on the user. Roughly one third of dental practices in the United States use
nitrous oxide, mainly to reduce anxiety patients may have regarding dental
treatment.
When used for these purposes, there are many benefits of nitrous oxide
with very few risks. The gas is administered through a mask placed over the
nose and the patient usually begins to feel sedated within 30 seconds to three or
four minutes. Once the appropriate dosage level is reached, the dentist will
proceed with treatment. After treatment is completed, the dentist will give the
patient pure oxygen to reverse the effects of sedation.
Intramuscular Sedation. Intramuscular sedation dentistry is a less
common form of sedation, but one that may suit some patients better than other
alternatives. The goal of intramuscular sedation is to ease a patient's concerns
about visiting a cosmetic dentist.
During intramuscular sedation drugs are injected into the muscle of the
upper arm or thigh. Within about 30 minutes, the patient is sedated and the
doctor begins the procedure.
Why is General Anaesthesia not used very much for dental work?
General anaesthesia is a procedure which is never without risk (including
the risk of death). As a result, the General Dental Council in the UK
recommends that "the decision to refer a patient for treatment under general
anaesthesia should not be taken lightly." "In assessing the needs of an individual
patient, due regard should be given to all aspects of behavioural management
and anxiety control before deciding to treat or refer for treatment under general
anaesthesia. General anaesthesia for dental treatment should only be
administered in a hospital setting with critical care facilities. All dentists
involved in arranging or providing treatment under general anaesthesia should
92
discuss with the patient advice and treatment options to avoid or reduce future
episodes of general anaesthesia. A dentist who refers a patient for treatment or
carries out treatment on a patient under general anaesthesia without ensuring
that the relevant conditions are met is liable to a charge of serious professional
misconduct."
Apart from the risk of death (which, while very small, is still significantly
higher than for conscious IV sedation), general anesthesia has a few major
disadvantages:
(1) Complications are more likely with GA compared with conscious
sedation both during and after the procedure. GA depresses the cardiovascular
and respiratory systems. For some groups of medically compromised patients, it
is contraindicated for elective procedures.
(2) It's not recommended for routine dental work like fillings. The
potential risk involved is too high to warrant the use of GA. For things like
fillings, a breathing tube must be inserted, because otherwise, little bits of tooth,
other debris or saliva could enter the airway and produce airway obstruction or
cause illnesses like pneumonia.
(3) Laboratory tests, chest x-rays and ECG are often required before
having GA, because of the greater risks involved.
(4) Very advanced training and an anesthesia team are required, and
special equipment and facilities are needed. GA introduces a number of
technical problems for the operator (i. e. dentist), especially when a "breathing
tube" is involved: the tongue is brought forward more into the dentist's way by
the airway tubing, the muscles are paralysed so the operator is working against a
dead weight all the time and there are postural problems because the patient
can't be moved about much. The operator can get very tired very quickly when
doing a session. It's physically the most demanding kind of dentistry (usually
standing, hot lights, compromised patient position).
(5) You can't drink or eat for 6 hours before the procedure (otherwise,
vomiting is possible and this would be extremely dangerous during GA).
(6) It's expensive.
(7) GA does nothing to reduce dental anxiety. The next time you need any
work, or even a routine check-up, you'll most likely be as afraid as ever. This
may not be applicable to all situations - as mentioned below, GA can be useful
or even indicated for certain situations.
How is it administered?
GA is usually started off with an injection
in the hand or arm. It can be supplemented by a face mask but if a face mask
is used you probably won't remember it.
If post-op pain is expected, the normal practice is to inject a long acting
local anaesthetic during the GA, so that when you wake up everything is nice
and numb for a good few hours (say 6 hours?) afterwards, which should give
you time to take some painkillers and allow them to kick in. It's much better to
93
premptively stop pain than it is to try to deal with it once it has started.
Complications that may occur during anesthesia may be either local or
general.
Local complications.
breaking an injection needle at a patient’s sudden move
misplacement of an injection solution
introduction of infection deeply into a tissue, or infecting a hematoma
resulting from a break in a blood vessel
damage of the periosteum or neural branch followed by pain, after a
deep puncture
ischemic necrosis of a tissue after application of a large amount of an
anesthetic and a vasoconstrictive substance
muscle contraction after anesthesia at foramen mandibulae. It may be
caused by a muscle injury, hematoma infection or toxic effects of the
anesthetic.
General complications.
Toxic reaction in case of exceeding a maximum dose of an anesthetic. It
can occur by a cumulative effect of two different anesthetics - absolute
overdosing - or by an accidental intravasation - relative overdosing.
Allergic reaction is quite frequent after application of procaine-like
anesthetics. Allergic reactions include hives with exanthema, angio-neurotic
edema, oral mucosa erythema etc. The most serious condition is the
anaphylactic shock with a sharp onset of the heart and respiratory insufficiency
(accelerated, later weakening breathing, heart arrhythmia or even heart arrest).
Therapy of general reactions to anesthetics should be carried out according to
general rules of reanimation, which every physician should be familiar with.
Relatively frequent reaction, which sometimes occurs even before an anesthetic
is applied, is fainting (peripheral collapse). It results from a sudden failure of
blood circulation into the brain, caused by a decrease of the peripheral resistance
of blood vessels due to vasodilatation. Numerous other factors play a role here,
such as mental stress, fear from a treatment, hypotonicity and hypoglycemia
caused by hunger, lack of sleep or fatigue. A patient turns pale, gets nausea and
loses his/her consciousness temporarily. These states can usually be managed
without a medication. A doctor should keep talking to a patient and try to calm
him/her down. A deep leaning over, with head close to the knees sometimes
works well, due to pressing of the splanchnic area together with a low position
of the head which increases blood circulation of the brain tissues. In all cases, a
patient’s clothing should be released and fresh air should be supplied. A patient
may as well be laid at the dentist’s chair, with his/her legs raised.
94
General anesthesia is а controlled state of unconsciousness accompanied
bу а partial оr complete loss of protective retlexes, including the ability to aintain an airway independently and respond to verbal commands. General anesthesia renders the patient unconscious through depression of the central nervous
system, thus eliminating patient cooperation as а factor. The administration of
general anesthesia is performed bу an anesthesiologist in the hospital operating
roоm. Local anesthesia is also administered at the treatment site.
TOOTH EXTRACTIONS
While there are many oral surgery procedures, some are mоrе commonly
performed than others.
Tooth extraction is an oral surgery procedure classified into three types:
simple, complicated, and impacted extractions. These аrе explained brietly in
the following paragraphs. Simple Extractions Simple extractions involve
removal of а tooth or root that does not require bone removal оr sectioning. The
deciduous (nonpermanent) or permanent tooth extracted is erupted and usually
diseased or malposed. Retained roots mау bе buried in the tissue and not visible
in the oral cavity. Retained root tips mау bе present because of fractured teeth,
advanced decay, оr аnу incomplete post-surgical procedure. They саn bе
identified оn radiographs.
Complicated extractions involve removal of а tooth or root that requires
surgical sectioning and/or bоnе removal.
Impacted extractions involve removal of а tooth that is partially or
completely covered bу bоnе and/оr soft tissue. This extraction mау involve
tissue incision, excision, or bone removal. Two types of impactions аrе
associated with оrаl surgery: soft tissue and bony impaction. Soft tissue - occurs
when the tooth is blocked frоm eruption due to the gingival tissue. It mау bе
partially erupted with а portion of the tooth visible in the mouth.
Extraction is performed for positional, structural, or economic reasons.
Teeth are often removed because they are impacted. Teeth become impacted
when they are prevented from growing into their normal position in the mouth
by gum tissue, bone, or other teeth. Impaction is a common reason for the
extraction of wisdom teeth. Extraction is the only known method that will
prevent further problems. Teeth may also be extracted to make more room in the
mouth prior to straightening the remaining teeth (orthodontic treatment), or
because they are so badly positioned that straightening is impossible. Extraction
may be used to remove teeth that are so badly decayed or broken that they
cannot be restored. In addition, patients sometimes choose extraction as a less
expensive alternative to filling or placing a crown on a severely decayed tooth.
In some situations, tooth extractions may need to be postponed
temporarily. These situations include:
Infection that has progressed from the tooth into the bone. Infections may make
anesthesia difficult. They can be treated with antibiotics before the tooth is
extracted.
95
The patient's use of drugs that thin the blood (anticoagulants). These
medications include warfarin (Coumadin) and aspirin. The patient should stop
using these medications for three days prior to extraction.
Patients who have had any of the following procedures in the previous six
months: heart valve replacement, open heart surgery, prosthetic joint
replacement, or placement of a medical shunt. These patients may be given
antibiotics to reduce the risk of bacterial infection.
Tooth extraction can be performed with local anesthesia if the tooth is
exposed and appears to be easily removable in one piece. An instrument called
an elevator (Fig. 22) is used to loosen (luxate) the tooth, widen the space in the
bone, and break the tiny elastic fibers that attach the tooth to the bone. Once the
tooth is dislocated from the bone, it can be lifted and removed with forceps.
If the extraction is likely to be difficult, the dentist may refer the patient to
an oral surgeon. Oral surgeons are specialists who are trained to give nitrous
oxide, an intravenous sedative, or a general anesthetic to relieve pain. Extracting
an impacted tooth or a tooth with curved roots typically requires cutting through
gum tissue to expose the tooth. It may also require removing portions of bone to
free the tooth. Some teeth must be cut and removed in sections. The extraction
site may or may not require one or more stitches to close the cut (incision).
Clinical features. There may be few or no symptoms.
Fig. 22 Elevators commonly used in oral surgery.
Before an extraction, the dentist will take the patient's medical history,
noting allergies and prescription medications. A dental history is also taken,
with particular attention to previous extractions and reactions to anesthetics. The
dentist may then prescribe antibiotics or recommend stopping certain
96
medications prior to the extraction. The tooth is x-rayed to determine its full
shape and position, especially if it is impacted.
If the patient is going to have deep anesthesia, he or she should wear loose
clothing with sleeves that are easily rolled up to allow for an intravenous line.
The patient should not eat or drink anything for at least six hours before the
procedure. Arrangements should be made for a friend or relative to drive the
patient home after the surgery.
An important aspect of aftercare is encouraging a clot to form at the
extraction site. The patient should put pressure on the area by biting gently on a
roll or wad of gauze for several hours after surgery. Once the clot is formed, it
should not be disturbed. The patient should not rinse, spit, drink with a straw, or
smoke for at least 24 hours after the extraction and preferably longer. Vigorous
exercise should not be done for the first three to five days.
For the first two days after the procedure, the patient should drink liquids
without using a straw, and eat soft foods. Any chewing must be done on the side
away from the extraction site. Hard or sticky foods should be avoided. The
mouth may be gently cleaned with a toothbrush, but the extraction area should
not be scrubbed.
Wrapped ice packs can be applied to reduce facial swelling. Swelling is a
normal part of the healing process. It is most noticeable in the first 48-72 hours.
As the swelling subsides, the patient may experience muscle stiffness. Moist
heat and gentle exercise will restore jaw movement. The dentist may prescribe
medications to relieve the postoperative pain.
Risks
Potential complications of tooth extraction include postoperative
infection, temporary numbness from nerve irritation, jaw fracture, and jaw joint
pain. An additional complication is called dry socket. When a blood clot does
not properly form in the empty tooth socket, the bone beneath the socket is
painfully exposed to air and food, and the extraction site heals more slowly.
Normal results
After an extraction, the wound usually closes in about two weeks. It takes
three to six months for the bone and soft tissue to be restructured. Complications
such as infection or dry socket may prolong the healing time.
Tooth extraction refers to painless removal of a whole tooth or a toothroot from its bony socket with minimal trauma to its investing tissues, so that
wound heals uneventfully and no postoperative prosthetic problem is created.
The branch of dentistry that deals with surgical, atraumatic and painless
removal of tooth is called Exodontia.
Indications for tooth removal:
90% of the teeth are removed due to dental caries (decay) and/ or
periodontal pathology.
Other reasons are:
1) Over retained deciduous teeth.
2) Impacted Teeth. Mainly, 3rd molar teeth and maxillary canines.
97
3) Mal-posed, transposed or supernumerary teeth. (As these teeth interfere with
oral hygiene maintenance and might cause ulcerations.)
4) Teeth might have to be removed in order to gain space to affect orthodontic
correction.
5) Teeth lying in the line of fracture, which, if not removed can provide access
to bacteria and hinder healing of the bone.
6) Tooth involved with cystic lesions or tumors.
7) Non strategic teeth are sometimes removed so that a proper prosthetic
appliance can be made.
8) Before therapeutic radiation.
9) Attempted extraction.
10) Fractured non- restorable tooth or a tooth- root.
11) Severe attrition, erosion, abrasion, hypoplasia, internal or external
resorption.
Contraindications:
They can be divided into absolute and relative contraindications.
A) Absolute contraindications:
Haemocoagulopathies, primary or secondary.
Uncontrolled hyperthyroidism.
Immuno compromised states
B) Relative contraindications:
Diabetes
Pregnancy
Myocardial infarction
Rheumatic fever
Angina
Hypertension
Cerebrovascualr diseases
Extraction of teeth. Dentists extract teeth for many reasons, but by far,
the most common is that the patient is in pain and wants to relieve the pain as
quickly, permanently and as inexpensively as possible. This does not mean that
there are not other ways of relieving the pain. But the other methods are likely to
be more expensive or inconvenient. Other reasons are:
The patient may choose extraction because the other alternatives are
simply too expensive.
The dentist may decide that the tooth is not repairable, or may be
impractical to repair under the circumstances, and extraction is the best of a
bunch of bad alternatives. This includes teeth that are decayed below the gum
line, or teeth that have lost too much bone due to periodontal disease.
Removal of the tooth may be a matter of health. This is the case in the
decision to remove impacted wisdom teeth, teeth associated with cysts or
tumors, or teeth that would otherwise compromise the patient's oral health if left
in place. In some instances, an infected tooth can even bring a patient close to
death by causing swelling that can stop breathing or initiating a brain abscess.
98
Teeth are frequently removed because they are crowded and their removal
would create a situation which could be repaired in their absence. Orthodontists
request extractions to give them more room to move teeth around. Dentists
sometimes remove crowded front teeth and replace them with bridges,
removable partial dentures or implants.
The types of extractions
1. Simple extractions. A Simple extraction is one in which the dentist can
remove the tooth simply by loosening the gums around it, grasping the crown
above the gum line with a plier-like forceps and then moving it side to side
until it loosens from the bone. Teeth are normally held into the bone by a thin
sheathe of soft tissue that separates it from the bone like a sock separates a foot
from a shoe. This sheathe is called the periodontal ligament, and it is this
structure which ultimately enables the dentist to remove the tooth. The key to
simple extractions is to rock the tooth side to side slowly enlarging the socket in
the bone while at the same time breaking the ligament which binds the tooth in
the socket.
2. Complex (surgical) extractions. Unfortunately, not all extractions can
be done by simply grasping the tooth with forceps and rocking it out. What if
there is nothing left above the gum line to grasp? Or what if the crown breaks
off leaving the roots still in the bone? These things can and do happen, and any
dentist that extracts teeth will have to deal with them routinely. In these cases, it
becomes necessary to surgically remove the tooth. This is frequently
accomplished by prying the root out using a sharp instrument that can be forced
between the root and the bone surrounding it. This technique is called
"luxation". In the case of multiple rooted teeth, the roots are first separated so
they can be removed individually. Unfortunately, not all roots or root fragments
may be removed in this fashion. This means that the dentist must make an
incision into the gums around the tooth and raise a flap of tissue exposing the
tooth and its surrounding bone.
Sometimes, after the flap is raised, there is enough tooth exposed to grab
and remove it as in a simple extraction (#1 above). Sometimes, the technique
described above as luxation may successfully remove the tooth. If luxation fails,
the dentist must take a handpiece (drill) and cut away some of the surrounding
bone in order to gain a purchase on the tooth. After the tooth has been pried out
of the artificially enlarged socket, the dentist then sutures (sews) the flap of
tissue back in place so that healing can proceed normally.
3. Impacted teeth. When a tooth does not fully erupt into the mouth, but
remains below the gums, it is said to be impacted. Impacted teeth can present
special health problems for most patients, and they are generally removed to
prevent future difficulties. The extraction of such teeth proceeds like the surgical
extraction explained above with a few modifications. Sometimes, the only
surgical procedure is the raising of the soft tissue flap. If after raising the flap,
the extraction can proceed as a simple extraction, the tooth is said to be a "tissue
99
impaction" because there was enough of the crown left above the bone to grab
and extract with forceps.
But many times the crown is submerged below the level of the bone. The
tooth may even be lying on its side under the bone which complicates the
extraction further. In these cases, not only must the dentist remove surrounding
bone in order to expose the tooth, but he must cut and break the tooth itself into
sections so that each section can be removed separately. Teeth in this condition
are said to be "bony impactions" and are further classified as vertical, horizontal
or angular depending on the angle of the tooth under the bone (Fig. 23)
Treatment planning. The information from the history and examination
is used to formulate the best plan for the patient. This will include measures for
adequate preparation for the procedure and also the selection of anaesthesia:
whether local anaesthesia, conscious sedation with local anaesthesia or general
anaesthesia. Anticipated difficulties are better discussed with the patient before
treatment rather than during treatment, when they may be perceived as excuses
for inadequate planning or experience.
Surgical techniques
Instrumental extraction. To extract a tooth from the alveolus, the
periodontal attachment must be disrupted and the bony dental socket enlarged to
allow withdrawal of the tooth. To achieve this, various instruments have been
developed:
• elevators: curved chisel-shaped instruments that fit the curvature of tooth roots:
an elevator has a single blade
• luxators: similar to elevators but finer blade
• forceps: have paired blades that are hinged to permit the root to be grasped
• peritome: has a finer blade with which to sever the periodontal attachment and
is preferred where it is important not to damage the bony support of the tooth,
for example when immediately replacing a tooth with a dental implant.
There are several different forceps extraction techniques described and so
some basic principles and guidance are required when learning. Forceps are used
to disrupt the periodontal attachment and dilate the bony socket either directly,
by forcing the blades between tooth and bone, or by moving the tooth root
within the socket, or both. Once this has been done, the tooth may be lifted from
its socket. The movements that are required to complete the extraction may be
described as a preliminary movement to sever the periodontal membrane and ge-
100
Fig. 23 Examples of various types of third molar impactions.
A. Vertical impaction with unfavourable root morphology requiring bone removal and
vertical sectioning.
B. Mesioangular impaction requiring bone removal and a mesial application point to elevate
and upright to remove.
C. Horizontal impaction requiring bone removal, sectioning of the crown to permit removal of
crown and then roots in stages.
D. Distoangular impaction requiring significant bone removal to permit elevation distally for
removal without
reimpaction.
nerally dilate the socket, followed by a second movement to complete the
dilation and withdraw the tooth. The first movement requires that force is
directed along the long axis of the tooth, pushing the blades of the forcep
towards the root apex. This force is then maintained during the second
movement, which is dependent on the tooth root and bone morphology. If a
tooth has a single round root, then it may be rotated. Where the buccal bone
plate is relatively thin, it may be possible to distort it significantly by moving the
forceps applied to the tooth root in a buccal direction. The second movement
depends on the tooth and may be described as:
101
upper incisors and canines: rotational
upper premolars: limited buccal and palatal
upper molars: buccal
lower incisors and canines: buccal
lower premolars: rotational
lower molars: buccal.
Alternative techniques for forceps movement are advocated by some,
including a 'figure of eight' movement to expand the socket for molar teeth.
Elevators may be used to carry out the first movement prior to completion of the
extraction with forceps. Sometimes teeth and roots may be removed with
elevators alone. There are many different designs of elevator. The most
commonly used are:
• Coupland's elevators: a straight blade in line with the handle; available in three
sizes referred to as 1, 2 and 3
• Cryer's elevators: a triangular blade at right angles to the handle; available as a
right and left pair
• Warwick James elevators: a small blade that is rounded at its tip rather than
pointed; this is set at right angles to the tip in a right and left pair, but a straight
Warwick James is also available.
It is important that elevators are used appropriately, with their blades
between root and bone rather than between adjacent teeth, or else both teeth will
be loosened.
There are many designs of forceps. The blades vary in size and shape
according to the root morphology of the tooth/teeth for which they are designed.
For example, lower molar forceps incorporate a right angle between blades and
handles, while the blades each have a central projection to accommodate the
bifurcation. Upper 'root' forceps have narrower blades than the equivalent upper
premolar forceps. It may be difficult to apply forceps to teeth that are outside of
a crowded dental arch and elevators may be more appropriate for initiating the
extraction or for the whole procedure. The applied force should be controlled
and limited when using both elevators and forceps so that the soft tissues are not
accidentally injured or the jaws fractured. Only with experience is it possible to
know that the usual force is not producing the expected result, when further
investigation is required with a radiograph (if not already available) or a
transalveolar approach required. The non-dominant hand is used to support the
mandible against the force of the first movement when extracting lower teeth. It
is also used to retract the intraoral soft tissues and, by supporting the adjacent
alveolus, provide feedback of movement as a measure of control. The patient
should have eye protection; if the treatment is carried out under local
anaesthesia, then the patient may be placed in a position between sitting up and
supine or treated supine. Treatment under conscious sedation or general
anaesthesia will dictate the supine position. Lower right quadrant extractions are
best performed with the operator standing behind the patient; for all other
extractions, the operator should stand in front of the patient.
102
Surgical removal of teeth. Teeth resistant to forceps extraction and those
that fracture during extraction need to be removed surgically. However, it may
be acceptable to leave a very small root apex if there is no associated periapical
pathology and the anticipated surgical morbidity is significant. The patient must
be told if any fragments are to be retained. Teeth are surgically removed by a
transalveolar approach.
Postoperative care. Control of postoperative pain is important. Some
clinicians prescribe antibiotics if bone removal is necessary. Patients should be
given a written set of postoperative instructions and these should be also given
verbally before the patient leaves.
Technique for surgical tooth removal by transalveolar approach
1. A mucoperiosteal flap is raised, with a broad base to ensure good blood
supply. Incisions should be full thickness and the flap should be retracted to
ensure good access and visibility of the area without causing undue trauma to
the soft tissues. Papilla should be included in the design of a flap and not divided
or they are unlikely to maintain their viability.
2. Bone is removed with an irrigated bur to permit adequate access for
application of elevators and for removal of the tooth. A chisel and mallet may be
used to remove bone if the operation is being undertaken under general
anaesthesia.
3. The tooth is divided with a bur as necessary.
4. Elevators are used to sever the periodontal membrane and dilate the socket;
the tooth is then removed.
5. The wound is cleaned with irrigation and bone is filed, as appropriate.
6. Haemostasis.
7. The wound is closed with sutures. The flap should be designed so that its
margins will rest on sound bone at closure.
On the day of treatment:
Do not rinse your mouth for at least 24 hours.
Avoid hot fluids, alcohol, hard or chewy foods. Choose cool drinks and
soft foods.
Avoid vigorous exercise.
Smokers should avoid smoking.
Should the wound start to bleed, apply a small compress. This can be
made from some cotton wool in a clean handkerchief. Place this on the
bleeding point and bite firmly on it for 5-10 minutes or longer if
necessary.
If you cannot stop the bleeding yourself, please seek professional advice.
Any pain or soreness can be relieved by taking the prescribed medication.
If none was prescribed, take tablets such as paracetamol (Panadol) 2
tablets every 4 hours as required. Do not take more than the recommended
number per day.
Starting 24 hours later
103
Gently rinse the wound with hot saltwater mouth rinses (or other rinse as
recommended) for a few days. This should be carried out three times a day after
each meal.
Complications of dental extractions
Postoperative pain. Discomfort after the surgical trauma of dental
extractions
is to be expected and may be alleviated with an analgesic such as paracetamol or
a non-steroidal antiinflammatory drug (NSAID) such as ibuprofen. Severe pain
after a dental extraction is unusual and may indicate that another complication
has occurred.
Postoperative swelling. Mild inflammatory swelling may follow dental
extractions but is unusual unless the procedure was difficult and significant
surgical trauma occurred. More significant swelling usually indicates
postoperative infection or presence of a haematoma. Management of infection
may require systemic antibiotics or drainage. A large haematoma may need to
be drained. Less likely is surgical emphysema.
Trismus. Trismus or limited mouth opening after a dental extraction is
unusual and is likely to be infective in origin.
Fracture of teeth. Teeth may fracture during forceps extraction for a
variety of reasons and this is not an unusual event. The crown may fracture
because of the presence of a large restoration, but this may not prevent the
extraction from continuing as the forceps are applied to the root. However, if the
fracture occurs subgingivally, then a transalveolar approach will be necessary to
visualise the root.
If a small (3 mm) root apex is retained after extraction, this may be left in
situ, providing it is not associated with apical infection. The patient must be
informed of the decision to leave the apex to avoid the morbidity associated with
its surgical retrieval and the decision recorded. Antibiotics should be prescribed.
Excessive bleeding. It may be difficult to gauge the seriousness of the
blood loss from the patient's history, because they are usually anxious. However,
it is important to establish whether or not the patient is shocked by measuring
the blood pressure and pulse. This can be done while the patient bites firmly on
a gauze swab to encourage haemostasis. Typically, if the systolic pressure is
below 100 mgHg and the heart rate in excess of 100 beats/min, then the patient
is shocked and there is an urgent need to replace lost volume. This may be done
by infusion of a plasma expander such as Gelofusine or Haemaccel or a
crystalloid such as sodium chloride via a large peripheral vein. For this purpose,
the patient should be transferred to hospital. More commonly, the patient is not
shocked and can be managed in the primary care setting.
The next step in management is to investigate the cause of the
haemorrhage by taking a history and carrying out an examination.
History
• Local causes
— mouthrinsing
— exercise
104
— alcohol
• General causes
— previous postextraction or surgical haemorrhage
— medications
— liver disease
— family history of disorders of haemostasis.
Examination. Determine the source of the haemorrhage by sitting the
patient upright (unless feeling faint) and using suction and a good light. This is
commonly from capillaries of the bony socket or the gingival margin of the
socket, or more unusually from a large blood vessel or soft tissue tear.
Achieve haemostasis. If the history has suggested a general cause, then
local methods will not adequately result in haemostasis and the patient should be
transferred to hospital where specialist haematological management is available.
Otherwise the following techniques are used:
• socket capillaries: pack the socket with resorbable cellulose, such as Surgicell
• gingival capillaries: suture the socket with a material that will permit adequate
tension, such as vicryl or black silk
• large blood vessel: ligate vessel, usually by passing a suture about the vessel
and soft tissues.
Dry socket (alveolar osteitis). In some cases, a blood clot may
inadequately form or be broken down. Predisposing factors of osteitis include
smoking, surgical trauma, the vasoconstrictor added to a local anaesthetic
solution, oral contraceptives and a history of radiotherapy. The exposed bone is
extremely painful and sensitive to touch.
Dry socket is managed by:
• reassuring the patient that the correct tooth has been extracted
• irrigation of socket with warm saline or chlorhexidine mouthrinse to remove
any debris
• dressing the socket to protect it from painful stimuli: bismuth-iodoformparaffin paste (BIPP) and lidocaine (lignocaine) gel on ribbon gauze are useful.
Postoperative infection. In some cases, sockets may become truly infected, with pus, local swelling and perhaps lymphadenopathy. This is usually localised to the socket and can be managed in the same way as a dry socket, although antibiotics may be necessary in some instances. A radiograph should be taken to exclude the presence of a retained root or sequestered bone. Positive evidence of such material in the socket indicates a need for curettage of the socket.
Osteomyelitis. Osteomyelitis is rare but may be identified by radiological
evidence of loss of the socket lamina dura and a rarefying osteitis in the
surrounding bone, often with scattered radio-opacities representing sequestra.
Damage to soft tissues. Crush injuries can occur to soft tissues when a
local or general anaesthetic has been used and the patient does not respond to the
stimulus and, therefore, inform the operator. This may happen to a lower
anaesthetised lip when extracting an upper tooth; the lip can be crushed between
forceps and teeth if it is not rotated out of the way.
105
Damage to nerves. Paraesthesia or anaesthesia can result from damage to
the nerves in the intradermal canal during extraction of lower third molars.
Opening of the maxillary sinus. Creation of a communication between
the oral cavity and maxillary sinus, an oroantral fistula (OAF), may result during
extraction of upper molar teeth.
Loss of tooth. A whole tooth may occasionally be displaced into the
maxillary sinus, when it is managed as for displacement of a root fragment. A
tooth may also be lost into the infratemporal fossa or the tissue spaces about the
jaws, but this usually only occurs when mucoperiosteal flaps are raised.
Loss of tooth fragment. Typically, a fractured palatal root of an upper
molar tooth is inadvertently pushed into the maxillary sinus by the misuse of
elevators. Rarely, a fragment may be lost elsewhere, such as into the inferior
alveolar canal.
Fracture of the maxillary tuberosity. Fracture of the maxillary
tuberosity can result from the extraction of upper posterior molar teeth.
Fracture of jaw. A fracture of the jaw is a rare event and is most likely to
be the result of application of excessive force in an uncontrolled way. More
commonly, small fragments of alveolar bone are fractured, which may be
attached
to the tooth root. Any loose fragments should also be removed.
Dislocation of the mandible. Dislocation may occur when extracting
lower teeth if the mandible is not adequately supported. It is more likely to occur
under general anaesthesia and should be reduced immediately.
Displacement of tooth into the airway. The airway is at risk when
extracting teeth on a patient in the supine position. It can be protected when the
patient is being treated under general anaesthesia but not when the patient is
conscious or being treated under conscious sedation. It is, therefore, essential
that an assistant is present and high velocity suction and an appropriate
instrument for retrieval of any foreign body are immediately available. A chest
radiograph is essential if a lost tooth cannot be found, to exclude inhalation.
Surgical emphysema. Air may enter soft tissues, producing a
characteristic crackling sensation on palpation. However, this is unlikely if a
mucoperiosteal flap has not been raised. Air-rotor dental drills should not be
used during surgery because they may force air under soft tissue flaps. The
patient should be reassured and antibiotics prescribed.
Impacted and ectopic teeth Assessment
In the context of teeth, the term ectopic is applicable to a tooth that is
malpositioned through congenital factors or displaced by the presence of
pathology. It includes impacted teeth. Impaction may occur because there is no
path of eruption because the tooth develops in an abnormal position or is
obstructed by a physical barrier such as another tooth, odontogenic cyst or
tumour.
Most commonly affected
• mandibular third molars
106
• maxillary third molars
• maxillary canines.
Less commonly affected
• mandibular second premolars
• supernumerary teeth.
An impacted tooth may be completely impacted, when entirely covered by
soft tissue and partially or completely covered by bone within the bony alveolus,
or partially erupted, when it has failed to erupt into a normal functional position.
The terms unerupted and partially erupted are commonly used for normally
developing as well as impacted teeth. It is important, therefore, to distinguish
between impaction and normal development.
Third molars. These usually erupt between 18 and 24 years but,
frequently, eruption occurs outside these limits. One or more third molars fail to
develop in approximately one in four adults. Impaction of third molars
predisposes to pathological changes such as pericoronitis, caries, resorption and
periodontal problems.
Impacted maxillary canines. These may be associated with resorption of
adjacent lateral incisor roots, dentigerous cyst formation and infection.
Impacted lower second premolars. These are often lingually positioned
and may have an unfavourable root morphology.
History and clinical examination. The patient may have noticed that a
tooth is missing or this may not be apparent until observed at a routine dental
examination. It is unusual for unerupted teeth to cause pain unless there is
associated infection. Pericoronitis can be associated with any impacted tooth but
is of particular concern when it involves the mandibular third molar because of
the greater potential to spread via the tissue spaces and compromise the airway.
On examination, missing teeth should be noted and also any caries or mobility
of adjacent teeth. Signs of infection will include swelling, discharge, trimus and
tender enlarged cervical lymph nodes.
Radiological examination. Radiological examination should be based
upon clinical history and examination. Routine radiographic examination of
unerupted third molars is not recommended. Radiological assessment is essential
prior to surgery but does not need to be carried out at the initial examination if
infection or other local problem is present. The views used are:
• periapical, dental panoramic tomography (DPT) (or lateral oblique) and, rarely,
computed tomography (CT) for lower third molars
• DPT (or lateral oblique, or adequate periapical) for upper third molars
• parallax films (two periapicals or one periapical and an occlusal film) for
maxillary canines
• periapical and true occlusal radiograph for mandibular second premolar; a DPT
(or lateral oblique) should be used if the periapical does not image the whole of
the unerupted tooth. Radiological assessment of impacted teeth should cover:
• type and orientation of impaction and the access to the tooth crown size and
condition root number and morphology alveolar bone level, including depth and
107
density follicular width periodontal status, adjacent teeth relationship or
proximity of upper teeth to the nasal cavity or maxillary antrum
• relationship or proximity of lower teeth to the interdental canal, mental
foramen, lower border of mandible.
Diagnosis. When documenting the diagnosis, it is important to state
'impacted tooth' and the problem associated with this tooth. The mere presence
of an impacted tooth may not in itself justify the treatment planned for the
patient. It is better therefore to state 'impacted lower third molar and recurrent
pericoronitis', for example.
Treatment options. The initial management of pericoronal infection may
include irrigation beneath the operculum, grinding the cusps (or extraction) of
any opposing tooth in the case of a lower third molar, and antibiotic therapy.
Review of the patient is necessary to assess the longterm management of the
impacted tooth or teeth.
Treatment options are:
observation
surgical removal
operculectomy
surgical exposure
surgical reimplantation / transplantation.
In the case of impacted third molars, the decision is usually between
observation or removal, as the outcomes for the alternative treatments offer
limited therapeutic success. The decision to recommend removal takes into
account the likely surgical morbidity and the risk of continuing and recurring
pathology. In the case of maxillary canines, surgical exposure is a good option if
there is sufficient space in the arch to accommodate the tooth or if space can be
created orthodontically. Lower second premolars may also be exposed, but this
is less commonly undertaken than for maxillary canines. The medical history,
social history and age of the patient may all have an influence on the decision
making.
Indications for removal of third molars. There has been disagreement
about the appropriateness of removing third molars without associated
pathology in order to prevent potential later pathology (prophylactic removal)
but there is no controversy about the value of removing teeth that are associated
with pathology. A working party (convened by the Faculty of Dental Surgery of
the Royal College of Surgeons of England) provided in 1997 guidance on best
practice. The National Institute for Clinical Excellence (NICE) issued similar
guidance in England and Wales in 2000. The latter stated that routine practice of
prophylactic removal of pathology-free impacted third molars is unacceptable
and that the surgical removal of third molars should be limited to patients with
evidence of pathology. Such pathology includes:
• second or subsequent episode of pericoronitis
• unrestorable caries
• non-treatable pupal/periapical pathology
108
• cellulitis / abscess / osteomyelitis
• internal/external resorption of the tooth or adjacent teeth
• fracture of the tooth
• disease of the follicle, including cyst /tumour
• tooth impeding surgery or reconstructive jaw surgery
• tooth involved in tumour or in field of tumour resection.
In Scotland, more detailed evidence-based guidelines were issued by the
Scottish Intercollegiate Guidelines Network in 2000. While being broadly in
agreement with the NICE guidance, these include a list of situations in which
removal of unerupted/ impacted third molars is not advisable.
• In patients whose third molars would be judged to erupt successfully and have
a functional role in the dentition.
• In patients whose medical history renders removal an unacceptable risk to the
overall health of the patient or where the risk exceeds the benefit.
• In patients with deeply impacted third molars with no history or evidence of
pertinent local or systemic pathology.
• In patients where the risk of surgical complications is judged to be
unacceptably high, or where fracture of an atrophic mandible may occur.
• Where surgical removal of a single third molar is planned under local
anaesthesia, the simultaneous extraction of asymptomatic contralateral teeth
shouldnot normally be undertaken. Impacted third molar teeth that are not to be
removedshould be kept under review to ensure that no pathological process
develops.
Surgical techniques
Lower third molar surgery. The area of bone that is removed and the path
of withdrawal of the tooth depends upon the type of impaction.
Upper third molar surgery. The procedure follows the same principles as
for lower third molars, although obviously no lingual nerve protection is
required and, frequently, bone removal is not necessary. It is important to
maintain good vision of the surgical site and to position an instrument carefully
to keep the soft tissue flap open and direct the elevated tooth into the mouth, to
prevent its entry into the infratemporal fossa.
Maxillary canines. A buccal or palatal approach is made, appropriate to
the position of the tooth. The palatal approach must take into account the greater
palatine artery, which is incorporated into a large flap design with the sacrifice
of the nasopalatine neurovascular bundle. Bone is then removed and the tooth
elevated and removed. If the tooth is to be exposed, the bone is removed without
damaging the tooth and a defect created when repositioning the flap, which is
maintained by interrupting healing by the placement of a pack, sutured in place.
If it is apparent that the tooth cannot be moved by orthodontic means, then it is
possible carefully to remove it with as little damage as possible to the
periodontal ligament and splint it into position in a surgically created socket.
This transplantation technique has become less popular over the years as it has
become apparent that the long-term success is not good and resorption
frequently occurs, albeit after some years.
109
Surgical technique for removal of lower third molar
1. A buccal mucoperiosteal flap is raised to provide adequate access.
2. A lingual flap is raised and the lingual nerve is protected with an appropriate
instrument. This aspect is controversial and some would avoid raising a lingual
flap and restrict their approach to the buccal only. This latter approach requires
tooth division more frequently and is carried out in an attempt to reduce the
incidence of lingual nerve damage and resulting sensory disturbance. The
avoidance of a lingual flap has been the popular technique in the USA, while
raising a lingual flap and protecting the nerve has been common in the UK, but
the results of clinical trials are leading to changes in practice.
3. Bone removal may be required and this may be undertaken with an irrigated
bur in a handpiece or a chisel. The lingual split technique involves the removal
of a segment of lingual bone plate with a chisel after the nerve has been
protected. The advocates of this technique suggest that while temporary nerve
damage may occur, permanent damage is reduced when compared with the use
of burs for bone removal.
4. The tooth may then need to be divided before elevation and removal.
5. The wound is irrigated and inspected before the soft tissues are closed with an
appropriate suture material. remove it with as little damage as possible to the
periodontal ligament and splint it into position in a surgically created socket.
This transplantation technique has become less popular over the years as it has
become apparent that the long-term success is not good and esorption frequently
occurs, albeit after some years.
Mandibular second premolars. It is important that the mental nerve is
identified and protected while raising a buccal mucoperiosteal flap. It is
frequently necessary to divide the tooth and remove the crown before the root
can be delivered by elevation.
Supernumerary teeth. Commonly, supernumerary teeth occur in the
anterior maxilla and are exposed via a buccal or palatal flap and bone removal.
It is important to identify the supernumerary teeth clearly before removal and
this can be difficult when there are also developing permanent teeth present.
Complications of treatment of impacted and ectopic teeth
The complications of the surgical removal of impacted teeth are the same
as those that may occur as a result of extraction of teeth with forceps or
elevators or the surgical removal of other teeth. However, in addition, sensory
nerve damage may occur when surgery involves the mental foramen area and
lower third molars.
The estimated incidence of nerve damage varies greatly between studies.
Sensory loss for the lingual and inferior alveolar nerves combined is
approximately 13%, with a substantial reduction to about 1% at 6 months after
surgery.
Wisdom teeth are known as third molars in dentistry. In the X-ray film
above, if you count the number of large teeth from the front of the mouth to the
back, you can see that the "third" ones are impacted (as defined above). They are
110
called wisdom teeth because they erupt at about the age of 17 or 18 when people
are supposed to begin to assume the mantle of adulthood (I can only assume that
this name must be a hangover from centuries ago when people only lived to 25).
During the course of evolution, our faces tended to get shorter, but the number
of teeth did not decrease as rapidly as the shortening of the jaws. Most people do
not have enough room in the dental arches for their wisdom teeth, and they tend
to remain fully or partially impacted, under the bone of the jaw, or at least partly
under the gums (as in the image above). In some cases, the wisdom teeth may
remain impacted all of a person's life without causing trouble, but in a high
stress society, these people are in the minority. What's stress got to do with it?
You'll see.
Pericoronitis. The image on the right shows angry, swollen gums just
behind a second molar. There is actually a third molar (wisdom tooth) buried
under the swollen gums. You might think that a tooth that is totally buried under
the gums should not come into contact with germs from the mouth, and thus
should not be prone to infection. Usually, however, the enamel on the crown of
the impacted wisdom tooth is in contact with the enamel on the crown of the
second molar, which is erupted and immediately in front of the wisdom tooth
(see arrow on illustration below). Gums cannot attach to enamel. Thus the gums
lie over the crown of the wisdom tooth like a glove lies over the hand, in close
approximation, but not attached to it. Germs can leak under the gums at the
place where the enamel of the second molar contacts the enamel of the wisdom
tooth, Therefore, there is almost always a communication between the germs
that live in the mouth and the space surrounding the wisdom tooth. It is a tooth
you cannot brush. When your body's resistance is normal, the germs surrounding
the impacted tooth are kept at bay by the body's normal immune system. But if
the body's resistance is decreased, through sickness or emotional stress, the
germs can get the upper hand and you find yourself with an infection around the
wisdom tooth. These infections are called "pericoronitis" which means
(appropriately), "an infection around the crown of an unerupted tooth".
The relationship of wisdom teeth to the sinuses
As you can see in the image to the right, the upper impacted wisdom tooth is in
very close approximation to the maxillary sinus. As a rule impacted upper
wisdom teeth cause few symptoms if no obvious oral infection is present. But in
the case of peircoronitis, the infection can sometimes be transferred to the sinus
causing typical sinus headaches and congestion. Conversely, the extraction of a
wisdom tooth in this location can occasionally cause problems with the sinus.
Most oral surgeons prefer to wait until the roots of the wisdom teeth are
mostly formed, at about age 17 or 18 before extracting wisdom teeth. The main
reason for this is that during this period, the normal forces of eruption have
generally allowed the tooth to erupt as much as is possible under the
circumstances. This means a simpler procedure involving less drilling of bone
and tooth in order to effect extraction.
111
Cysts Aside from pericoronitis, there are two other complications
associated with impacted wisdom teeth. They both involve the uncontrolled
expansion of the follicle (the space in the bone where the tooth was originally
formed). This follicle is lined with cells which are supposed to transform into
the lining of the sulcus of the gums when the tooth erupts. But if they are kept
submerged for too long, they sometimes forget their original mission and begin
to produce fluid which expands the follicle causing a cyst.
Amyloblastoma. The second, very rare complication arising from
uncontrolled follicular growth is a form of tumor called amyloblastoma. This
tumor is not considered a cancer because it does not tend to metastasize (spread
to other areas of the body), but it is locally invasive which means that it grows
uncontrollably and can cause major damage and weakness in the bone if it is not
thoroughly removed. Amyloblastoma is most likely to attack young adult males.
It is less frequent in females or older people of either sex. Since it is always
associated with an impacted tooth, usually a wisdom tooth, (but not always, as
seen in the images above) it rarely occurs before the age of 18. It is difficult to
remove entirely, and the surgeon will usually perform a wide excision (ie. he
takes a lot of extra bone along with the tumor) just to be sure that he has rem
112
Complications:
Intra Operative Complications:
Failure of Anesthesia / persistence of pain.
Failure to remove tooth with either forceps or elevators.
Syncope happens because of sudden fall in blood pressure and subsequent
cerebral hypoxia.
Excessive bleeding,
Fracture of tooth being extracted, roots of the tooth being extracted,
Maxillary Tuberosity, adjacent or opposing tooth, alveolar bone or jaw
bone.
Laceration of soft tissue.
Fistulation of antrum in case of upper maxillay molars.
Dislocation of adjacent tooth or temporomandibular joint.
Damage to the nerve branches.
Displacement of tooth or fragment of tooth or tooth- root in to soft tissue,
tissue spaces, Antrum.
Asphyxia or inhalation of tooth or a fragment.
Broken instrument.
Trismus, Microstomia and transposed or crowded teeth.
Cardiac Arrest.
Respiratory Arrest.
Complication arising from anesthetic agent and injection technique.
Post op complications:
Reactionary Hemorrhage and secondary hemorrhage.
Oedema.
Pain
Trismus
Ulceration
Dry socket
Fibrous healing of bone
Parasthesia, transient anesthesia or permanent anesthesia.
Surgical emphysema
Complications after extractions
1. Bleeding . It is possible to bleed to death following the extraction of a
tooth. But it almost never happens. All you have to do is follow directions #1
and #2 above and the bleeding will stop. The only patients that may still be in
danger from excessive bleeding are those who are taking anticoagulant drugs
(blood thinners) like Coumadin or Heparin for cardiovascular problems, or
people with bleeding disorders like Hemophilia or related clotting cascade
disorders . These patients should consult their physicians before having a tooth
extracted. People taking aspirin and other non steroidal anti inflammatory drugs
(NSAID's) like Advil or Aleve may experience prolonged bleeding times, but in
my experience, these drugs have never presented a problem as long as the
patient keeps the extraction site covered with gauze to stem the bleeding. The
blood WILL clot eventually!
2. Infection. The mouth is alive with bacteria, especially in people with
poor oral hygiene. Infection is a constant problem after extractions, and most
dentists have developed a personal protocol on whether or not a particular
patient needs preventive antibiotics. People who present at the office with
swollen faces, teeth tender to light pressure, swollen gums or tongue, or
bleeding and pus around a tooth are generally already infected. They should
expect to be given prophylactic (preventive) antibiotics after an extraction.
Patients may develop infections after an extraction even if they were
not infected before the extraction. This is a common complication and is due
to the fact that that the mouth is teeming with bacteria and cannot be
sterilized prior to the extraction. (They are NOT due to any error on the part
of the dentist!) The first sign of an infection after an extraction is often
renewed bleeding after 48 hours. The bleeding is not generally severe, but it
is an indication that the patient should return to the dentist's office for
evaluation and possibly a prescription for antibiotics. Other signs of infection
include renewed swelling around the extraction site and surrounding parts of
the face, as well as increased pain after 48 hours. Signs of infection two
days after an extraction should be attended to as soon as possible. Some
dentists will give a patient an antibiotic and send them home for several days
to allow the infection to clear before attempting the extraction. The reason
for this is because the local anesthesia does not work as well in acid
environments and it may take a lot of shots to get the patient numb. However,
if the dentist gives enough anesthesia, it is possible to extract a tooth under
113
such circumstances. In general, I have never found that extraction of a tooth
in the presence of an active infection has presented special problems as long
as the patient takes the antibiotics prescribed faithfully.
It is NOT necessary to take antibiotics after every extraction. A simple
extraction in a clean, uninfected mouth generally does not require
prophylactic antibiotics.
Whenever the extraction requires the cutting of any tissue (see surgical
and impacted extractions above), it is generally a good idea to give
prophylactic antibiotics, and the patient SHOULD fill the prescription and
take the drug faithfully, or he may suffer an extended convalescence.
3. Dry Sockets. A Dry Socket, while not potentially life threatening like
bleeding or infections, is one of the most painful, common, debilitating and
dreaded post extraction problems encountered in dentistry. They are much more
common following the extraction of lower teeth than they are after extraction of
upper teeth. They can happen after even the simplest of extractions. If you
follow all of the post surgical directions listed above, you have done the most
anyone can do to prevent them. Unfortunately, no matter how hard you try, you
may still get one. If you get one, it is not (necessarily) your fault. Nor is it the
fault of the dentist. They are a quirk of nature. You may THINK you are going
to die. You won't!
The two classes of patients who are most prone to dry sockets are those
who smoke during the first 48 hours after the extraction, and persons who
tend to constantly grind and clench their teeth.
A dry socket is a condition in which the blood clot that forms in the
extraction site becomes detached from the walls of the socket, or dissolves
away leaving the bare bone exposed to saliva and the foods you eat. The
bone becomes inflamed due to bacteria and chemicals in the mouth, and this
inflammation is persistent and painful. The pain is "deep pain". That is, it
comes from tissues buried deep in the body, and your brain has no experience
of pain from these regions. When the brain receives pain signals through
these unusual channels, it is unsure of the exact location of the pain, so it tells
you that the pain is coming from areas on that side of your face and head that
are far removed from the actual source. Pain like this is called "referred"
pain. It seems to shoot up the side of the head, or makes your eye ache.
How are dry sockets treated? Left alone, dry sockets will always
heal. It takes a month or two, and the pain is persistent for the entire period of
healing. Antibiotics are not useful in curing a dry socket, and the usual pain
medications are not very effective. It is better to go back to the dentist who
extracted the tooth and let him or her "pack" the socket. This is a procedure
done (usually) without anesthesia even though it can be painful, because it
does not take too long, and the pain relief is almost complete, beginning an
hour or so after the socket is packed. The first packing will provide relief for
about 24 hours. As you return to the dentist and the old packing is removed,
114
the socket washed out and new packing is placed in, each succeeding packing
debrides (cleans) the socket and renews the pain relief. A second packing
may last 24 to 48 hours, and succeeding packings last longer still. Within a
week (or sometimes more depending on the severity of the dry socket), The
socket begins to heal from the bottom up and can be left empty without pain.
4. Broken Jaws. Yes, it does occasionally happen. The fracture of a lower
jaw is unusual, principally because dentists who extract teeth routinely do not
place great force on any instrument to remove a tooth. Teeth are generally
"finessed out" with a minimum of pressure applied to the jaw through the
surgical instruments. There are, however, some situations in which a dentist can
look at the x-ray and see that the jawbone that surrounds the tooth is much more
fragile than is usually the case, and will usually warn the patient that fracture of
the jaw is a possibility. People are not like cars, every one identical. Everyone is
unique and presents unique circumstances under which the dentist must labor.
The chances that the removal of any given tooth will result in a fractured
lower jaw run about the same for any dentist who attempts the extraction.
That particular patient is usually more prone than other people to a broken jaw
due to any traumatic incident such as a traffic accident or a blow to the jaw
during a sporting event. Unfortunate, but true, and a fact of life for any dentist
who extracts teeth.
5. Sinus perforation. The image to the right is a detail from a panoramic
film. The roots of the upper back teeth are always in close approximation to the
maxillary sinus. Since everyone is built differently, The roots of the teeth may
actually appear to be inside the sinus. There is always a thin wall of bone
between the root and the sinus, but is can be very thin indeed. Most of the time,
the bone remains intact, but upon occasion, a piece of the bone separating the
root from the sinus may break off and be removed with the tooth. This creates a
direct connection between the sinus and the mouth! That means that you would
be unable to suck on a straw, because air would rush into your mouth from your
nose through the socket.
Sometimes a sinus perforation will go unnoticed by the dentist or the
patient. If the perforation is small, the only symptom could be a nosebleed. If
this happens, call the dentist so he can prescribe the proper drugs so that healing
can proceed normally
When a sinus perforation occurs, the dentist will prescribe an antibiotic to
prevent infection and a decongestant to keep the sinuses clear during healing.
The patient bites on his gauze as is usual after any extraction, and a clot will
form in the socket as usual. If nothing disturbs the clot, it will organize during
healing and close the perforation. Dry sockets rarely happen after extraction of
upper teeth unless the patient smokes.
It is IMPERATIVE, however that the patient do NOTHING that could
disturb the clot.
Do not suck on anything for at least a week. This puts pressure on the clot
115
and could dislodge it into the mouth.
Do not smoke the longer you wait the better. This will dissolve the clot, or
could even suck it out of the socket.
Do not blow up balloons or anything else. This puts pressure on the clot
and could dislodge it into the sinus.
Avoid sneezing. This explosive event will definitely dislodge the clot.
In the case of very large perforations, or in case the clot dislodges and a
perforation between the sinus and the mouth remains after healing, It may
be necessary to perform a further surgical procedure in order to draw a
flap of gum tissue over the perforation to close it permanently.
6. Sequestrii (Broken bone fragments that come out weeks after the
extraction, but are often mistaken for pieces of tooth.)
Whenever a dentist extracts a tooth, it requires that the bone that used to
hold the tooth be expanded, or sometimes even fractured to allow the tooth to
slip out of the socket. Most of the time, these fractures are of the type known as
"greenstick" fractures which means they are only partial fractures immediately
around the top of the socket leaving the bone fragments still attached to the main
body of the bony structure beneath. In some instances, these greenstick fractures
coalesce to release a bone fragment completely from the underlying bony
structure. Even when this happens, the bone fragments tend to heal and reattach
to the main body of the bone during healing.
In the oral cavity, however, the presence of oral bacteria, as well as
noxious chemicals from the foods we eat and cigarettes we smoke can cause the
healing to cease. This is what causes dry sockets. Bony fragments that do not
heal properly often loose their blood supply and become "necrotic" (dead
tissue). Thus, the body begins the process of ejecting them from the healing
socket, a process known as sequestration. The process can be painful, and
sometimes requires the dentist to reenter the socket to remove the sequestrum.
When the sequestrum comes out on its own, the patient often mistakes this piece
of bone for a piece of tooth that the dentist left in the socket.
Sequestrii are a normal complication of extractions. They are often
unavoidable, and undetectable at the time of the extraction. They are not
considered to be a mistake the dentist made. Once the sequestrum is gone, the
healing resumes, the pain subsides and all is well.
7. Retained roots (Pieces of tooth left in the bone by the dentist)oved it all.
Do ALL extracted teeth HAVE to be replaced?
The removal of any tooth has consequences, some of which are important
enough to cause you to seriously consider replacing that tooth with a removable
or fixed alternative. If it's one of your top front teeth, then esthetic
considerations will probably cause you to want to replace it. But even then, if
you don't care about how you look, leaving the space will not kill you. The x-ray
116
below shows what happens to the adjacent teeth if a first molar is extracted
when a patient is very young. There IS tilting of the teeth and a small collapse of
the occlusion, but it is not especially bvious when you look at the teeth in the
mouth. I am going to guess that at least a third of my adult patients have lost
back teeth in the past and have never had them replaced. A vast majority suffer
no major problems eating, speaking or esthetically (The way they look). On the
other hand, a few, especially some women, tend to develop the joint problems,
headaches, neck aches or ear aches typical of TMJ. If they use a lot of sugar,
they are more prone to ectopic decay (explained below). In addition, many of
these people who later want to repair the damage caused by the loss of the tooth
find that repair is much more expensive because of the movement in the
adjacent and opposing teeth.
The removal of any tooth will always cause destabilization of the
remaining teeth and over a period of years, every tooth in your mouth will
move in response to its loss, at least a little. The amount of movement
depends upon several factors:
Your age: The younger you are when the tooth is removed, the
more quickly and severely the rest of your teeth will move in
response.
The position of the tooth in the mouth: The loss of any back tooth (the
canine tooth and behind) will have a greater effect on the movement of the
remaining teeth than the loss of a front tooth. The removal of the last tooth in the
arch will not effect the position of any tooth in front of it. It may, however allow
hypereruption ("extrusion") of the tooth above or below the missing tooth if that
tooth does not make contact with a tooth in the opposite arch. Finally, the
majority of the movement in the remaining teeth happens on the same side as the
missing tooth. Teeth on the oposite side of the dental arch are effected, but not
nearly as much.
Wisdom teeth. Wisdom teeth usually emerge from the gum between the
ages of 17 and 24. They are the last of the molar teeth, which are the large
grinding teeth at the back of the mouth. Some people never develop wisdom
teeth and others have up to four - one in each corner of the mouth.
Wisdom teeth usually cause no problems. They are described as impacted
when there is not enough space for them at the back of the mouth. Impacted
wisdom teeth can cause pain, swelling, infection or damage to the teeth next to
them. If the gum around the wisdom tooth is swollen the jaw may become stiff
and sore. Infection at the back of the mouth can cause bad breath and a bad taste.
The surgical removal (extraction) of one or more wisdom teeth can relieve
these problems. However, removing the wisdom teeth does not usually improve
crookedness or crowding of other teeth.
If you have problems such as infection, cysts, tooth decay or gum disease
around a wisdom tooth you may think about having it removed.
117
If you have impacted wisdom teeth that are not causing problems, you
don't need to have them removed.
Alternatives: Having wisdom teeth removed is often the only way to
permanently relieve painful symptoms. Although antibiotics can provide
temporary relief, pain tends to flare up again in the future.
In some cases, where a wisdom tooth is causing pain because it is pressing
into the surrounding gum, removal may not be necessary - an operation to cut
back the gum may be all that is needed. However, this alternative is not suitable
for everyone.
Operation:You may have your wisdom teeth removed under local
anaesthesia by a dentist or oral surgeon. This means you are awake, but the area
around the wisdom tooth is completely numb. Sedative drugs can be given with
a local anaesthetic to help you relax during the procedure.
Sometimes wisdom teeth are removed under general anaesthesia. This
means you are asleep during the procedure. This has to be done in hospital,
usually as a day case. Typically, you will be asked not to eat or drink for about
six hours before general anaesthesia. However, some anaesthetists allow a few
sips of water until two hours beforehand.
The operation will not start until the anaesthetic has taken effect. It is
often necessary to make a small cut in the gum over the wisdom tooth, and to
remove some bone so that the tooth can be lifted out. Stitches are usually put in
to help the gum heal.
Eating and drinking. To begin with, you should eat soft foods, gradually
returning to a normal diet once your jaw feels less stiff. Don't drink alcohol or
hot fluids such as tea or coffee, and don't eat spicy food or smoke until the gum
has fully healed. These can make the wound bleed and will delay healing.
Bleeding. If your gum bleeds, fold a clean handkerchief or piece of gauze,
place it on the bleeding gum and bite on it for at least 20 minutes. Don't rinse
your mouth out or lie down until the bleeding has stopped.
Most people experience no problems after having their wisdom teeth removed.
However, contact your dentist immediately if you develop any of the following
symptoms:
bleeding that doesn't stop after applying pressure, or that lasts for more
than half an hour
difficulty in breathing or swallowing
severe pain that is not helped by painkillers
high temperature
face continues to swell three days after surgery
Risks: The extraction of wisdom teeth is a commonly performed and
generally safe procedure. For most people, the benefits - treatment of pain,
118
decay and infection - are greater than any disadvantages. However, in order to
make an informed decision and give your consent, you need to be aware of the
possible side-effects and the risk of complications.
Side-effects. These are the unwanted but mostly temporary effects of a
successful procedure. Examples of side-effects include feeling sick as a result of
a general anaesthetic and occasional bleeding from the gums, which can last 12
hours or more. You may have some facial swelling, pain and jaw stiffness for up
to two weeks.
Complications. Complications are when problems occur during or after
the operation. Most people are not affected. The main possible complications of
any surgery include excessive bleeding during or soon after the operation,
infection, and an unexpected reaction to the anaesthetic. Complications may
require further treatment such as having another operation to stop bleeding, or
antibiotics to treat an infection.
Specific complications of having wisdom teeth extracted are uncommon
but may include accidental damage to other teeth.
It's possible to develop a condition called dry socket. This is when the
blood clot breaks away from the wound exposing the bone and nerves to air,
food and fluids. This can cause pain and delay healing. This usually occurs two
days after surgery and can last about 5 to 7 days.
Occasionally nerves in the jaw can be damaged, either by the surgery or
by swelling afterwards. This can cause temporary numbness or "pins and
needles" in the lower lip or tongue after having lower wisdom teeth removed.
There's a small chance this altered sensation could be permanent.
The risk of complications depends on the exact position of the wisdom
teeth, the type of anaesthetic used and other factors such as your general health.
This is one of the reasons why we have not included statistics here. Ask your
surgeon to explain how these risks apply to you.
Methods:
Closed method (forcep extraction, intraalveolar method): having separated
the rest of the periodontium from bone and tooth, the tooth is delivered with the
help of a forcep.
Open method (extra alveolar): Persiosteum is separated from the bone, the
investing bone is cut and the tooth is removed with the help of a forceps or
elevator in toto or in pieces.
The dentist has a variety of means at his/her disposal to address bleeding,
however, it is important to note that small amounts of blood mixed in the saliva
after extractions are normal—even up to 48 hours after extraction.
Often dictated by the amount of surgery performed to extract a tooth (e.g.
surgical insult to the tissues both hard and soft surrounding a tooth). Generally,
when a surgical flap must be elevated periosteum covering the bone is thus
injured), minor to moderate swelling will occur. A poorly-cut soft tissue flap, for
119
instance, where the periosteum is torn off rather than cleanly elevated off the
underlying bone will often increase such swelling. Similarly, when bone must be
removed using a drill, more swelling is likely to occur.
Maxillary sinus sits right above the roots of maxillary molars and
premolars. There is a bony floor of the sinus dividing the tooth socket from the
sinus itself. This bone can range from thick to thin from tooth to tooth from
patient to patient. In some cases it is absent and the root is in fact in the sinus. At
other times, this bone may be removed with the tooth, or may be perforated
during surgical extractions. The doctor typically mentions this risk to patients,
based on evaluation of radiographs showing the relationship of the tooth to the
sinus. It is important to note that the sinus cavity is lined with a membrane
called the Sniderian membrane, which may or may not be perforated. If this
membrane is exposed after an extraction, but remains intact, a “sinus exposed”
has occurred. If the membrane is perforated, however, it is a “sinus
communication”. These two conditions are treated differently. In the event of a
sinus communication, the dentist may decide to let it heal on its own or may
need to surgically obtain primary closure—depending on the size of the
exposure as well as the likelihood of the patient to heal. In both cases, a
resorbable material called “gelfoam” is typically placed in the extraction site to
promote clotting and serve as a framework for granulation tissue to accumulate.
Patients are typically provided with prescriptions for antibiotics that cover sinus
bacterial flora, decongestants, as well as careful instructions to follow during the
healing period.
Nerve injury: This is primarily an issue with extraction of third molars,
however, can technically occur with the extraction of any tooth should the nerve
be in close proximity to the surgical site. Two nerves are typically of concern,
and are found in duplicate (one left and one right side): 1. the inferior alveolar
nerve, which enters the mandible at the mandibular foramen and exits the
mandible at the sides of the chin from the mental foramen. This nerve supplies
sensation to the lower teeth on the right or left half of the dental arch, as well as
sense of touch to the right or left half of the chin and lower lip. 2. The lingual
nerve (one right and one left side), which branches off the mandibular branches
of the trigeminal nerve and courses just inside the jaw bone, entering the tongue
and supplying sense of touch and taste to the right and left half of the anterior
2/3 of the tongue as well as the lingual gingiva (i.e. the gums on the inside
surface of the dental arch). Such injuries can occur while lifting teeth (typically
the inferior alveolar), but are most commonly caused by inadvertent damage
with a surgical drill. Such injuries are rare and are usually temporary, but
depending on the type of injury (i.e. Seddon classification: neuropraxia,
axonotmesis, & neurotmesis), can be prolonged or even permanent.
Displacement of tooth or part of tooth into the maxillary sinus (upper
teeth only). In such cases, almost always the tooth or tooth fragment must be
retrieved. In some cases, the sinus cavity can be irrigated with saline (antral
lavage) and the tooth fragment may be brought back to the site of the opening
through which it entered the sinus, and may be retrievable. At other times, a
120
window must be made into the sinus in the canine fossa—a procedure referred
to as “Caldwell luc”.
Wisdom tooth removal. Wisdom teeth usually emerge from the gum
(erupt) between the ages of 17 and 24. They are the last of the molar teeth,
which are the large grinding teeth at the back of the mouth. Some people never
develop wisdom teeth, others have up to four - one in each corner of the mouth.
Impacted teeth
The surgical removal (extraction) of one or more wisdom teeth can relieve
these problems. However, removing the wisdom teeth does not usually improve
crookedness or crowding in other teeth.
People who have problems such as infection, cysts or tumours, tooth
decay, or gum disease around a wisdom tooth should think about having it
removed.
What are the alternatives?
Having wisdom teeth removed is often the only way to permanently
relieve painful symptoms. Although antibiotics can provide temporary relief, the
symptoms tend to flare up again in the future.
In some cases, where a wisdom tooth is causing pain because it is pressing
into the surrounding gum, removal may not be necessary - an operation to cut
back the gum may be all that is needed. However, this alternative is not suitable
for everyone.
The operation. Many people have their wisdom teeth removed under local
anaesthesia by a general dentist or oral surgeon. This means that they are awake,
but the area around the wisdom tooth is completely numb. Sedative drugs can be
given with local anaesthesia to help people relax during the procedure.
Some people have their wisdom teeth removed under general anaesthesia.
This means that they are asleep throughout the procedure. This has to be done in
hospital, but it's almost always carried out as a day case, requiring no overnight
stay. Typically, patients are asked not to eat or drink for about six hours before
general anaesthesia. However, some anaesthetists allow a few sips of water until
two hours beforehand.
The operation will not start until the anaesthetic has taken effect. It is
often necessary to make a small cut in the gum over the wisdom tooth, and to
remove some bone so that the tooth can be lifted out. Stitches are usually put in
to help the gum heal.
What to expect afterwards
It will be necessary to rest for a while after general anaesthesia or
sedation. The jaw may feel stiff and sore, but painkillers will help to relieve
discomfort.
Most people can go home as soon as they have recovered from the anaesthesia. However, if you have had general anaesthesia or sedation, you will need
to arrange for someone to drive you home and stay with you for at least 24
hours.
121
General anaesthesia can temporarily affect co-ordination and reasoning
skills, so you should not drive, drink alcohol, operate machinery or sign legal
documents for 48 hours afterwards.
You may be given painkillers, antibiotics and mouthwash solutions to take
home. Once home, the painkillers should be taken as advised by the surgeon and
nurses. Any pain, swelling or stiffness is usually at its worst two or three days
after the operation and then gradually improves.
Do not vigorously rinse your mouth out during the first 24 hours because
this disturbs the blood clots that are part of the healing process. After meals,
rinse gently with warm salt water (one teaspoon of table salt to a glass).
At first, it may be possible to feel small fragments of bone with your
tongue. These are the edges of the tooth socket and will soon disappear as the
gum heals.
Depending on the type of stitches used, they may need to be removed
(arrangements will be made for this to be done). If dissolvable stitches have
been used, they will disappear 7 to 10 days after the operation.
To begin with, you should eat soft foods, gradually returning to a normal
diet once any jaw stiffness has settled. Very hot drinks and spicy food can
increase pain and bleeding and should be avoided until the gum has healed.
Drinking alcohol and smoking should also be avoided as they can increase
bleeding and delay healing.
Anyone who experiences increased bleeding should fold a clean
handkerchief or piece of gauze, place it on the bleeding gum and - in a sitting
position - bite on it for at least 20 minutes. It is important not to rinse your
mouth out or lie down.
Most people experience no problems following an operation to remove
wisdom teeth. However, contact your dentist or the hospital immediately if you
develop any of the following:
bleeding that doesn't stop after applying pressure, or that lasts for more
than half an hour
difficulty breathing or swallowing
a face that continues to swell more than three days after the operation
a fever or high temperature
severe pain that is not relieved by painkillers
These symptoms may indicate that you have an infection or another
problem.
Side-effects and complications
The extraction of wisdom teeth is a commonly performed and generally
safe procedure. For most people, the benefits - treatment of pain, decay and
infection - are greater than any disadvantages. However, in order to make an
informed decision, anyone deciding whether or not to have this procedure needs
to be aware of the possible side-effects and the risk of complications.
Side-effects
122
These are the unwanted but usually mild and temporary effects of a
successful procedure. Examples of side-effects include feeling sick as a result of
the anaesthetic and occasional bleeding from the gums, which can last 12 hours
or more. There may also be some facial swelling, pain and jaw stiffness, which
can last for several days.
Complications
Complications are problems that can occur during or after the operation.
Most people are not affected. The main possible complications of any surgery
include excessive bleeding during or soon after the operation, infection, and an
unexpected reaction to the anaesthetic. Complications may require further
treatment such as having another operation to stop bleeding, or antibiotics to
treat an infection.
Specific complications of having wisdom teeth extracted are uncommon
but may include accidental damage to other teeth.
Occasionally nerves in the jaw can be damaged, either by the surgery or
by swelling afterwards. This can cause temporary numbness or "pins and
needles" in the lower lip or tongue after lower wisdom teeth have been removed.
In a small number of cases this altered sensation is permanent.
The risk of complications depends on the exact position of the wisdom
teeth, the type of anaesthetic used and other factors such as the person's general
health. Your surgeon will be able to explain how these risks apply to you.
Surgical removal of third molars
The surgical removal of teeth is one of the four surgical operations
included in both top 10 day case and inpatient NHS procedures for England and
Wales. The other three procedures, for 1989-90, were endoscopic operations on
the upper gastrointestinal tract and bladder and evacuation of the contents of the
uterus. Surprisingly, in the last year for which statistics are available (1989-90)
more than twice as many people (60 000) were admitted for the surgical removal
of teeth as were treated as day cases (28 000). For inpatient procedures, the
surgical removal of teeth was ninth in frequency behind vasectomy. The surgical
removal of third molars (wisdom teeth) accounted for 70% of these procedures
in 1989-90. In addition, 67 000 people had their third molars removed by dental
practitioners in the general dental service and 22 000 had their third molars
removed in the private sector. The total cost of third molar removal in the NHS
in 1989-90 was estimated as pounds sterling 23.3m and in the year ended 30
June 1992 was pounds sterling 22m in the private sector. In the hospital service,
patients waiting for third molar removal account for up to 90% of patients on
waiting lists in oral and maxillofacial surgery. Although patient throughput has
increased year on year since 1985, in 1990 the oral and maxillofacial surgery
waiting lists remained among the longest of any surgical specialty.
Despite the very large number of third molars that are being removed,
audit suggests that rates of surgical intervention could be reduced and that more
rational decision making is needed. Although prophylactic removal has
previously been criticised and the cost-benefit ratio of this procedure is very
poor, little evidence exists of a link between levels of morbidity and
123
intervention. Wide small area variation in operation rates for the South West
Regional Health Authority has been reported, and recent comparisons of
treatment decisions with National Institutes of Health consensus criteria for
intervention have shown that about a fifth of patients not meeting these criteria
were nevertheless scheduled for surgery.
A recent literature review concluded that "prophylactic surgery is not an
appropriate management strategy for third molars." The wholesale removal of
unerupted teeth seems as inappropriate as the wholesale removal of tonsils and
adenoids. In terms of health gain, the scales are loaded against intervention even
in the presence of mild pericoronitis (inflammation around the crown).
Previously, prophylactic surgery has been justified on the basis that third
molars have no role in the mouth, notwithstanding that few people would
contemplate the prophylactic removal of their appendix, which, unlike many
unerupted third molars, communicates with the alimentary tract throughout life.
Prophylactic removal has also been justified on the basis that unerupted teeth
contributed to facial pain and even that the presence of an unerupted tooth
weakens the lower jaw such that it is likely to fracture; no objective evidence
exists to substantiate these assertions.
No reliable evidence is available on trends in the incidence and severity of
infections associated with the eruption of third molars, but the number of third
molars surgically removed by family dental practitioners has increased by 30%
since 1988. Good reasons exist why the number of impacted unerupted teeth are
increasing, including improved dental health leading to fewer extractions of
standing teeth and therefore decreased space for third molars to erupt.
Surveillance has also improved, as more people now attend for dental treatment;
payment by capitation has been introduced into dentistry, and the use of panoral
x ray machines is increasing.
The indications for removing third molars was the subject of a National
Institutes of Health consensus conference held in the United States in 1979. The
consensus criteria for surgical intervention were recurrent pericoronitis, caries
not amenable to restorative measures, dentigerous cyst, internal or external
resorption, and periodontal disease to which the third molar was contributing.
Overall, pericoronitis is the reason for intervention in about one fifth of
removals, though a study of more than 16 000 lower third molars showed that
only 8% had been removed for this problem.
Recent evidence suggests that the teeth at most risk are partially erupted,
vertically placed mandibular third molars.
The prevalence of periodontitis associated with third molars is also low reportedly about 5% in studies of 1200 and 1800 impacted third molars. In
relation to resorption of the adjacent second molar, prevalence has been
estimated at about 2%. Crowding of anterior teeth has previously been
attributed, at least in part, to the eruption of third molars, but recent findings and
reviews all strongly suggest no causal link. The prevalence of cystic change has
been found to be about 2-4%. There are many reports of an association between
facial pain and the presence of unerupted third molars, though there is no
124
evidence of a causal link. There is enormous potential for mistaken diagnoses
and unnecessary surgery: regular dental surveillance, both clinical and
radiological, is the cornerstone of modern preventive dentistry, and facial pain is
a common complaint, particularly in young adults. Radiological surveys of the
mouth and jaws have shown that about one in five people in their 30s have at
least one unerupted third molar and that these can remain in situ throughout life
without pathological change.
The complications associated with the removal of unerupted third molars
should not be underestimated. The surgery entails incision, stripping of
periosteum, bone and tooth removal, and suturing. Pain, swelling, and trismus
are almost universal after this procedure, and the incidence of both inferior
dental and lingual nerve damage is high. After surgical removal of lower third
molars, 5-15% of patients suffer some numbness of the anterior two thirds of
tongue and ipsilateral lower lip, and lingual numbness is permanent in about
0.5% of cases.
Surprisingly, until very recently no studies of the preferences of patients
have been carried out. Recent evidence, however, suggests that the
disadvantages and complications of surgery are generally considered by patients
as more serious than those of non-intervention. In any event, the prophylactic
removal of third molars should be abandoned. If surgery was carried out only
where National Institutes of Health consensus criteria existed then surgical
morbidity and costs would be reduced substantially
DISEASES OF MUCOUS MEMBRANE OF ORAL CAVITY
Inflammation of the mucous lining of any of the structures in the mouth,
which may involve the cheeks, gums, tongue, lips, and roof or floor of the
mouth. The word "stomatitis" literally means inflammation of the mouth. The
inflammation can be caused by conditions in the mouth itself, such as poor oral
hygiene, poorly fitted dentures, or from mouth burns from hot food or drinks, or
by conditions that affect the entire body, such as medications, allergic reactions,
or infections.
Description. Stomatitis is an inflammation of the lining of any of the softtissue structures of the mouth. Stomatitis is usually a painful condition,
associated with redness, swelling, and occasional bleeding from the affected
area. Bad breath (halitosis) may also accompany the condition. Stomatitis affects
all age groups, from the infant to the elderly.
Causes and symptoms. A number of factors can cause stomatitis; it is a
fairly common problem in the general adult population in North America.
Poorly fitted oral appliances, cheek biting, or jagged teeth can persistently
irritate the oral structures. Chronic mouth breathing due to plugged nasal
airways can cause dryness of the mouth tissues, which in turn leads to irritation.
Drinking beverages that are too hot can burn the mouth, leading to irritation and
pain. Diseases, such as herpetic infections (the common cold sore), gonorrhea,
measles, leukemia, AIDS, and lack of vitamin C can present with oral signs.
125
Other systemic diseases associated with stomatitis include inflammatory bowel
disease (IBD) and Behçet's syndrome, an inflammatory multisystem
disorder of unknown cause.
Diagnosis. Diagnosis of stomatitis can be difficult. A patient's history may
disclose a dietary deficiency, a systemic disease, or contact with materials
causing an allergic reaction. A physical examination is done to evaluate the oral
lesions and other skin problems. Blood tests may be done to determine if any
infection is present. Scrapings of the lining of the mouth may be sent to the
laboratory for microscopic evaluation, or cultures of the mouth may be done to
determine if an infectious agent may be the cause of the problem.
Treatment. The treatment of stomatitis is based on the problem causing it.
Local cleansing and good oral hygiene are fundamental. Sharp-edged foods such
as peanuts, tacos, and potato chips should be avoided. A soft-bristled toothbrush
should be used, and the teeth and gums should be brushed carefully; the patient
should avoid banging the toothbrush into the gums. Local factors, such as illfitting dental appliances or sharp teeth, can be corrected by a dentist. An
infectious cause can usually be treated with medication. Systemic problems,
such as AIDS, leukemia, and anemia are treated by the appropriate medical
specialist. Minor mouth burns from hot beverages or hot foods will usually
resolve on their own in a week or so. Chronic problems with aphthous stomatitis
are treated by first correcting any vitamin B12, iron, or folate deficiencies. If
those therapies are unsuccessful, medication can be prescribed which can be
applied to each aphthous ulcer with a cotton-tipped applicator. This therapy is
successful with a limited number of patients. More recently, low-power
treatment with a carbon dioxide laser has been found to relieve the discomfort of
recurrent aphthae. Major outbreaks of aphthous stomatitis can be treated with
tetracycline antibiotics or corticosteroids. Valacyclovir has been shown to be
effective in treating stomatitis caused by herpesviruses.
Patients may also be given topical anesthetics (usually a 2% lidocaine gel)
to relieve pain and a protective paste (Orabase) or a coating agent like
Kaopectate to protect eroded areas from further irritation from dentures, braces,
or teeth.
Alternative treatment. Alternate treatment of stomatitis mainly involves
prevention of the problem. Patients with such dental appliances as dentures should visit their dentist on a regular basis. Patients with systemic diseases or chronic medical problems need to ask their health care provider what types of oral
problems they can expect from their particular disease. These patients must also
contact their medical clinic at the first sign of problems. Common sense needs to
be exercised when consuming hot foods or drinks. Use of tobacco products
should be discouraged. Alcohol should be used in moderation. Mouthwashes
and toothpastes known to the patient to cause problems should be avoided.
Botanical medicine can assist in resolving stomatitis. One herb, calendula
(Calendula officinalis), in tincture form (an alcohol-based herbal extract) and
diluted for a mouth rinse, can be quite effective in treating aphthous stomatitis
and other manifestations of stomatitis.
126
More recently, a group of researchers in Brazil have reported that an
extract made from the leaves of Trichilia glabra, a plant found in South
America, is effective in killing several viruses that cause stomatitis.
Prognosis. The prognosis for the resolution of stomatitis is based on the
cause of the problem. Many local factors can be modified, treated, or avoided.
Infectious causes of stomatitis can usually be managed with medication, or, if
the problem is being caused by a certain drug, by changing the offending agent.
Prevention. Stomatitis caused by local irritants can be prevented by good
oral hygiene, regular dental checkups, and good dietary habits. Problems with
stomatitis caused by systemic disease can be minimized by good oral hygiene
and closely following the medical therapy prescribed by the patient's health care
provider.
Thrush is a form of stomatitis caused by fungi and characterized by
cream-colored or bluish patches on the tongue, mouth, or pharynx.
-Aphthous Stomatitis
A specific type of stomatitis presenting with shallow, painful ulcers. Also
known as Stomatitis or inflammation of the lining of the mouth, gums, or
tongue.
Recurrent aphthous ulcers (RAUs), or canker sores, are among the most
common oral mucosal lesions physicians and dentists observe. RAU is a
disorder of unknown etiology that causes clinically significant morbidity. One to
several discrete, shallow, painful ulcers are visible on the unattached mucous
membranes. Individual ulcers typically last 1-2 weeks. Large ulcers may last
several weeks to months.
Although the process is self-limited, in some individuals, the ulcer activity
is almost continuous. Similar ulcers can be noted in the genital region. Behзet
syndrome and inflammatory bowel disease are systemic diseases associated with
oral and genital RAUs.
Pathophysiology: RAU is classically divided into 3 clinical forms: RAU
minor, RAU major, and herpetiform RAU. RAU affects the following
nonkeratinized or poorly keratinized surfaces of the oral mucosa:
Labial and buccal mucosa
Maxillary and mandibular sulci
Unattached gingiva
Soft palate
Tonsillar fauces
Floor of the mouth
Ventral surface of the tongue
RAU minor is the most common form, accounting for 80% of all RAUs.
Discrete, painful, shallow, recurrent ulcers measuring 3 mm to smaller than 1
cm in diameter characterize this form. At any time, 1-5 ulcers can be present.
RAU minor occurs on the labial and buccal mucosa and on the floor of the
mouth. Lesions heal without scarring within 7-10 days.
127
RAU major. RAU is formerly known as periadenitis mucosa necrotica
recurrens. This form is less common than the others and is characterized by oval
ulcers 1-3 cm in diameter. In this relatively severe form, 1-10 major aphthae
may be present simultaneously. Ulcers are large and deep, they may coalesce,
and they often have a raised and irregular border. On healing, which may take as
long as 6 weeks, the ulcers leave extensive scarring, and severe distortion of oral
and pharyngeal mucosa may occur. This form most commonly affects the lips,
the soft palate, and the fauces.
Herpetiform RAU. This least common form has the smallest of the
aphthae, measuring 1-3 mm in diameter. The aphthae tend to occur in clusters
that may consist of tens or hundreds of minute ulcers. Clusters may be small and
localized, or they may be distributed throughout the soft mucosa of the oral
cavity.
Frequency:
In the US: RAUs are the most common oral mucosal disease in North
America. They affect 20% of the population, with the incidence rising to more
than 50% in certain groups of students in professional schools. Children from
high socioeconomic groups may be affected more than those from low
socioeconomic groups.
Internationally: RAUs occur worldwide and are reported on every
populated continent. RAUs affect 2-66% of the international population.
Mortality/Morbidity: Unless RAU is associated with a systemic disease,
such as Behзet syndrome or inflammatory bowel disease, it rarely leads to
clinically significant morbidity or mortality.
Sex: In children and in some adult communities who are affected, the
incidence of RAU is higher in female individuals than in male individuals.
Age:
RAU minor is the most common form of childhood RAU. About 1% of
American children may have RAUs, with onset before age 5 years. The
percentage of patients who are affected rises with age.
RAU major has a typical onset after puberty and persists for as long as 20
years.
Herpetiform RAU first occurs in the second decade of life; 67-85% of
persons have onset when younger than 30 years. The frequency and the severity
of episodes may increase during the third and fourth decades and then decrease
with advancing age.
Clinic
RAUs consist of 1 or several rounded, shallow, punched-out appearing,
painful oral ulcers that recur at intervals of a few days to a few months. To
evaluate oral ulcers as RAUs, ascertain the following information:
Nature of the lesions (number, size, duration, recurrence)
The prodromal stage begins with a pricking or burning sensation on the
mucosa.
The ulcers develop within 24-48 hours.
128
Pain lasts 3-4 days or until a thicker fibrinous cover develops or early
epithelialization occurs.
Healing is complete in 7-10 days.
Age of the patient at onset
Cutaneous or mucosal changes
Symptoms of other organ system involvement
Current medications
Host factors associated with RAU
Genetic - Family history evident in some cases
Hematinic deficiency - Iron, folic acid, or vitamin B-12 deficiencies
possible
Immune dysregulation - Possible role
Stress - Physical or emotional stress often reported by patients as
associated with recurrent outbreaks
Environmental factors associated with RAU
Local, chemical, or physical trauma may initiate ulcer development in
patients who are susceptible (pathergy).
Allergy may stimulate an outbreak.
The role of microbial infection is debated.
HIV infection (associated with lesions)
Aphthouslike oral ulcerations involving all 3 types of RAUs are observed.
About 66% of patients who are HIV positive have herpetiform and major RAUs.
Ulcerations must be distinguished from those caused by HIV medications and
fungal, viral, or bacterial infections.
Behзet syndrome (associated with lesions)
This complex, multisystemic inflammatory disorder of unknown cause is
characterized by recurrent oral aphthae and at least 2 of the following findings:
genital aphthae, synovitis, cutaneous pustular vasculitis, posterior uveitis, or
meningoencephalitis.
Oral aphthae of Behзet syndrome are similar to those in RAUs, though
they are more extensive and frequent.
The incidence is highest in Japan, Southeast Asia, the Middle East, and
southern Europe and in persons aged 30-40 years.
Behзet syndrome is strongly associated with human leukocyte antigen
B51 (HLA-B51).
Gluten-sensitive enteropathy
Less than 5% of patients with RAUs have gluten-sensitive enteropathy
(GSE), also known as celiac disease, or other minor mucosal abnormalities of
the small intestine.
Bowel symptoms may not be present, but patients may have folate
deficiency, and they sometimes have reticulin antibodies.
Physical: Regardless of the clinical form of RAU, ulcers are confined to
the nonkeratinized mucosa of the mouth, sparing the dorsum of the tongue, the
attached gingiva, and the hard palate mucosae that are keratinized. Although
129
patients often have submandibular lymphadenopathy, fever is rare. Most patients
are otherwise well.
RAU minor is characterized by 1-5 discrete, shallow ulcers smaller than 1
cm in diameter.
The ulcers are covered by a yellow-gray pseudomembrane (fibrinous
exudate) and are surrounded by an erythematous halo.
RAU major is characterized by oval ulcers that are larger (1-3 cm in
diameter) and deeper than those observed in RAU minor.
The ulcers may coalesce and often have a raised, irregular border.
Herpetiform RAU
Herpetiform RAU is characterized by crops of smaller ulcers measuring 3
mm in diameter, with sometimes more than 100 lesions present at 1 time.
The ulcers can coalesce to produce a widespread area of irregular ulceration.
Causes: Although the clinical characteristics of RAU are well defined, the
precise etiology and the pathogenesis of RAU remain unclear. Many
possibilities have been investigated. RAU is a multifactorial condition, and it is
likely that immune-mediated destruction of the epithelium is the final common
pathway in RAU pathogenesis. Host risk factors associated with RAU are
described below.
Genetics
A family history of RAUs is evident in some patients. A familial
connection includes a young age of onset and symptoms of increased severity.
RAU is highly correlated in identical twins.
Associations between specific HLA haplotypes and RAU have been
investigated. No consistent association has been demonstrated, most likely
because of the lack of any immunogenetic basis for RAU. However, host
susceptibility is clearly variable, with a polygenic inheritance pattern, and
penetrance depends on other factors.
Hematinic deficiency
In several studies, hematinic (iron, folic acid, vitamin B-12) deficiencies were
twice as common in patients with RAUs than in control subjects. As many as
20% of patients with RAU had a deficiency.
Serologic workup is warranted. Hemoglobin and RBC indices are not
sufficient in all cases.
Immune dysregulation
At present, no unifying theory of the immunopathogenesis of RAU exists.
Immune dysregulation may play a role.
Cytotoxic action of lymphocytes and monocytes on the oral epithelium
seems to cause the ulceration, but the trigger remains unclear.
On histologic analysis, RAU consists of mucosal ulcerations with mixed
inflammatory cell infiltrates. T-helper cells predominate in the preulcerative and
healing phases, whereas T-suppressor cells predominate in the ulcerative phase.
Other findings associated with immune dysregulation include the
following:
Reduced response of patients' lymphocytes to mitogens
130
Circulating immune complexes
Alterations in the activity of natural-killer (NK) cells in various stages of
disease
Increased adherence of neutrophils
Release of tumor necrosis factor-alpha (TNF-alpha)
Significant involvement of mast cells in the pathogenesis of RAU
Microbial infection
Researchers disagree about the role of microbes in the development of
RAUs. Emphasis has been on a microbial agent as a primary pathogen or an
antigenic stimulus.
Numerous studies have failed to provide strong evidence to support the
role of herpes simplex virus (HSV), human herpes virus (HHV), varicella-zoster
virus (VZV), or cytomegalovirus (CMV) in the development of aphthous ulcers.
RAU may be a T-cell–mediated response to antigens of Streptococcus sanguis
that cross-react with the mitochondrial heat shock proteins and induce oral
mucosa damage.
Helicobacter pylori has been detected in lesional tissue of ill-defined oral
ulcers, but the frequency of serum immunoglobulin G (IgG) antibodies to H
pylori is not increased in RAU.
Lab Studies:
CBC determination
Measurement of erythrocyte sedimentation rate (ESR)
Determination of iron, ferritin, folate, and vitamin B-12 levels
Potassium hydroxide (KOH) examination of the lesion
Tzanck smears, viral cultures, or even skin biopsy to exclude HSV
Other Tests:
The following procedures may be indicated if other disease is suspected:
Colonoscopy
Biopsy with hematoxylin-eosin stains and cultures
Swab the ulcer to obtain material for a polymerase chain reaction to
identify H pylori DNA.
Medical Care: RAUs are treated by using a variety of agents for palliative,
prophylactic, and curative purposes. Many of the treatments are used without
substantial research demonstrating therapeutic results.
Therapy for RAU must be directed by the extent of the condition, as
determined by the patient and the clinician. Patients often complain of great pain
when clinical examination reveals only a minor ulcer of 1-2 mm in diameter. In
addition, the frequency and the extent of involvement should direct therapy.
Topical regimens
Anti-inflammatory (eg, corticosteroids) and immunomodulatory agents
(eg, retinoids, cyclosporin) are used initially.
Topical gels
Creams
131
Pastes
Ointments
Sprays
Rinses
Adjuvant rinses limit inflammatory effect and reduce bacterial counts.
Chlorhexidine gluconate
Betadine, tetracycline, and dilute salt water rinses
Dilute hydrogen peroxide
Topical lidocaine or benzocaine
Systemic agents
Colchicine 0.6 mg 3 times a day (tid)
Cimetidine 200 mg 2 or 4 times a day (bid/qid)
Azathioprine (Imuran) 50 mg per day (qd)
Thalidomide (This is the only treatment the US Food and Drug
Administration [FDA] had approved for the treatment of major aphthae in
individuals with HIV infection.)
Miscellaneous
Bismuth subsalicylate (Kaopectate) may protect raw mucosa and
accelerates reepithelialization.
Multivitamins with iron are recommended but do not have any clear
benefit.
Eliminate sodium laurel sulfate from the patient's use.
Surgical Care: No surgical treatment has been used effectively because of
the recurrent nature of RAUs.
Diet:
An elimination diet may help control outbreaks by revealing allergic stimuli that
stimulate oral lesions.
A gluten-free diet helps patients with GSE (celiac disease) control outbreaks of
aphthae.
Patients with oral lesions should adhere to a diet of soft foods.
Advise avoidance of salt and spices to prevent unnecessary aphthae irritation.
Some patients report aphthae after exposure to English walnuts or pineapple. In
such cases, remission may be achieved by avoiding the inciting agent.
Conditions related to friction or trauma
Frictional keratosis
Chronic mechanical, thermal or chemical trauma may induce a
keratinising response in buccal mucosa (which is normally non-keratinising) and
hyperkeratosis
(excessive keratinisation) elsewhere. This may occur through activation of the
genes for keratin. The keratin becomes swollen, resulting in a spongy
appearance. Diagnosis is clinical and treatment normally involves eliminating
the cause and reviewing to ensure resolution. There may be a local cause such as
a sharp tooth or it may be habit related. Biopsy is undertaken where doubt exists.
The principal histopathological features are:
132
• regular epithelial maturation pattern
• hyperkeratosis, usually hyperparakeratosis
• parakeratin layer appears macerated and bacterial plaque is adherent
• acanthosis (widening of prickle cell layer).
Smoker's palatal keratosis. Smoker's palatal keratosis is also known as
nicotinic stomatitis. It is associated with any smoking habit but tends to be most
florid in pipe smokers. The diagnosis is restricted to palatal lesions. The
principal features are:
• palatal mucosa appears white and crazed as a result of keratosis
• red spots occur through blockage of minor salivary gland ducts
• histopathology shows keratin plugs in duct openings
• reversible if smoking habit stopped
• not regarded as a potentially premalignant disorder.
Fibrous hyperplasia and neoplasia. Chronic irritation to the oral mucosa
is common and often results in fibrous hyperplasia. Elimination of the cause of
irritation may reverse the process, resulting in shrinkage or resolution. Many
fibrous hyperplastic lesions are excised, however, because this is a simple and
rapid method of treatment. All such tissue should be forwarded for
histopathological examination to confirm the diagnosis.
Fibroepithelial polyp. A fibroepithelial polyp is typically a firm sessile
or pedunculated polyp that arises most commonly on the labial and buccal
mucosa as a result of mechanical trauma from the teeth or dentures. It is easily
excised under local analgesia but will recur unless the source of trauma is
corrected. Histopathologically there is a core of dense fibrous tissue covered by
stratified squamous epithelium. The latter often shows keratinisation, reactive to
trauma. Secondary ulceration may be seen.
Denture irritation hyperplasia. Denture irritation hyperplasia often
forms in relation to denture flanges that have become overextended because of
alveolar resorption. Folds of fibroepithelial tissue form in the vestibule.
Papillary hyperplasia may be seen in the palatal mucosa covered by a poorly
fitting denture.
Connective tissue neoplasms. Connective tissue neoplasms are
uncommon in the oral mucosa, but benign tumours including lipoma, neuroma
and fibroma may occur and have the appearance of fibrous hyperplasia.
Malignant soft tissue neoplasms are extremely rare but may arise from any
connective tissue in the oral cavity.
Ulceration. Oral uiceration is commonly caused by mechanical trauma. It
is also associated with systemic diseases, drug side effects and with infections.
Traumatic uiceration. An ulcer is a breach in the integrity of the
covering epithelium. Traumatic uiceration is common in the oral cavity. The
most frequent cause is mechanical injury from the teeth; such ulcers occur on
the buccal mucosa, lateral tongue and lower lip in the occlusal plane. Illfitting
dentures may also cause traumatic uiceration. Ulcers at other sites may be
caused by habits (e.g. fingernail picking in children) or even deliberate selfharm. Sharp foodstuffs may cause traumatic uiceration of the palate. Thermal
133
injuries are common at this site from over-hot drinks. Chemical causes of
traumatic uiceration include placing aspirin in the vestibule, and rinsing with
astringent chemicals. Traumatic ulcers are common on the lower lip and may
follow mechanical or thermal injury after inferior alveolar nerve block.
Clinical features
On clinical examination, traumatic ulcers typically are painful and surrounded
by erythema. The base is covered by fibrinous exudate and at a later stage by
granulation tissue and regenerating epithelium. Shape and location often give a
clue as to the cause. On gentle palpation, traumatic ulcers lack induration and
are tender.
Management. Management is to elicit an accurate history, document the
features of the ulcer, eliminate the cause if possible, provide symptomatic
treatment and review to ensure that healing takes place. Any ulcer that does not
heal within 3 weeks should be considered as suspicious and referred for
specialist opinion to exclude carcinoma.
Drug-related uiceration An increasing number of drugs are associated
with oral uiceration as an unwanted effect. Examples include nicorandil,
indometacin (indomethacin) and phenytoin. These tend to produce solitary or
multiple ulcers, often recurring at fixed sites. Where such a drug reaction is
suspected, the possibility of changing the medication believed to be responsible
should be raised with the patient's general practitioner, who needs to balance any
risks associated with such a change against the benefits to the patient. Cytotoxic
drugs cause oral uiceration through toxicity to the rapidly turning over cell
population in the oral epithelium. Direct application of legal and illegal drugs
for extended periods to the oral mucosa can produce severe uiceration at the site.
Infections.
Bacterial infections Bacterial infections of the oral mucosa are rare.
Treponema pallidum causes syphilis and mucosal lesions include primary
chancres, secondary snail track ulcers and tertiary areas of focal necrosis
(termed gumma). Syphilic leukoplakia may also result. Tuberculosis infection is
usually secondary to pulmonary lesions and presents as granular ulceration of
the posterior palate and dorsal tongue. Raised red-white mucosal plaques termed
lepromas are seen in established leprosy.
Viral infections Herpes simplex. Oral involvement in herpes simplex
(HSV) infection is commonly encountered, especially in children, and is most
often due to HSV type 1.
Primary herpetic gingivostomatitis. Initial infection results in primary
herpetic gingivostomatitis. Grey blisters, which rapidly break down to form
small ulcers, may be present anywhere on the oral mucosa and most frequently
involve the gingivae. Crusted blisters may also appear on the circumoral skin.
Infection is usually accompanied by a febrile illness, and bilateral tender
cervical lymphadenopathy is frequently present. Infection can be spread to the
fingers and conjunctiva by direct contact with the oral lesions, and advice should
be given to avoid this. Resolution occurs within 2 to 3 weeks. Rest, maintaining
fluid intake, chlorhexidine mouthwash to prevent secondary infection of the oral
134
lesions and advice about cross-infection risks should be given. Infants under 6
months are at special risk of developing central nervous system infection and
contact with infected siblings should be avoided. Prescription of systemic
aciclovir is only of benefit in the early stages of the infection; a reasonable guide
to this is the presence of intact vesicles. Diagnosis is usually made on clinical
grounds, where doubt exists, serology performed on samples of acute and
convalescent (2-3 weeks after onset of symptoms) blood should reveal a
significant rise (of the order of fourfold or greater) in IgG antibodies against
HSV in the later sample. Swabs, for culture, and smears for cytology (showing
ballooning of epithelial nuclei and/or multinucleate epithelial cells) may be
useful but usually only if taken early in the course of the disease, ideally from an
intact vesicle.
Herpes labialis (cold sores).
After primary infection, HSV may remain in the trigeminal ganglion and
low levels of virus are shed into the axoplasm thereafter. Local factors, such as
exposure of the lip to intense sunlight, and systemic factors, such as depressed
general immunity, result in herpes labialis (cold sores) in about 20-30% of
individuals. Crusted vesicular patches appear on the lips, nose and circumoral
skin. Aciclovir or penciclovir cream applied immediately to new sores is an
effective therapy. In a small number of individuals, recurrent intra-oral HSV
infection may occur, lesions most commonly affect the hard palate and attached
gingivae. Severe recurrent HSV infection may occur in the
immunocompromised and further investigation to exclude this possibility should
be performed.
Herpes zoster. Herpes zoster, the causative agent of chickenpox and
shingles, may involve the oral mucosa. Shingles tend to affect one or more
dermatomes of the trigeminal nerve and is an important cause of facial pain. In
the oral mucosa, rashes of grey vesicles restricted to the distribution of the
sensory nerves are seen. High-dose systemic aciclovir or famciclovir is usually
prescribed.
Coxsackievirus. Coxsackieviruses cause hand, foot and mouth disease and
herpangina. These manifest as vesicular eruptions and the management is
conservative. Lifelong immunity is normally conferred.
Epstein-Barr virus. Epstein-Barr virus causes hairy leukoplakia.
Human papillomavirus. Human papillomaviruses produce focal
proliferative
lesions referred to as squamous papillomas (warts). They may be sessile or show
finger-like projections. Histologically, fronds of keratotic squamous epithelium
are supported by delicate fibrovascular stroma. Papillomas on the fingers can be
the source of human papillomavirus, particularly for lesions on the lips and
circumoral skin. Orogenital transmission is also possible. Intra-oral papillomas
can be readily excised under local analgesia.
Human immunodeficiency virus. Initial infection by HIV may be
asymptomatic or may cause a febrile illness with diarrhoea. An oral eruption
clinically similar to primary herpetic stomatitis may occur at this time.
135
Numerous manifestations of established HIV infection are recognised. There are
several with strong associated oral manifestations.
Kaposi's sarcoma. This is caused by human herpesvirus 8 (HHV8), which
is endemic in Mediterranean regions. Initial mucosal lesions are flat brown
spots, which show haemosiderin deposition and vascular proliferation on biopsy.
They progress into raised, nodular purple-red lesions, most often found on the
palate, retromolar areas and gingivae. Oral lesions may precede the appearance
of skin lesions.
Hairy leukoplakia. This lesion has been associated with progression from
HIV infection to AIDS (acquired immunodeficiency syndrome) as the CD4 T
lymphocyte count falls. It is caused by proliferation of Epstein-Barr virus in the
lateral tongue epithelium and rarely elsewhere in the oral cavity. Warty ridged
or smooth white plaques are typical: sometimes extended papillary projections
are seen. The lesion is also found in HIVnegative immunosuppressed patients,
for example in renal transplant recipients. No treatment is required and the
lesion is not premalignant.
Erythematous candidiasis. This is a frequent manifestation of HIV
infection. It presents as white speckles on an erythematous background. Tongue
and palate are frequently affected. Treatment can be a problem because of the
development of resistant fungal strains in some patients. Hyperplastic and
pseudomembranous forms of candidiasis are also common in HIV infection.
HIV-related gingivitis. This may resemble acute necrotising ulcerative
gingivitis or present as a red lesion, termed linear gingival erythema.
HIV-related periodontitis. This manifests as unusual focal alveolar
destruction. Severe alveolitis and osteomyelitis with sequestration of teeth and
surrounding tissue may be seen in patients with advanced AIDS.
Other mucosal manifestations in HIV infection. Purpura results from
thrombocytopaenia; bacillary angiomatosis, atypical ulceration, melanotic
pigmentation, unusual infections and multiple viral papillomas are also seen.
Fungal infections. Candida albicans is the most common cause of fungal
infection in the oral cavity. It is a commensal organism carried by roughly half
the population and disease is caused by opportunistic overgrowth. Oral
candidiasis has been described as the 'disease of the diseased'. Local or systemic
predisposing factors should be identified and corrected whenever possible.
Diagnosis is generally based on clinical features and can be confirmed by
laboratory methods using material from oral swabs, smears or rinses. Where
quantification is required, saliva samples, the oral rinse or the imprint culture
techniques may be used. Other Candida sp. May also cause oral infection,
particularly in the immunocompromised. Treatment is based on the use of
topical antifungal agents. Both amphotericin and nystatin are available in the
form of lozenges/pastilles or a suspension; higher concentrations of nystatin are
sustained when pastilles are used. Patients suffering from xerostomia may find
suspensions or miconazole gel more pleasant to use; similarly this gel is
convenient for the treatment of denture-induced stomatitis as it can be applied
directly to the fitting surface of the denture. In addition to the use of an
136
antifungal agent, the patient should be advised to leave the denture out at night.
Acrylic dentures should be soaked overnight in a 0.1% solution of hypochlorite.
If a cobalt chromium denture is worn, it should be soaked for 15 minutes twice
daily in chlorhexidine. These measures should eradicate those microorganisms
adherent to the denture, which may be more heavily colonised than the mucosa.
Systemic antifungal agents (e.g. fluconazole) should be reserved for those cases
of oral candidiasis that do not respond to topical antifungals. There are an
increasing number of reports of resistance to azole antifungal drugs. These drugs
play a significant role in the treatment of candidal infections in the
immunocompromised. Before prescribing triazole or imidazole antifungal
agents, including miconazole, care should be taken to check for possible drug
interactions.
Angular cheilitis. Candida sp. alone, bacteria alone or a combination of
Candida and bacteria (Staphylococcus aureus, (5-haemolytic streptococci) may
cause angular stomatitis. Unless the classic golden yellow crusts associated with
S. aureus are present, treatment should be commenced with antifungal drugs,
e.g. a combined miconazole/hydrocortisone cream (miconazole has some
antibacterial properties). When clinical features indicate S. aureus infection,
mupirocin or fusidic acid creams are appropriate. If infra-oral candidiasis is
present, this must be treated concurrently or recurrence of the angular stomatitis
will occur. Iron deficiency is a significant aetiological factor in angular cheilitis.
Aspergillosis. Aspergillus sp. infection is sometimes encountered in the
maxillary sinus in severe immunosuppression or in association with zinccontaining endodontic material inappropriately extruded through the roots of
maxillary molars.
Chronic hyperplastic candidiasis. Oral white and red lesions may be seen
in oral infections with C. albicans. Chronic hyperplastic candidiasis is a
particular form of candidiasis that presents as a persistent white plaque that
cannot be scraped off. Smoking and continuous denture wearing are the main
predisposing factors, although the condition may also be associated with
reduced immunity.
Clinical features
• Dense white rough or nodular patch
• Typically found on the buccal mucosa adjacent to the angle of the mouth
• Often bilateral, may be multifocal
• Associated with smoking and poor denture hygiene habits.
Histopathological features
• Epithelial acanthosis and parakeratosis resulting in broad, blunt rete processes
• Candidal pseudohyphae penetrate the parakeratin layer
• Neutrophils form microabscesses in the parakeratin layer
• Intense diffuse chronic inflammatory infiltrate present in the lamina propria
• May regress following elimination of local predisposing factors and antifungal
therapy (often systemic antifungal drugs are used)
• If microscopic dysplasia found (-40% cases) then the lesion may be clinically
classified as candidal leukoplakia.
137
Median rhomboid glossitis. Median rhomboid glossitis is an abnormality
of the midline dorsal tongue where a lozenge-shaped, smooth or nodular red
flecked area of depapillated mucosa is found. A corresponding area of erythema
may be present on the palate. It is often (but not always) associated with
candidal infection and Candida can often be recovered. Further investigation
and treatment are usually unnecessary. Treatment, with topical antifungal
agents, is only normally indicated if the lesion gives rise to discomfort.
Candidiasis, Mucosal
Background: Candidosis describes a group of yeastlike fungal infections
involving the skin and mucous membranes. Infection is caused by Candida
species, typically, Candida albicans.
By tradition, the most commonly used classification of oral candidosis
divides the infection into 4 types including (1) acute pseudomembranous
candidosis (thrush), (2) acute atrophic (erythematous) candidosis, (3) chronic
hyperplastic candidosis, and (4) chronic atrophic (erythematous) candidosis.
Chronic hyperplastic candidosis was further subdivided into 4 groups
based on localization patterns and endocrine involvement including (1) chronic
oral candidosis (candidal leukoplakia), (2) endocrine candidosis syndrome, (3)
chronic localized mucocutaneous candidosis, and (4) chronic diffuse candidosis.
Thrush (acute pseudomembranous candidiasis) is the term used for the
multiple white-fleck appearance of acute candidiasis, which purportedly
resembles the appearance of the bird with the same name.
Erythematous candidosis is the term used for the red lesions of
candidiasis.
Pathophysiology: C albicans is the predominant causal organism of most
candidosis. Other species, including Candida krusei, have appeared in persons
who are severely immunocompromised. Candida glabrata is an emerging cause
of oropharyngeal candidosis in patients receiving radiation for head and neck
cancer. In patients with HIV infection, new species, such as Candida
dubliniensis and Candida inconspicua, have been recognized.
C albicans is a harmless commensal organism inhabiting the mouths of
almost 50% of the population; however, under suitable circumstances, it can
become an opportunistic pathogen. A suitable circumstance may be a
disturbance in the oral flora or a decrease in the immune defenses.
Acute pseudomembranous candidosis (thrush) . Thrush may be observed
in healthy neonates or in persons in whom antibiotics, corticosteroids, or
xerostomia disturb the oral microflora. Oropharyngeal thrush occasionally
complicates the use of corticosteroid inhalers. Immune defects, especially HIV
infection, immunosuppressive treatment, leukemias, lymphomas, cancer, and
diabetes, may predispose patients to candidal infection.
Erythematous candidosis. Erythematous candidosis may cause a sore red
mouth, especially of the tongue, in patients taking broad-spectrum
antimicrobials. It also may be a feature of HIV disease. Median rhomboid
glossitis is a red patch occurring in the middle of the dorsum in the posterior
138
area of the anterior two thirds of the tongue and especially is observed in
smokers and in those with HIV disease.
Chronic mucocutaneous candidosis. Chronic mucocutaneous candidosis
(CMC) describes a group of rare syndromes, which sometimes include a
definable immune defect, in which persistent mucocutaneous candidosis
responds poorly to topical treatment. Generally, the more severe the candidosis,
the greater the likelihood that immunologic defects (particularly of cellmediated immunity) can be identified. Recent studies suggest a defect in
cytokine (interleukin 2 and interferon) production in response to candidal and
some bacterial antigens, with reduced TH1 lymphocyte function and enhanced
TH2 activity (and increased interleukin 6), and reduced serum levels of
immunoglobulin G2 and immunoglobulin G4.
Sex: Candidosis is reported equally in males and females worldwide,
except in areas where males with HIV infection outnumber females.
Age: Candidosis predominantly occurs in middle-aged or older persons;
however, in those with HIV infection, candidal infection primarily occurs in the
third and fourth decades.
Causes: Members of the genus Candida are the causal organisms of
candidosis. Secretion of antimicrobial proteins and peptides is decreased in
saliva of patients with oral candidosis. The following factors affect candidal
carriage and infection:
Factors predisposing individuals to oral candidal infections are as follows:
Broad-spectrum antimicrobial therapy may predispose individuals to
stomatitis or glossitis caused by C albicans.
Topical, systemic, and aerosolized corticosteroid use may result in oral
yeast infection.
Smoking predisposes individuals to chronic atrophic candidosis and other
forms of candidosis.
Drugs with xerostomic adverse effects (eg, psychopharmaceuticals) are
associated with oral candidosis. Xerostomia (as in Sjugren syndrome and
after radiotherapy) predisposes individuals to candidosis.
Immunologic disorders: Candidosis is common in patients with HIV
infection and other secondary immunodeficiencies, including blood
dyscrasias, diabetes, and malignant disease.
CMC can be a feature of primary immune defects such as severe
combined immune deficiency syndrome.
Diabetes may predispose individuals to candidosis.
Lab Studies:
Quantitative saliva culture is useful in the diagnosis of oral candidosis.
Immunologic tests
Serologic tests
Hematologic investigations
Because oral candidosis frequently is associated with HIV disease,
nutritional deficiencies, diabetes, or blood dyscrasias, estimates of
139
corrected whole blood folate, vitamin B-12, serum ferritin, glucose,
hemoglobin, lymphocyte, and WBC counts can be important.
Tests, such as lymphocyte function, serum immunoglobulins, calcium
status, or parathyroid hormone levels, are unnecessary except in CMC.
Tests of thyroid or adrenocortical function are warranted in selected
individuals, since endocrine disorders may be associated with oral
candidosis.
HIV testing may be indicated.
Procedures:
Histopathology: Although swabs and smears are essential for a
microbiological diagnosis of a number of types of oral candidosis, when
candidal leukoplakia (chronic hyperplastic candidosis) is suspected, a biopsy
specimen should be taken
Medical Care: Attention to the underlying cause helps avoid prolonged or
repeated courses of treatment. If antibiotics or corticosteroids (oral or inhaled)
are the probable cause, reducing the dose or changing the treatment may help.
Intermittent or prolonged topical antifungal treatment may be necessary when
the underlying cause is unavoidable or incurable.
In patients with severe immunosuppression, prevention of colonization
and infection is the goal because the oropharyngeal region may be the primary
source of initial colonization and allows subsequent spread of the infection.
Denture plaque often contains Candida species. To prevent denture-induced
stomatitis, denture cleansing that includes removal of candidal organisms is a
necessary and important factor..
Chlorhexidine oral rinses also may be of some benefit in the control of
oral candidosis. It is important to note that clinical cure is not synonymous with
mycologic cure.
Diet: High-sucrose diets should be avoided. Drug Category: Antifungals -Currently available azoles are imidazoles (eg, clotrimazole, miconazole,
econazole, ketoconazole) and triazoles (eg, fluconazole, itraconazole), which are
synthetic antifungals with broad-spectrum activity against a number of yeasts
and fungi including candidal organisms. They are fungistatic and expensive.
They inhibit fungal cytochrome P450-dependent enzymes, which are essential
catalysts for the 14-demethylation of lanosterol in sterol biosynthesis and block
synthesis of ergosterol, the principal sterol in fungal cell membranes. One
adverse effect of azoles is accumulation of precursors of ergosterol, which may
have effects on their own.
Diazoles (eg, ketoconazole, miconazole) have more effect on mammalian
cytochromes than do triazoles (eg, fluconazole, itraconazole) and tend to have
more severe adverse effects. None of the azoles is entirely benign.
Hepatotoxicity may be common to all of them, and the potential for endocrine
toxicities exists, particularly at high doses. As with any new agent, novel
toxicities may be discovered.
140
Azoles are effective antifungals, but resistance increasingly is reported.
Development of cross-resistance of C albicans to different azoles during
treatment with a single azole derivative has been described.
Drug Name Clotrimazole (Lotrimin, Mycelex, Femizole, Gyne-Lotrimin)
-- Broad-spectrum antifungal agent that inhibits yeast growth by altering cell
membrane permeability, causing death of fungal cells. Reevaluate diagnosis if
no clinical improvement after 4 wk. Used as a topical agent only because of GI
and neurologic toxicity. As a 10-mg troche used 5 times/d, clotrimazole is
effective against oral candidiasis in some patients who are
immunocompromised. Less effective than other azoles in patients with HIV
infection.
Adult Dose For 10-mg troches: Hold in mouth and allow to dissolve over
a single 15- to 30-min period 5 times/d
Pediatric Dose
Children: Not establishedAdolescents: Administer as in
adults
Name Miconazole (Absorbine, Femizole, Lotrimin, Monistat, Maximum
Strength Desenex) -- For topical treatment of candidosis such as angular
stomatitis. Miconazole lacquer is effective for treatment of chronic atrophic
candidosis; chewing gum may be effective against intraoral candidosis.
Available for parenteral use against systemic mycoses, but injection contains
polyethoxylate castor oil, which may provoke allergic reactions.
Adult Dose Cream and lotion: Apply to affected areas bid for 2-6
wkPowder: Spray or sprinkle liberally on affected area bid
Pediatric Dose
Administer as in adults
Drug Name Econazole (Spectazole) -- Effective in cutaneous infections.
Interferes with RNA and protein synthesis and metabolism. Disrupts fungal cell
wall membrane permeability, causing fungal cell death.
Adult Dose Apply sparingly on affected areas qd/bid
Pediatric Dose
Administer as in adults
Prognosis:
Good for most infections in the immunocompetent host, but in patients who are
immunocompromised, antifungal resistance is commonplace
Oral tuberculosis.
The incidence of tuberculosis is once again on the increase in the UK
Multidrug resistant strains of Mycobacterium tuberculosis are emerging
Tuberculosis should always be considered as a possible cause of chronic
oral ulceration
Tuberculosis is a major cause of ill health and death worldwide, but has
been declining in incidence in industrialised countries until recently. A steady
decline in the numbers of tuberculosis notifications in England and Wales
ceased in the mid-1980s and increases were seen in the late 1980s and early
1990s.
141
The clinical presentation of tuberculosis may take many forms. However,
with the decline in numbers, tuberculous lesions of the oral cavity have become
so rare that they are frequently overlooked in the differential diagnosis of oral
lesions.
Primary tuberculosis in the oral cavity is a rare entity. Usually, the
microorganisms need a disruption of the oral mucosa to become pathogenic. In
this article the authors describe a clinical case of primary oral tuberculosis, on a
female of 52 years-old who suffered an exodontia 20 days before. The bacteria
identificated was Mycobacterium tuberculosis hominis. The microbiologic
identification is essential to assure the efficacy of the treatment.
Tuberculosis is one of the major causes of ill health and death worldwide.
Nevertheless, tuberculous lesions of the oral cavity are rare and can be a
diagnostic challenge, particularly in young immunocompetent patients. Most of
the cases are secondary to pulmonary disease and the primary form is
uncommon. In this paper, we present a case of primary oral tuberculosis,
affecting the floor of mouth in a 13-year-old Brazilian male patient. Oral
Diseases (2005) 11, 50–53 Keywords: tuberculosis; infectious disease; oral
lesion Introduction Tuberculosis is a re-emerging infectious granulomatous
disease caused mainly by Mycobacterium tuberculosis, an acid-fast bacillus that
is transmitted primarily via the respiratory route. Less frequently, tuberculosis
may also be caused by other two species of bacteria, M. bovis and M. africanum
(Samaranayake, 2002). According to the World Health Organization,
tuberculosis is responsible for death of approximately 2 million people each year
and it is estimated that between 2002 and 2020, approximately 1 billion people
will be newly infected, over 150 million people will get sick, and 36 million will
die because of tuberculosis. Tuberculosis has a definitive affinity for the lungs
causing primary disease. However, any part of the body can be affected,
including the mouth and normally these lesions are secondary to lung disease
(de Aguiar et al, 1997). Reports have shown that oral lesions occur in 0.05–5%
of the patients with uberculosis and frequently are secondary affecting more
usually elderly patients. On the other hand, the primary form is uncommon and
more usually affects young patients (Mignogna et al, 2000).
Tuberculosis is a re-emerging infectious disease, and infection by
Mycobacterium tuberculosis has been increasing in immunocompromised hosts,
including elderly persons. M. tuberculosis-infected persons may receive dental
treatment. To evaluate the risk of M. tuberculosis infection in dental clinics, we
examined the detection rates of M. tuberculosis in sample of mixed saliva,
dental plaque, extracted teeth, caries lesions, and denture plaque by nested
polymerase chain reaction (PCR). The detection rates by PCR in samples from
mixed saliva, dental plaque, caries lesions and denture plaque obtained from
tuberculosis patients were 98.0%, 92.0%, 89.0%, and 100%, respectively. The
detection rates by the culture method were 17.3%, 2.0%, 0%, and 0%,
respectively. M. tuberculosis also was detected from the nontuberculous
mycobacteria-infected
group.
Strains
of
Actinomyces
naeslundii,
Porphyromonas gingivalis, and Fusobacterium nucleatum inhibited the growth
142
of clinical strains of M. tuberculosis, but strains of Streptococcus mutans,
Streptococcus sanguinis, and Actinobacillus actinomycetemcomitans did not.
The present study concludes that the PCR method is essential for detecting
M. tuberculosis in oral samples.
The incidence of tuberculosis decreased significantly in the world after
the Second World War because of the development of various chemotherapeutic
agents and better nutrition and environmental improvement. However, the
reduction rate of Mycobacterium tuberculosis infection has decelerated recently.
Despite the World Health Organization's declaration that the spread of
tuberculosis is a global re-emerging infectious disease and the implementation
of strong tuberculosis-control initiatives, this highly infectious disease continues
to affect all vulnerable populations, including the elderly population. Numerous
outbreaks have been reported since 1985. Some reasons for the increase in
outbreaks include a pandemic infection by human immunodeficiency virus
(HIV) and increasing numbers of immunocompromised hosts. Infection by
multidrug- resistant tubercle bacillus (MDR-TB) is also a serious problem,
especially for immunocompromised individuals.
In dental clinics, oral health workers are at high risk for M. tuberculosis
infection because of close contact with patients and aerosol spread during the
dental treatment process. The guidelines for prevention of M. tuberculosis
infection from the Center of Disease Control at 1994 state that the risk of
infection by M. tuberculosis is low in health-care facilities, but relatively high in
dental care facilities. Patients infected with M. tuberculosis may visit dental
clinics with unrecognized symptoms of the infection. For prophylaxis against
infection by M. tuberculosis in dental clinics, it is essential to perform simple
and rapid screening for this microorganism in oral samples. For detecting
M. tuberculosis infection, conventional methods such as Ziehl–Neelsen stain or
culture with Ogawa medium are usually used. However, the detection sensitivity
of the staining is not high, and the culture method requires more than 3 weeks.
Recently, polymerase chain reaction (PCR) had enabled a rapid and simple
diagnostic system for the detection of M. tuberculosis.
In this study, we screened for M. tuberculosis in samples of mixed saliva,
dental plaque, caries lesions, and denture plaque and found that the PCR method
is essental for the detection of this microorganism in oral samples.
Subjects. A total of 93 tuberculosis patients hospitalized in the respiratory
unit of Tokyo City Fuchu Hospital from October 1997 to December 1998 were
recruited for this study. Informed consent for this survey was obtained from all
patients. The patients were diagnosed with tuberculosis by X-ray and tubercline.
None of the patients was HIV-seropositive, nor were any severely
immunocompromised patients included in this study. Of the 93 study subjects,
67 were men and 26 women, with an average age of 55. (range: 19–93).
Samples from 75 of the patients were tested for the presence of M. tuberculosis
before chemotherapy. Ten patients were also tested for the presence of
M. tuberculosis after they had finished chemotherapy. Eight tuberculosis
patients, who were found to be infected by nontuberculous mycobacteria such as
143
Mycobacterium avium by culture,were also tested for M. tuberculosis. In
addition, 10 healthy individuals were also tested for the presence of M.
tuberculosis by PCR.
To detect M. tuberculosis by culture we used Ogawa egg medium (Eiken
Chemical, Co., Ltd, Tokyo). The PCR method is described below.
Sample collection
From 75 tuberculosis patients, specimens from 50 dental plaques, 16
carious lesions, 75 mixed saliva, and seven denture plaques were examined by
culture and those from 37 dental plaques, nine carious lesions, 52 mixed saliva,
and four denture plaques were examined by PCR in this study. Saliva samples
were also obtained from patients infected with nontuberculous mycobacteria and
patients after chemotherapy. Samples of dental plaque were isolated by spoon
excavator from the mandibular right first molar. If the tooth was absent, samples
were obtained from the first molar in the maxilla or on the opposite side of the
mouth. Unstimulated saliva from the patients was also collected in test tubes.
Obtained samples were suspended in sterilized saline. To examine
contamination in caries lesions, extracted teeth with developed caries were
crushed and suspended in sterilized saline. The samples for the PCR method
were stored at –80°C until they were used for experiments.
Detection of M. tuberculosis by nested PCR
Each sample was treated with the N-acetyl-L-cysteine-NaOH (NALCNaOH) method and inoculated on Ogawa egg medium. For detection by PCR,
obtained samples were sedimented at 18 120 Ч g for 10 min. The precipitate was
treated with 0.1N NaOH-2 M NaCl-0.5% SDS and incubated at 95°C for
15 min. After removing the sediment, the sample was treated with
phenol:chloroform:isoamyl alcohol and then precipitated with ethanol. This
precipitate was dissolved with distilled water. Detection of M. tuberculosis was
performed by nested PCR according to the method described by Pierre et al. The
primers used in this study are listed in. Briefly, 10 μl of obtained sample was
added to a PCR reaction mixture containing Geneamp PCR gold buffer, 200 μM
dNTP, 1 μM specific primers, and 0.025 U/μl of AmpliTaq Gold. PCR reactions
were carried out using Gene Amp PCR System 9700 (PE Biosystems, Foster
City, CA) under the following conditions: denaturation at 95°C for 9 min, and
50 cycles of denaturation at 95°C for 1.5 min, hybridization at 60°C for 2 min,
and elongation at 72°C for 2 min with a 4-s increase in each step. PCR products
were electrophoresed on 2% agarose and visualized as a 155 bp band under UV
light after staining with ethidium bromide. Validation of the primer was
performed using typical oral bacteria such as Streptococcus mutans Ingbritt,
Streptococcus sobrinus 6715, Streptococcus sanguinis ATCC10556,
Streptococcus salivarius ATCC 9758 Actinomyces naeslundii ATCC 15987,
ATCC 19246, Actinobacillus actinomycetemcomitans 310a, Porphyromonas
gingivalis ATCC 33277, W83, Fusobacterium nucleatum 21, Prevotella
intermedia ATCC 25611, Prevotella oris ATCC 33573, Campylobacter rectus
ATCC 33238, Bacteroides forsythus ATCC 43037, Selenomonas sputigena
ATCC 33612, Treponema socranslii ATCC subsp. scoranskii ATCC 35536,
144
Treponema vincentii ATCC 35580, Treponema pectinovorum ATCC 33768 and
Treponema denticola ATCC 33520, ATCC 35405.
Growth inhibition of M. tuberculosis by oral bacteria
Growth inhibitory activity of oral bacteria against isolated M. tuberculosis
strains was assayed by the stab culture method described previously. Eight
strains of six oral bacterial species, including A. naeslundii ATCC 15987,
P. gingivalis ATCC 33277 and FDC381, and F. nucleatum ATCC25586,
S. mutans Ingbrid, S. sanguinis ATCC10556, A. actinomycetemcomintans
SUNY465 and ATCC 43718, were used for this experiment. The oral bacterial
strains were placed on Trypticase soy agar (BBL, Cockysville, MD) to a depth
of approximately 2 mm. These stab culture plates were incubated for 5 days in
an anaerobic chamber containing N2: 80%, H2: 10% and CO2: 10%. After the
culture plates were placed under ultraviolet light for 5 h, isolated M. tuberculosis
strains in Middlebrook 7H9 broth containing 1% agar were overlaid on the
plates. After incubation for 2 weeks, growth inhibition of isolated
M. tuberculosis strains was examined.
Sensitivity of isolated M. tuberculosis strains to chemotherapeutics
We detemined the sensitivities of seven chemotherapeutic drugs to
M. tuberculosis strains isolated in saliva samples from nine patients undergoing
chemotherapy. The examined chemotherapeutic drugs were streptomycin, paminosalicylic acid (PAS), isoniazid, kanamycin, ethionamide, rifampicin and
ethanbutol.
Syphilis
The lesion that develops at the primary site of infection with Treponema
pallidum is the chancre. Usually this occurs on the genitalia, but if patients have
engaged in oral sex, then the chancre may occur in the oral cavity. The
commonest site is on the lips, but chancres may also be found within the mouth.
Chancres associated with primary syphilis heal spontaneously. When,
however, the disease enters its secondary phase, patients may suffer oral
manifestations. Most common are 'snail-track' ulcers on the soft tissues of the
mouth.
A minority of patients with untreated syphilis go on to develop a tertiary
disease, long after initial infection. This is considered to be a hypersensitivity
reaction rather than an infectious process and is characterised by the
development of large areas of ulceration known as gummas. These frequently
affect the oral cavity.
Syphilis can be spread during the practice of dentistry by direct contact
with mucosal lesions of primary and secondary syphilis or blood and saliva from
infected patients. The dentist also can play an important role in the control of
syphilis by identification of the signs and symptoms of syphilis, patient
education, and referral. The incidence of syphilis and the impact of control
measures are presented with the emphasis on the past 5 years. The signs and
symptoms of primary, secondary, latent, and late (tertiary) syphilis are reviewed.
145
Current medical treatment is presented. The oral manifestations of syphilis are
discussed as well as the dental management of the infected patient.
Syphilis of the mouth cavity
Untreated syphilis or lues is a chronic infectious disease. It is caused by
treponema pallidum, which is most commonly transmitted by sexual contact and
occasionally by blood transfusion or by intrauterine infection. If the disorder is
not treated, its clinical course can be chronic, persisting for decades. During this
time, a variety of morphological signs occur depending on the stage of the
disease.
CASE REPORT: We describe a case of tertiary syphilis in the oropharynx with
a defect of the soft palate. In a 37-year-old woman, the first symptom was a
dryness of the throat followed by a feeling of foreign body in the palate area.
The patient had a history of sexual contact with a man who had had syphilis ten
years ago, and our initial suspicion was confirmed by a final diagnosis of tertiary
syphilis. Signs of primary or secondary syphilis were not observed.
RESULTS: In the course of diagnostic procedures both further manifestatons of
syphilis and other infectious or malignant causes were excluded. The serological
results showed a typical constellation of Treponema and non-Treponema serum
reactions. The histopathological examination of an exploratory excision from the
soft palate showed granulomatous changes with peripheral participation of
plasma cells. We initiated appropriate antibiotic therapy, using clemizole
penicillin G over a period of 21 days, which induced healing of the soft palate.
CONCLUSIONS:
A defect of the soft palate was diagnosed as a very rare sign of tertiary syphilis.
Aspects of oral syphilis.
The incidence of sexually transmitted diseases recently increased in the
United States and Europe due to migration, increase in high-risk behavior, and
abandonment of safer sex practices at the advent of anti-retroviral combination
therapy for human immunodeficiency virus infection. This article presents four
cases of primary oral anti perioral syphilis with differential diagnoses. It is
important to bear this reappearing infection in mind to avoid latent infection.
Resembling common oral infections, the primary affect disappears
spontaneously, and the infection enters the second stage. The patient remains
infected, may further spread the disease, and risks severe organ damage from
long-standing infection. The antibiotic cure is inexpensive and safe and spares
the patient mucous patches and gumma residuals, apart from severe general
sequelae such as thoracic aorta aneurysm and neurosyphilis. However,
compliance problems jeopardize clinical and serologic follow-up.
Background: Syphilis is a venereal disease that can also be acquired by
exposure to infected blood. The organism can cross the placenta and infect the
unborn child.
Untreated syphilis progresses through 4 stages: primary, secondary, latent,
and tertiary stages. Known as a great imitator, patients with syphilis can be a
diagnostic challenge because of their wide-ranging clinical presentations.
146
Pathophysiology: Three genera of spirochetes cause human infection: (1)
Treponema, which causes syphilis, yaws, and pinta; (2) Borrelia, which causes
Lyme disease and relapsing fever; and (3) Leptospira, which causes
leptospirosis.
Transmission occurs by penetration of the spirochetes through mucosal
membranes and abrasions on epithelial surfaces. Incubation time from exposure
to development of primary lesions averages 3 weeks but can range from 10-90
days. Lesions develop at the primary site of inoculation. Pathologically, the
primary lesion of syphilis is a focal endarteritis and periarteritis. Rabbit studies
show that spirochetes can be found in the lymphatic system as early as 30
minutes after primary inoculation, suggesting that syphilis is a systemic disease
from the outset.
Sex: Male-to-female ratios of primary and secondary syphilis infection
increased from 1.6:1 in 1965 to nearly 3:1 in 1985. Since then, the ratio has
decreased, reaching a nadir in 1994-95. From 2000-2002, the ratio has increased
to 1985 levels.
Age: Syphilis is most common during the years of peak sexual activity.
Most new cases occur in men and women aged 15-40 years. The highest
infection rates occur in people aged 20-29 years. Causes: Syphilis is caused by
the spirochete T pallidum, a thin helical cell approximately 0.15 m X 6-50
m. T pallidum is a labile organism that cannot survive drying or exposure to
disinfectants; thus, fomite transmission (eg, from toilet seats) is virtually
impossible. It is solely a human pathogen and does not naturally occur in other
species. T pallidum has, however, been cloned in Escherichia coli and has been
used experimentally in rabbits. Transmission occurs by penetration of the
spirochetes through mucosal membranes and abrasions on epithelial surfaces. It
is primarily spread through sexual contact but can be spread by exposure to
blood products and transferred in utero.
Risk factors
Unprotected sex, promiscuous sex, and IV drug use are the major risk factors.
Health care workers are at occupational risk.
Studies:
Immunofluorescence staining of mucocutaneous lesions or dock-filled
microscopy demonstrates the spirochete in primary, secondary, and early
congenital disease.
Serologic reaginic tests
The fluorescent treponemal antibody absorption (FTA-ABS) test is
reactive in 85% of primary cases, in 99-100% of secondary cases, and in 95% of
latent or late cases. It should be used as a confirmatory test for positive VDRL
or RPR test findings.
The T pallidum hemagglutination (TPHA) test and the T pallidum particle
agglutination (TPPA) test are generally used for screening.
Treponemal enzyme immunoassay (EIA/immunoglobulin G [IgG],
immunoglobulin M [IgM]) tests may be performed.
147
Testing must be performed more than once in patients diagnosed with latent
syphilis in order to exclude laboratory error.
Imaging Studies:
Obtain a chest radiograph for patients with tertiary syphilis to screen for aortic
dilation.
CT scan and MRI of the head and body may be employed to document the
complications of tertiary syphilis.
Other Tests:
Slit-lamp examination and ophthalmic assessment can differentiate between
acquired and congenital syphilis (presence of interstitial keratitis) in patients
with latent infection of uncertain duration.
Procedures:
Lumbar puncture
CNS invasion by treponemes occurs in 30-40% of patients with primary or
secondary syphilis; however, no studies show this to be a predictor of poor
neurologic outcome.
Lumbar puncture (LP) is not indicated for patients with early syphilis. Current
guidelines in clinical infectious diseases state that physicians should evaluate
cerebrospinal fluid (CSF) in individuals with latent syphilis of unknown
duration or with late latent syphilis if (1) treatment fails, (2) neurologic or ocular
symptoms are present; or (3) the patient has underlying HIV infection. Patients
with high titers on serological tests (≥1:32) have only a relative indication for
performing an LP.
Examination of the CSF should include the VDRL test, cell count, and
protein level. Abnormalities of any of these measurements combined with a
suggestive history and examination strongly indicate the presence of
neurosyphilis. Derangements of these values are consistently found in
neurosyphilis. The presence of a positive VDRL test result is indicative of active
disease. A positive polymerase chain reaction (PCR) test finding is sensitive in
detecting past invasion of the CNS but does establish whether the organisms are
still alive.
Histologic Findings: The primary lesion of syphilis is a chancre.
Histologically, it is characterized by mononuclear leukocytic infiltration,
macrophages, and lymphocytes.
The inflammatory reaction of secondary syphilis is histologically similar to that
of the primary chancre but is less intense.
In tertiary syphilis, histological examination shows palisaded macrophages and
fibroblasts as well as plasma cells surrounding the margins. Treponemes are rare
in these lesions and typically cannot be cultured or visualized.
Medical Care:
Clinical and serologic conversions are the endpoints of medical treatment.
Obtain follow-up VDRL test levels to document treatment efficacy.
Surgical Care:
Surgical care is reserved for treating the complications of tertiary syphilis (eg,
aortic valve replacement).
148
Consultations:
Infectious disease consultation may be required for difficult or complex cases.
Consult dermatology, vascular surgery, ophthalmology, and neurology to assist
with the variable presentations
INFLAMMATORY DISEASES OF MAXILLOFACIAL REGION
Inflammatory processes of maxillofacial region.
They caused by microbes, the majority from which in usual conditions are
not pathogenic and live on skin and mucous membrane of oral cavity. In case of
injury of skin, mucous membrane, marginal periodontium, and also destruction
of hard substances of tooth with opening of its cavity these microbes spread into
deep tissues. Their further destiny can be various. In one cases they perish in a
zone of entrance under influence of protective factors, in others - with lymph
reach lymph nodes where there is they are fixed and destroyed. If lymph nodes
are not able to fix and destroy completely microbes, the last get into a blood
vessels and with blood can be brought in any organ of the body. However other
outcome is often observed. Microbes adapt to new conditions of existence, start
to multiply. Some of them produce toxic proteins called exotoxines which have
antigenic properties and can be harmful to different tissues.
As a result of destruction of cells from them endotoxines are
discharged. Causing alteration of tissue structures, toxins cause development of
the inflammatory reaction and local infectious process begins. It depends on
virulence of infection (pathogenic properties of microorganisms, and their
quantity), and also on the level of immune reactance of a macroorganism
Depending on localization of an entrance for microbes the infectiousinflammatory processes of GFR are devided into odontodenic, stomatogenic,
tonsilogenic, rhinogenic and dermatogenic infections. Inflammatory processes
of gnathofacial region more often are of odontogenic origin.
Classification of odontogenic inflammatory diseases of maxillofacial (gnathofacial) area
A.Apical and marginal Periodontitis
1) Acute (Serous and purulent)
2) Chronic in a stage of an aggravation
3) Chronic (granulating, granulomatous, and fibrous)
B.Periostitis of jaws
C.Osteomyelitis of jaws depending on
a. clinical process:
process:
1) sharp
2) subacute
3) chronic
4) chronic in stage of aggravation
b. character of spread of the
1) limited
2) diffuse
149
D. Abscesses and phlegmons of
1. Face
2. Paragnathic
3. Floor of the oral cavity
4. Parapharyngeal
5. Tongue
6. Neck
E. Lymphadenitis of the face and neck
Soft tissue infections of the face.
Infection sited at a tooth
Acute alveolar abscess. A common dental emergency facing the dentist is
a patient with an acute alveolar abscess. There are a number of possible
conditions that may lead to an abscess, including:
• periapical periodontitis
• periodontal disease
• pericoronitis
• infection of a cyst of the jaws.
Epidermoid (sebaceous) cysts in the facial skin may become infected and
be confused with infections of dental origin, according to their site, although a
punctum marking the blocked keratinous outflow may be obvious.
Clinical features
There is severe pain that is not well localised, although the affected tooth
is painful to touch when the abscess follows periapical periodontitis. The tooth
is non-vital to simple tests and a history of trauma to a tooth may be implicated.
More commonly, the tooth is carious on examination. Without treatment, the
infection spreads through bone and periosteum producing a soft fluctuant
swelling, which may be present in the buccal sulcus or occasionally in the
palate. As soon as the abscess spreads out of bone and into soft tissues, there is a
reduction in the pain experienced.
An abscess following periodontal disease is likely to result in a mobile
tooth that is tender to percussion. The tooth may remain vital and any swelling is
often nearer the gingival margin rather overlying the periapical region. Pus may
exude from the gingival margin.
Trismus and cervical lymphadenopathy are signs of local spread of
infection. Pyrexia and tachycardia are signs of systemic toxicity.
Radiology. While the acute abscess may be very obvious clinically,
radiological signs vary enormously depending upon the pre-existing pathosis.
An abscess may develop from a tooth with no previous chronic periapical lesion;
here the most that may be visible is a loss of periapical lamina dura. Where a
periapical granuloma or radicular cyst was present beforehand, the well-defined
margin of the
radiolucency tends to be lost. Such an ill-defined periapical radiolucency would
be described as a rarefying osteitis.
150
Pathology. An abscess may be defined as a pathological cavity filled
with pus and lined by a pyogenic membrane. The latter classically consists of
granulation tissue but in a rapidly expanding lesion it may simply be a rim of
inflammatory cells. The soft tissue surrounding an alveolar abscess may become
swollen as a result of the inflammatory exudation and reactive to bacterial
products, which have diffused from the abscess.
Management. The principle of treatment is to establish drainage of pus. In the
case of a periapical abscess, this may be accomplished via the root canal after
opening this up through the crown of the tooth with an air-rotor drill. This does
not require local anaesthesia as the tooth is non-vital, although it is impor-tant
not to apply pressure to the tooth (as it may be exquisitely tender to percus-sion)
by cutting tooth tissue slowly with a sharp bur. Alternatively, the tooth is extracted to gain adequate drainage. This may be undertaken under regional lo-cal
anaesthesia, with or without conscious sedation, or using general anaesthe-sia.
Spread of infection to facial tissues.
Lymphatic spread of infection. The lymphatic system is frequently
involved in infections and gives an indication as to the pattern of spread.
Enlargement and tenderness of nodes, described as lymphadenitis, is common,
although inflammation of the lymphatic vessels, described as lymphangitis,
may occur and can be seen as thin red streaks through the skin.
Spread of infection through tissue spaces. In addition to spread through
the lymphatic system, infection in the soft tissues of the face also spreads along
fascial and muscle planes. These potential tissue spaces usually contain loose
connective tissue and can be described anatomically.
Floor of mouth tissue spaces (Fig. 24). The mylohyoid muscle divides
the sublingual and submandibular spaces although they are continuous around
its posterior free edge. The submental space is situated below the chin and
between the anterior bellies of the digastric muscles. There are no restrictions on
the spread of infection between the two submandibular spaces and the submental
space; consequently, it can spread across the neck below the inferior border of
the mandible.
Fig. 24 Potential tissue spaces about the floor of the mouth.
151
Other tissue spaces of importance (Fig. 25)
Fig. 25 Potential tissue spaces about the posterior mandible.
Buccal spaces. These are located in the cheek on the lateral side of
buccinator muscle. Submasseteric tissue spaces lie between the masseter muscle
and the ramus of the mandible. The pterygomandibular spaces lie between the
medial surface of the mandible and the medial pterygoid muscle. The
infratemporal space is the upper part of the pterygomandibular space and closely
related to the upper molar teeth. The parotid space lies behind the ramus of the
mandible and about the parotid gland.
Pharyngeal tissue spaces. Of these, the parapharyngeal spaces are the
most important in terms of spread of infection from the teeth and jaws. These
spaces lie lateral to the pharynx and are continuous with the retropharyngeal
space, to where infection may spread. The retropharyngeal space lies behind the
pharynx and in front of the prevertebral fascia. The peritonsillar space lies
around the palatine tonsil between the pillars of the fauces.
Hard palate area. There is no true tissue space in the hard palate because
the mucosa is so tightly bound down to periosteum, but infection can strip away
some of this and permit formation of an abscess.
Types of facial infection
Maxillary infections. The spread of periapical infection may be predicted
by the relationship of the buccinator muscle attachment to the teeth. Infection
from molar teeth usually spreads buccally or labially into the sulcus but may
spread above the muscle into the superficial tissues of the cheek, where it can
spread over a wide area with little to contain it. Infection frequently spreads to
the palate from lateral incisors because of the palatal inclination of the root.
Occasionally, infection may also spread palatally from a palatal root of a molar
152
or premolar. The canine root is long and infection may spread superficially to
the side of the nose rather than intra-orally
Mandibular infections. Periapical infection may similarly spread
according to muscle attachments. Infection from incisors usually spreads labially
into the sulcus but may spread to the chin between the two bellies of the
mentalis muscle. Infection from the canine may spread into superficial tissues
because root is long. Premolars and molars show spread of infection into the
buccal sulcus leading to intra-oral or extra-oral spread according to the relation
to the attachment of buccinator. Similarly, second mandibular molar teeth have
more lingually placed roots and may, therefore, result in either sublingual or
submandibular spread depending on the relative position of the mylohyoid
muscle.
Cellulitis. Cellulitis is a spreading infection of connective tissue typical of
streptococcal organisms. It spreads through the tissue spaces as described above
and usually results from virulent and invasive organisms. The clinical features
are those of a painful, diffuse, brawny swelling. The overlying skin is red, tense
and shiny. There is usually an associated trismus, cervical lymphadenopathy,
malaise and pyrexia. The swelling is the result of oedema rather than pus and
may be extensive when it involves lax tissues such as in the superficial mid-face
about the eyes. Cellulitis usually develops quickly, over the course of hours, and
may follow an inadequately managed or ignored local dental infection. If the
infection spreads to involve the floor of mouth and pharyngeal spaces, then the
airway can be compromised. Initially, the floor of the mouth will be raised and
the patient will have difficulty in swallowing saliva; this pools and may be
observed running from the patient's mouth. This sign indicates the need for
urgent management. Cellulitis involving the tissue spaces on both sides of the
floor of mouth is described as Ludwig'sangina.
Cavernous sinus thrombosis. Rarely infection in the tissues of the face
may spread intracranially via the interconnecting venous system. This is more
likely with the upper face via the facial vein to the cavernous sinus. While rare,
cavernous sinus thrombosis is life threatening.
Management of infections about the face. A clinical and radiographic
examination of the mouth should be carried out to identify potential causes such
as carious or partly erupted teeth or retained roots. The patient may need to be
admitted to hospital if they are unwell or there are signs of airway compromise.
A differential white cell count may indicate an increase in neutrophils. A blood
glucose investigation may be carried out to exclude an underlying undiagnosed
diabetes mellitus. Blood cultures should be performed if there is a spiking
pyrexia or rigors. Intravenous antibiotics such as penicillin together with
metronidazole should then be started, as well as fluids to rehydrate the patient,
analgesics and an antipyretic. Erythromycin or clindamycin may be appropriate
if the patient is allergic to penicillin.
Drainage. Drainage should be established by opening or extracting the
tooth or management as appropriate, such as for pericoronitis as described
above. If there is an associated fluctuant swelling, then this may be incised and
153
drained. This can be undertaken with ethyl chloride topical anaesthesia, local
anaesthesia (carefully injected into overlying mucosa and not into the abscess)
or general anaesthesia as appropriate. Drainage should not be delayed if the
patient does not show signs of improvement. This may need to be under general
anaesthesia if it is anticipated that local anaesthesia would be ineffective
because of exquisite tenderness of the tooth or the extent of the swelling. The
causative carious or impacted tooth or retained root should be removed at the
same time. If trismus is a feature, intubation of the trachea will be difficult and
the patient's airway will be at risk on induction of anaesthesia. Such patients
may need to undergo fibreopticassisted intubation while awake or sedated, prior
to induction of anaesthesia. Drainage of tissue spaces may require extra-oral
skin incision, blunt dissection to open abscess locules and insertion of a drain
such as a Yates to permit continued drainage for 24-48 hours. Pus is sent to the
microbiology laboratory for investigation of antibiotic sensitivity. When
draining a cellulitis, little pus will be found, but tissue fluid will be released. In
the case of Ludwig's angina, incisions are made bilaterally to drain the
submandibular spaces via an extra-oral approach, and the sublingual spaces via
an intra-oral approach. The mortality of Ludwig's angina has reduced from 75%
before the advent of antibiotic use to 5%. A drain may be placed through the
skin to protrude intra-orally. If the airway is at risk, the patient will remain
intubated postoperatively and return to the intensive care unit for ventilation.
Chronic infection
Acute infections may become chronic if treatment is inadequate. A
persistent sinus may form, permitting intermittent discharge of pus. This may be
intra-oral or extra-oral. The chronic infection may revert to an acute situation
should the discharge be interrupted in any way.
Other infections and Inflammations
Actinomycosis
Clinical features. Infection with Actinomyces species, most commonly
A.israelii, may involve the cervicofacial and abdominal regions as well as skin
and the lungs. The cervicofacial region is, however, the most commonly affected
and acute infection here may be indistinguishable from an acute dentoalveolar
abscess. There may be a history of trauma. Multiple discharging sinuses are a
classic sign of chronic actinomyocosis infection.
Pathology. Actinomycosis is characterised by the presence of masses of
the filamentous anaerobic bacteria visible in sections stained with haemotoxylin
and eosin but more readily distinguished on Gram staining (Gram positive).
These masses may be partially calcified and visible to the naked eye as bright
yellow ('sulphur') granules. Diagnosis is made on clinical grounds accompanied
by Gram staining, culture and sensitivity testing performed on a sample of pus.
This is of particular importance as it is unusual for Actinomyces species to be the
only bacteria present. Within the tissues, the masses of bacteria lie in areas of
154
suppuration surrounded by acute inflammatory cells. The adjacent granulation
tissue often shows considerable fibrosis.
Management. Any related dental cause is treated and swellings are
incised and drained as necessary. A 3-week course of penicillin is used for acute
infections and a 6-week course is used for chronic infections. Alternatively,
erythromycin, tetracycline or clindamycin may be used.
Osteomyelitis
Osteomyelitis is inflammation of the medullary cavity of a bone caused
by an infection. It is quite rare but is seen particularly in those patients whose
defence against infection is compromised because of local or systemic factors. It
is a serious condition requiring urgent specialist management. In contrast,
localised bone infection following tooth extraction is referred to as osteitis or
dry socket and can be managed in primary care.
Acute osteomyelitis
Clinical features. Symptoms are of pain, tenderness and swelling in the
affected area. As such, these symptoms are essentially those of an acute dental
infection. The mandible is affected more frequently than the maxilla. An
important symptom is a developing numbness over the chin as a result of mental
nerve involvement.
Radiology. The typical feature is a rarefying osteitis (see acute periapical
periodontitis, above). This may extend through a large area of bone, involving
the inferior dental canal and lower cortex of the mandible. Fig. 26
Fig. 26 Radiograph of acute osteomyelitis. This occurred
after extraction of the first molar.
Pathology. True acute osteomyelitis is rare because spread of infection
into bone is usually a chronic process or develops on a background of chronic
inflammation. Acute osteomyelitis is considered to involve rapid spread of
infection within the marrow spaces. By the time that the bone matrix is affected,
the condition is classified as chronic osteomyelitis.
Management. Benzylpenicillin or clindamycin and metronidazole are
normally started and altered as necessary according to the results of pus
155
sensitivity testing. The patient may require hospital admission for incision and
drainage, but it is preferable to limit any dentoalveolar surgery to the extraction
of grossly mobile and non-vital teeth. Antibiotics should be continued for at
least 2 weeks after control of the acute infection.
Chronic osteomyelitis.
The natural course of acute osteomyelitis is that it develops into a chronic
disease with pus accumulation and the formation of islands of necrotic bone
(sequestra). Predisposing factors are depressed immune or inflammatory
response, for example diabetes or long-term corticosteriod use and bone
abnormalities such as Paget's disease or cemento-osseous dysplasia.
Clinical features
Pain and swelling are always present, although this is likely to be less
severe than in the acute form. Paraesthesia tends to persist in mandibular lesions.
One or more soft tissue sinuses are typically present, draining pus. The affected
bone may become enlarged owing to periosteal reaction.
Radiology. In addition to the rarefying osteitis seen in acute lesions,
irregular radio-opaque areas (sequestra) surrounded by radiolucencies are
visible. A late sign of chronic osteomyelitis is radiological evidence of periosteal
new bone, visible as one or more thin shells of radio-opacity at the lower border
of the mandible or above the buccal/ lingual cortices. (Fig. 27)
Fig. 27. Occlusal radiograph of the lower right molar region in
chronic osteomyelitis. Note the bone destruction within the
jaw and sequestration lingually. Buccally there is periosteal
new bone formation.
Pathology. Fragments of dead bone (sequestra) are characterised by the
presence of empty osteocyte lacunae and degenerative changes to the matrix.
Often the surfaces are scalloped as a result of previous osteoclastic activity. If a
sequestrum communicates with the exterior, then bacterial plaque may form on
some surfaces. Sequestra may be surrounded by necrotic debris and acute
inflammatory cells. Pus may fill the adjacent marrow spaces while granulation
tissue infiltrated by chronic inflammatory cells is present at the junction between
156
vital and non-vital bone. Osteoblasts rim the surface of the surrounding vital
bone and both endosteal and subperiosteal bone deposition may be seen.
Management. Antibiotics are given as for acute infection. Any sequestra
that have not spontaneously separated should be surgically removed. Quite
extensive sequestrectomy may be necessary, which may necessitate subsequent
reconstruction. Hyperbaric oxygen therapy may be helpful in difficult cases. The
patient breathes 100% oxygen in a special chamber for a prescribed number of
sessions.
Garre's osteomyelitis
Carre's osteomyelitis is a chronic sclerosing osteomyelitis with a
proliferative periostitis. This rare condition is usually associated with either a
chronic periapical periodontitis or, sometimes, a chronic pericoronitis.
Clinical features. This condition is usually seen in children and younger
adults in the body and ramus of the mandible. Swelling is the principal feature.
Symptoms and signs of an overlying periapical periodontitis will usually be
present.
Radiology. There is an area of sclerosing osteitis in the mandible.
Periosteal new bone will be evident at the periphery of the jaw.
Pathology. Carre's osteomyelitis is characterised by the formation of
periosteal new bone. The latter is trabecular in nature; cortical bone is lacking
and there may be 'onion skin layering' of the reactive bone.
Management. Removal of the diseased tooth will result in resolution,
with gradual remodelling of the bone cortex eventually resulting in restoration
of the normal contour.
Garre’s Schlerosing Osteomyelitis or Proliferative periostitis:
First introduced by Carl Garre 1893 as an irritation induced focal
thickening of the periosteum and cortical bone of the tibia.
It occurs primarily in children and young adults and is characterized by a
localized, hard, nontender, unilateral body swelling of the lateral and inferior
borders of the mandible. No skin involvement, no lymphadenopathy, no fever
and no leukocytosis occur. Usually a carious first molar may be present.
Radiographically the characteristic “onion skin” appearance is evident.
Since it is considered to be response to a low grade infection or irritation that
influences the potentially active periosteum of young individuals to lay down
new bone, treatment is directed towards removal of the source of inflammation.
Rarely surgical recontouring may be required.
Condensing Osteitis ( focal schlerosing ostemyelitis):
Localized area of bone sclerosis associated with the apex of a carious
tooth and periapical periodontitis. Lesion after treatment of the source may
regress or remain as a bone scar.
157
Osteoradionecrosis
Clinical features. A reduction in vascularity, secondary to endarteritis
obliterans, and damage to osteocytes as a consequence of ionising radiotherapy
can result in radiation-associated osteomyelitis or Osteoradionecrosis. The
mandible is much more commonly affected than the maxilla, because it is less
vascular. Pain may be severe and there may be pyrexia. The overlying oral
mucosa often appears pale because of radiation damage. Osteoradionecrosis in
the jaws arises most often following radiotherapy for squamous cell carcinoma.
Scar tissue will also be present at the tumour site, often in close relation to the
necrotic bone.
Radiology. Osteoradionecrosis appears as rarefying osteitis within which
islands of opacity (sequestra) are seen. Pathological fracture may be visible in
the mandible.
Pathology. The affected bone shows features similar to those of chronic
osteomyelitis. Grossly, the bone may be cavitated and discoloured, with
formation of sequestra. Acute inflammatory infiltrate may be present on a
background of chronic inflammation, characterised by formation of granulation
tissue around the non-vital trabeculae. Blood vessels show areas of endothelial
denudation and obliteration of their lumina by fibrosis. Small telangiectatic
vessels lacking precapillary sphincters may be present. Fibroblasts in the
irradiated tissues lose the capacity to divide and often become binucleated and
enlarged.
Management. Prevention of Osteoradionecrosis is vital. Patients who
require radiotherapy for the management of head and neck malignancy should
ideally have teeth of doubtful prognosis extracted at least 6 weeks prior to
treatment. However, a delay to starting the radiotherapy is unacceptable and if
teeth are extracted only within a couple of weeks of treatment,
Osteoradionecrosis may still result. This risk may have to be taken. There are
also other factors that increase the risk of developing Osteoradionecrosis, such
as the dose of radiation, the area of the mandible irradiated and the surgical
trauma involved in the dental extractions. Patient factors, such as age and
nutrition, and others that have a bearing on wound healing will also influence
the risk. A more conservative approach to preradiation extractions can be
adopted in the maxilla. When extraction of teeth is required in patients who have
had radiotherapy to the jaws, a specialist opinion should be sought. Surgical
management of Osteoradionecrosis is similar to osteomyelitis. Sometimes, the
changes can be extensive, necessitating partial jaw resection to remove all
necrotic bone.
Periostitis
Proliferative periostitis sometimes causes jaw swelling and ulceration.
The condition arises as a result of chronic irritation to the periosteum, often from
foreign material, which enters through an ulcer. Vegetable pulse material is the
best-known example. Leguminous grains from cooked food accumulate under
158
an ill-fitting denture and are forced into the periosteum. The resulting chronic
inflammatory processes, including a foreign body reaction to the starch, cause
cortical erosion and periosteal swelling. The lesion, which may be mistaken for
a malignant process clinically, is referred to as a vegetable pulse granuloma.
Augmentation materials that have been implanted into the jaws sometimes
become infected and cause periostitis and osteitis. A rare cause of periosteal
expansion is metastatic deposition of carcinoma.
Periostitis Ossificans (PO) is a non-suppurative type of Osteomyelitis,
commonly occurring in children and young adults, in mandible. The most
common cause for PO is periapical infection of mandibular first molar.
Radio graphically PO is characterized by the presence of lamellae of newly
formed periosteal bone outside the cortex, giving the characteristic appearance
of ‘onion skin’.
Case reports.
Two male children 11 years of age reported to the Department of Oral
Medicine with a painless and persistent bony hard swelling in the mandible,
with a short duration. Both the patients had grossly decayed mandibular
permanent first molar tooth with periapical infection and buccal cortical plate
expansion. The radiographic study revealed different appearances, the
Orthopantomograph of case I showed a single radiopaque lamella outside the
lower cortical border, without altering original mandibular contour and in case
II showed a newly formed bony enlargement on the outer aspect of the lower
cortical border without altering the original madibular contour. Occlusal
radiograph of both the patients showed two distinct radiopaque lamellae of
periosteal bone outside the buccal cortex .
Kawai classified PO of mandible into type I and type II, based on whether
the original contour of mandible is preserved or not. Each type is further
classified into two sub types. In case I, the orthopantomographic appearance is
characteristic of type I-1, but the appearance in occlusal radiograph is
characteristic of type I-2.
In case II, the appearances in both the radiographs are characteristic of type I-2.
Conclusions.
Apart from the typical onion skin appearance, PO shows various other
radiographic appearances.
The radiographic appearance of Periostitis Ossificans may reflect the
duration, progression and the mode of healing of the
disease process.
The radiographic classification of PO depends on the type of radiographs
taken for evaluation.
Periostitis ossificans (PO) is a type of chronic osteomyelitis, more
popularly known as Garre’s osteomyelitis. Other names attributed to PO in the
literature are nonsuppurative ossifying periostitis, osteomyelitis with
proliferative periostitis, nonsuppurative sclerosing osteomyelitis, and chronic
sclerosing inflammation of the jaw. Garre was not responsible for the
159
description of the disease that now bears his name. In his original publication
there is no mention of periostitis, periosteal duplication, or onion-skin
appearance. Garre himself had no access to pathologic specimens for
microscopic examination.
The term ‘Garre’s osteomyelitis’ as synonymous to PO may therefore be
an improper designation and the term periostitis ossificans may be preferred. PO
is most commonly reported in body of mandible. Most reported cases are
unifocal and unilateral,but at least one case involving four quadrants has been
reported. Apart from the mandible, the most common bone to be involved in
PO is the femur. The cause of inflammatory subperiosteal bone production in
PO affecting mandible or maxilla is spread of infection from the nonvital teeth
perforating the cortex and becoming attenuated, which in turn stimulates bone
formation by the periosteum.
Classification of PO based on the radiographic appearance. TYPE I
(Original contour of mandible preserved) TYPE II (original contour of mandible
is lost)
Subtype 1 Single lamella seen as a radiopaque line of periosteal new bone
overlying the original cortex separated by a radiolucent line. Newly formed
bony enlargement with resorption of original cortex and osteolytic areas usually
visible.
Subtype 2 Visible hemi-elliptical newly formed bony enlargement, well
outlined with a thin cortical surface located on the outer aspect of original cortex
producing an onion skin appearance. Deformation with a homogeneously dense
osteosclerotic bone that made original cortex discernible. This subtype
occasionally showed duplication of newly formed periosteal bone on the outer
aspect of the deformed mandible
Classification of Gross Periostitis Ossificans – GPO.
Type A Showing an onion skin appearance, resulting from a nonvital
tooth or following extraction of a tooth.
Type B Consolidation form shows fine bony spicules perpendicular to
bone surface.
Type C Consolidation form shows coarse trabeculation with wide marrow
spaces.
Type D Shows more osteosclerotic and osteolytic changes in the affected
medullary bone and disappearance of original cortex
or loss of the original bone contour.
The periosteal reaction may be in single or multiple layers. The apparent
duplication is understood to be a result of periodic exacerbation and remission of
the infection, which causes repeated perforation of the outermost new bone with
restripping of the periosteum resulting in repetitive layering of bone. The
radiographic appearance of PO includes radiopaque laminations that are roughly
parallel to each other and to the underlying cortical surface, giving an onion-skin
appearance. Laminations vary from 1 to 12 in numbers. Radiolucent separation
is present between new bone and original cortex. Within the new bone, areas of
160
small sequestra or osteolytic radiolucencies may also be found. There will be
patchy increased radiopacity overlying the lesion in cases involving the buccal
cortex. This is a result of attenuation of X-ray image by the reactive bone. The
classification of PO of the mandible by Kawai is shown in Table 1. In this
system of classification, PO has been classified into two types, each with two
subtypes, based on whether the original contour of mandible is preserved or not.
Type I lesions are of shorter duration than type II. Both the subtypes of
type I PO occurs in the early stages of mandibular osteomyelitis. With adequate
treatment there can be complete resolution of PO type I cases; however, if the
disease continues, type I-1 may progress to type II-2 and type I-2 to type II-1,
followed by type II-2. In type II cases where there has been loss of mandibular
contour, deformity remains even when normal bony architecture has been
restored and the disease process has been resolved Mandibular osteomyelitis
with bulbous bony enlargement in young patients is referred as gross periostitis
ossificans (GPO). Kawai et a .'s classification of this more severe form of the
disease is shown in Table 2. Type A is associated with carious tooth or followed
extraction of tooth, showing onionskin appearance. In 36·8% of types B and C,
no infectious source could be identified; it was suspected that it could be caused
by a developing unerupted tooth or a dental follicle. Type D was seen in the
chronic stage.
When other imaging modalities such as scintigraphy are used in cases of
PO, these have shown intense uptake of Tc 99 m methylene diphosphonate at
regions of interest. A computerized tomographic study has demonstrated cortical
thickening, which appeared as sclerosis and periosteal new bone formation
perpendicular to the cortex, which assumed a sunburst appearance.
The following case reports describe the occurrence of PO in two
paediatric patients, with contrasting radiographic findings.
The etiological factors for PO include periapical infection of mandibular
molars, periodontal infection, untreated fractures, developing tooth follicle,
unerupted teeth, previous extraction site, pericoronitis, buccal bifurcation cyst,
lateral inflammatory odontogenic cyst, and nonodontogenic infection. In some
cases there may not be any demonstrable aetiological factor and these are termed
idiopathic. Of these, the most common aetiological factor is said to be the
periapical infection of mandibular first molar. In both of our cases the source of
infection for PO was a nonvital carious mandibular first molar.For unknown
reasons PO is most common in the mandible, and has been especially related to
the left side of the mandible. A previous study showed 82·7% of periostitis
occurs along the lower border of mandible, 43% in buccal cortex, and 6·5% in
lingual cortex. In our cases there was involvement of lower border of body of
the mandible and the buccal cortex without involvement of the lingual
aspect.Radiographically, both our cases showed evidence of periapical infection
in their first mandibular molar.The affected area showed increased radio density,
which may be attributed to osteosclerosis. In case I, the orthopantomograph
showed a single radiopaque lamella separated from the original lower cortical
border of mandible by a fine radiolucent line. As the cortical border was not
161
destroyed and the mandibular contour was unaffected, it may be categorized
radiographically as type I-1. But occlusal radiographs taken for the same case at
the same visit showed fusiform bony mass with two radiopaque lamellae outside
the original buccal cortex, giving an onion-skin appearance more characteristic
of type I-2. This clearly indicates that this radiographic classification of PO may
be at least partly dependent on the type of radiograph taken. There may be
radiopaque sequestra or osteolytic lesion within PO lesion. In our case I, there
was an area of osteolytic radiolucency in the periapical region and in the newly
formed bone on the buccal aspect of 46 region. In case II, the
orthopantomograph showed an ill-defined area of uniform osteosclerosis and a
bony enlargement on the outer aspect of the original cortex of lower border
without alteration of the contour. So, radiographically, this may be considered as
type I-2. In the occlusal view it showed duplication of cortical lining and
homogenously dense osteosclerotic bone. Based on the radiographic
classification of GPO,both our cases correspond to type A, both showing onionskin appearance in the occlusal radiograph and being associated with nonvital
carious mandibular first molars.Both of our cases of PO were probably of
relatively short duration (3 months and 45 days) and were in the early stages of
the disease. The discrepancy in their radiographic appearances may be attributed
to the difference in the duration, progression and mode of healing of the disease
process in two individuals.Radiological differential diagnosis of PO includes
infantile cortical hyperostosis, Ewing’s sarcoma,osteosarcoma, etc., none of
which fitted the clinical and radiographic features of the two cases reported
here.PO in young persons is, in general, curable with early diagnosis and
adequate treatment; however, if the correct diagnosis is delayed by more than 6
months, it may progress into a persistent and deforming stage. Elimination of the
source of infection either by extraction or endodontic therapy of the offending
tooth may be accompanied by antibiotic therapy in the early stages. Once the
cause is removed the bone will remodel itself gradually and the original facial
symmetry will be restored. This remodeling of the bone may be helped by the
overlying muscle pull, which is attached to it. Successful resolution may be
more difficult in severe and long standing cases of PO. Both of our cases of PO
were probably of relatively short duration (3 months and 45 days) and were in
the early stages of the disease. The discrepancy in their radiographic
appearances may be attributed to the difference in the duration, progression and
mode of healing of the disease process in two individuals. Radiological
differential diagnosis of PO includes infantile cortical hyperostosis, Ewing’s
sarcoma, osteosarcoma, etc., none of which fitted the clinical and radiographic
features of the two cases reported here. PO in young persons is, in general,
curable with early diagnosis and adequate treatment; however, if the correct
diagnosis is delayed by more than 6 months, it may progress into a persistent
and deforming stage. Elimination of the source of infection either by extraction
or endodontic therapy of the offending tooth may be accompanied by antibiotic
therapy in the early stages. Once the cause is removed the bone will remodel
itself gradually and the original facial symmetry will be restored. This
162
remodeling of the bone may be helped by the overlying muscle pull, which is
attached to it. Successful resolution may be more difficult in severe and long
standing cases of PO.2 The radiographic appearance of periostitis ossificans
may reflect the duration, progression and the mode of healing of the disease
process. 3 The radiographic classification of PO may depend at least partly on
the type of radiographs taken for evaluation.
Proliferative periostitis of Garru is described as a productive and
proliferative inflammatory response of periosteum to infection or other irritation.
This can be odontogenic or non-odontogenic in nature. This is a case report of
an odontogenic periostitis resulting from periapical inflammation of endodontic
origin. It was successfully treated by nonsurgical endodontics. Antibiotic
therapy was not used during the treatment of this patient.
Epidemiology of odontogenic periostitis
The frequency of odontogenic periostitis (o.p.) observed during 13
consecutive years was assessed according to sex, age, and the affected pairs of
teeth, in subjects admitted to an outpatient department in an area with an
unsatisfactory dentist/population ratio. O.p. was less frequent in the older age
groups, even after adjustment for the decreasing number of subjects with
advancing age. The observed frequencies of o. p. were related in groups with a
range of 10 years, both in men and women to the number of teeth present, i. e.
"at risk". The last-mentioned figures were calculated for the individual pairs of
teeth relying on a population model. the maximum risk of the individual tooth
pairs was not found for all teeth in the same age classes but varied, partly in
accordance with the caries susceptibilities, though this item was not the
determining factor. O. p. is an avoidable sequel of penetrating caries, and as
such is one of the consequences of the inadequate health education of the public.
Lymphotropic therapy for acute purulent odontogenic maxillary periostitis
The structure of the gingival mucosa was studied by optic microscopy in
patients with acute purulent odontogenic maxillary periostitis treated
traditionally and receiving lymphotropic therapy. Lymphotropic administration
of the antibiotic during 2 days resulted in less pronounced dilatation of the
interstitial spaces and lymph vessels adjacent to the molars and higher counts of
lymphocytes, monocytes, and macrophages. This indicated high efficiency of
lymphotropic therapy of acute purulent maxillary periostitis for molars.
Microcirculation parameters and tissue leukocyte cytogram in gingival mucosal
tissue adjacent to the canines and premolars differed negligibly in patients
treated by different methods.
Osteomyelitis of jaws
By strict definition: Osteomyelitis is an inflammation of the medullary
portion of the bone. However the process rarely is confined to the endosteum
and usually affects the cortical bone and the periosteum.
Therefore osteomyelitis may be considered an inflammatory condition of bone
that usually begins as an infection of the medullary cavity which rapidly
163
involves the haversian system and quickly extends to the periosteum of the area.
The infection becomes established in the calcified portion of bone when pus in
the medullary cavity and beneath the periosteum compromises or obstructs the
blood supply. Following ischemia the infected bone becomes necrotic.
Osetomylitis of the jaws differs in many important aspects from the one
found in long bones.
Classification of ostemyelitis:
Multiple classification systems have been proposed:
Waldvogel classification
In 1970, Waldvogel described the first long bone osteomyelitis staging
system. He described 3 categories of osteomyelitis, as follows: hematogenous,
contiguous focus, and osteomyelitis associated with vascular insufficiency.
Hematogenous osteomyelitis is predominantly encountered in the pediatric
population; 85% of patients are younger than 17 years. This form of
osteomyelitis is more common in males at any age. The bone infection usually
affects the long bones in children, while in adults, the lesion is usually located in
the thoracic or lumbar vertebrae.
Osteomyelitis secondary to a contiguous focus of infection can derive
from a direct infection of bone, from a source outside the body (eg, soft tissue
trauma, open fracture, surgery), or from the spread of infection from an adjacent
focus (eg, soft tissue infection, dental abscess, decubitus ulcer). Contiguous
focus osteomyelitis has a biphasic age distribution: the infection occurs in
younger individuals secondary to trauma and related surgery and in older adults
secondary to decubitus ulcers and infected total joint arthroplasties.
Osteomyelitis associated with vascular insufficiency is usually seen in
individuals with diabetes mellitus. Of the 31 patients in Waldvogel's study with
this form of osteomyelitis, 25 had diabetes, 5 had severe atherosclerosis not
related to diabetes, and one had vasculitis secondary to rheumatoid arthritis. All
of the infections affected the toes, metatarsals, tarsals, or hindfoot. Most patients
in this group were aged 40-70 years.
Waldvogel's remains the primary osteomyelitis classification system.
However, it is an etiologic classification system that does not readily lend itself
to guiding surgical or antibiotic therapy. As a result, other classification systems
have been developed to emphasize different clinical aspects of osteomyelitis.
Kelly classification
The Kelly classification system divides osteomyelitis in adults into 4
categories, as follows:
Hematogenous osteomyelitis
Osteomyelitis in a united fracture (fracture with union)
Osteomyelitis in a nonunion (fracture with nonunion)
Postoperative osteomyelitis without fracture
This classification system emphasizes the etiology of the infection and its
relationship to fracture healing.
Weiland classification
164
Weiland defined chronic osteomyelitis as a wound with exposed bone,
positive bone culture results, and drainage for more than 6 months. A similar
wound with drainage for less than 6 months was not considered to be a site of
chronic osteomyelitis. The infection was further divided on the basis of soft
tissue and the location of bone involved, as follows:
Type I osteomyelitis is defined as open exposed bone without evidence of
osseous infection but with evidence of soft tissue infection.
Type II osteomyelitis consists of circumferential, cortical, and endosteal
infection. Radiographs demonstrate a diffuse inflammatory response, increased
bone density, and spindle-shaped sclerotic thickening of the cortex. Other
radiographic findings included areas of bony resorption and often a sequestrum
with a surrounding involucrum.
Type III osteomyelitis consists of cortical and endosteal infection associated
with a segmental bone defect.
Ger classification
The Ger classification system addresses the physiology of the wound as it
relates to osteomyelitis. The categories include simple sinus, chronic superficial
ulcer, multiple sinuses, and multiple skin-lined sinuses. If the wound is not
appropriately treated, the bone infection cannot be arrested. Early coverage of
open tibial fractures with soft tissue prevents the later development of
osteomyelitis, ulceration, and, perhaps, nonunion.
May classification
The May classification system focuses on the status of the tibia after soft
tissue and skeletal debridement (May, 1989). This system is useful in
determining the length of rehabilitation that will be needed, under ideal
conditions, before the patient will be able to ambulate without upper extremity
aids.
Type I osteomyelitis is defined as an intact tibia and fibula capable of
withstanding functional loads (rehabilitation time, 6-12 wk).
Type II osteomyelitis consists of an intact tibia with bone graft only needed for
structural support (rehabilitation time, 3-6 mo).
Type III osteomyelitis demonstrates a tibial defect of 6 cm or less with an intact
fibula (rehabilitation time, 6-12 mo).
Type IV consists of a tibial defect greater than 6 cm and an intact fibula
(rehabilitation time, 12-18 mo).
Type V osteomyelitis consists of a tibial defect greater than 6 cm long with no
usable intact fibula (rehabilitation time, 18 mo or more).
May's classification system with the estimated time for rehabilitation
assists the decision-making process in patients with posttraumatic tibial
osteomyelitis. However, many factors, including age, metabolic status, the
mobility of the patient's foot and ankle, neurovascular integrity, and the patient's
motivation, can greatly affect the time necessary for rehabilitation.
Gordon classification
The Gordon system classifies infected tibial nonunions and segmental defects on
the basis of the osseous defects (Gordon, 1988):
165
Type A includes tibial defects and nonunions without significant segmental loss.
Type B includes tibial defects greater than 3 cm with an intact fibula.
Type C includes tibial defects of greater than 3 cm in patients without an intact
fibula.
The Gordon classification system correlates with the prognosis for
successful free muscle transportation (ie, the microvascular movement of a
muscle flap). Once the wound and infection have been successfully treated with
staged microvascular muscle transplantation, the nature of the underlying
osseous problem will dictate the clinical results.
Cierny-Mader classification
The Cierny-Mader system is a good model for the diagnosis and treatment
of long bone osteomyelitis, since it permits stratification of infection and the
development of comprehensive treatment guidelines for each stage. The CiernyMader classification is based upon the anatomy of the bone infection and the
physiology of the host. The stages are dynamic and may be altered by therapy
outcome or change in host status. The classification is determined by the
condition of the disease process itself, regardless of its etiology, region, or
chronicity. The anatomic types of osteomyelitis are medullary, superficial,
localized, and diffuse.
Stage 1, or medullary osteomyelitis, denotes infection confined to the
intramedullary surfaces of the bone. Hematogenous osteomyelitis and infected
intramedullary rods are examples of this anatomic type.
Stage 2, or superficial osteomyelitis, is a true contiguous focus infection of
bone; it occurs when an exposed infected necrotic surface of bone lies at the
base of a soft tissue wound.
Stage 3, or localized osteomyelitis, usually is characterized by a full-thickness
cortical sequestration that can be removed surgically without compromising
bony stability.
Stage 4 or diffuse osteomyelitis is a through-and-through process that usually
requires an intercalary resection of the bone to arrest the disease process.
Diffuse osteomyelitis includes those infections with a loss of bony stability
either before or after debridement surgery.
The patient is classified as an A, B, or C host.
An A host is a patient with normal physiologic, metabolic, and immunologic
capabilities.
A B host has systemic compromise, local compromise, or both.
The C host is a patient in whom the morbidity of treatment is worse than that of
the disease itself.
The terms acute osteomyelitis and chronic osteomyelitis are not used in
this staging system, since areas of macronecrosis must be removed regardless of
the duration of an uncontrolled infection. This classification system aids in the
understanding, diagnosis, and treatment of bone infections in children and
adults. It is used mainly to stratify the infection in research protocols but is also
being built into many new research protocols.
A simpler classification scheme characterizes osteomyelitis as:
166
Suppurative and non suppurative and as acute, subacute and chronic.
Osteoradionecrosis.
Other special and less common forms are:
Syphilitic, tuberculous, brucellar, fungal, viral chemical, Eschericia coli and
Salmonella ostemyelitis.
Predisposing Factors:
The low incidence of osteomyelitis of the jaws is remarkable considering
the high frequency and severity of odontogenic infections. This low
incidence is a result of fine balance between the host resistance and the
virulence of the microorganism.
The virulence of the microorganisms in addition to any conditions altering
the host defense mechanism and alteration of jaw vascularity are
important in the onset and severity of ostemyelitis.
Systemic conditions that alter the host’s resistance and influence
profoundly the course of the disease include: diabetis mellitus,
Autoimmune disorders, agranulocytosis, anemia, especially sickle cell,
leukemia, AIDS, syphilis, malnutrition, chemotherapy therapy for cancer,
steroid drug use.
The importance of controlling these conditions in order to achieve proper
response from the treatment of ostemyelitis cannot be emphasized
enough.
Alchohol and tobacco use are frequently associated with osetomyelitis.
Conditions that alter the vascurarity of bone predispose patients to
develop osteomyelitis; those include: radiation, osteoporosis,
osteopetrosis, Paget’s dz, fibrous dysplasia, bone malignancy and bone
necrosis caused by mercury, bismuth and arsenic.
Etiology and pathogenesis:
In infants and children ostemyelitis occurs most commonly in long bones
primarily form hematogenous spread. While long bone ostemyelitis in adults
and the majority of cases of jaw osteomyelitis is initiated by a contiguous focus.
In the jaws contiguous spread of odontogenic infections that originate from
pulpal or periapical tissues is the primary cause of the dz.
Trauma, especially not treated compound fractures, is the second leading
cause.
Infection from periostitis after gingival ulcerations, lymph nodes, infected
furuncles or lacerations and hematogenous origin account for an additional small
number in jaw ostemyelitis.
The extensive blood supply of the maxilla makes it less prone to
ostemyelitis when compared to the mandible. The thin cortical plates and the
porosity of the medullary portion preclude infections from becoming contained
in the bone and facilitate spread of edema and purulent discharge into adjacent
tissues.
167
The mandible in this aspect resembles long bones with a medullary cavity,
dense cortical plates and well defined periosteum. The bone marrow is
composed of sinusoids rich is reticuloendothelial cells, erythrocytes,
granulocytes, platelets, osteblastic precursors as well as cancellous bone, fat
tissue and blood vessels.
The bone marrow is lined by the endosteum a membrane of cells
containing large numbers of osteoblasts. Bone spicules radiate centrally from the
cortical bone to produce a scaffold of interconnecting trabeculae.
The cortical bone has a distinctive architecture that includes longitudinally
oriented haversian systems (osteons). Each osteon has a central canal and blood
vessel that provide nutrients by means of canaliculi to osteocytes contained
within lacunae.
Volkmann’s canals create a complex interconnecting vascular and neural
network that nourishes bone and allows for repair, regeneration and function
demands. These canals connect the central canals among them and with the
periosteum and the marrow spaces.
An outer fibrous layer and an inner layer of osteogenic cells that consists
the periosteum, envelopes the cortical bone.
Compromise of blood supply is critical factor in establishment of
osteomyelitis.
The primary blood supply to the mandible is from the inferior alveolar
artery, while the periosteal supply is a secondary source.
The venous drainage from the mandible is directed to the pharyngeal
plexus and to the external jugular.
Acute inflammation that causes hyperemia increased capillary
permeability and infiltration of granulocytes is the process that leads to
ostemyelitis.
Proteolytic enzymes are released and along with destruction by bacteria
and vascular thrombosis ensue cause tissue necrosis.
If this pus is not walled off by the host and an abscess is not created or if
the pus does not escape to surrounding soft tissue from the medullary bone then
the process of ostemyelitis is initiated.
Necrotic tissue, dead bacteria within WBCs (pus) accumulate increasing
intramedullary pressure resulting in vascular collapse, venous stasis and
ischemia.
Pus travels through the Haversian system and the nutrient canals and
accumulates beneath the periosteum which gets elevated from the cortex and
further decreases the blood supply.
The inferior alveolar neurovascular bundle is compressed further
accelerating thrombosis and ischemia and results in osteomyelitis induced
inferior alveolar nerve dysfunction.
If the pus continues to accumulate then the periosteum is penetrated and
mucosal and cutaneous abscess and fistulas may develop.
The periosteum in children is less well bound to the cortical bone thus
allowing for more extensive elevation.
168
As host defenses are more effective and the therapy becomes more
effective the process may become chronic.
Inflammation regress and granulation tissue forms, new vessels lyse bone
and necrotic bone becomes separated from the viable bone (sequestra).
Small sections of bone may become completely lysed while larger ones may
become isolated by a bed of granulation tissue encased in a sheath of new bone
(involucrum).
Sequestra may follow any of the following routes: may be revascularized,
remain quiescent, resorb, or become chronically infected requiring surgical
removal for complete resolution of the infection.
When the involocrum is penetrated by channels, called cloacae, the pus escapes
to the epithelial surface creating fistulas.
Microbiology:
Appropriate collection and transportation of cultures are essential in
accurate diagnosis and initiation of appropriate therapy. Repeated cultures,
especially in cases of chronic osteomyelitis and chronic antibiotic therapy, are
paramount for identification and isolation of the involved pathogen.
Appropriate collection and handling of specimen along with culture of all
sequestra cannot be emphasized enough.
Staphylococcus Aureus and epidermis were, until recently, estimated to be
involved in jaw osteomyelitis 80-90% of the times. With more sophisticated
methods of collection and appropriate handling of cultures a-hemolytic
streptococcus is recognized as the primary organism along with oral anaerobes
e.g. Peptostreptococcus, Fusobacterium and Provotela species.
It is recognized that if staph is isolated in cultures probably originates
from skin contamination or through fistulas.
Eikenella corrodens is isolated in high percentages form cultures along
with Klesbsiella, Pseudomonas and Proteus species.
Antibiotic treatment should therefore be directed towards Streptococcus
spp and anaerobes and not towards staph.
Clinical findings: 4 clinical types are observed so the findings are
different for each one.
1. Acute suppurative;
2. Secondary chronic, a form that begins as acute and becomes chronic;
3. Primary chronic, it manifests no acute phase;
4. Nonsuppurative.
A subacute phase exists in which the there are no acute symptoms, but
there is production of pus and extension into adjacent tissues.
Acute suppurative (acute intramedullary osteo):
Deep intense Pain.
High intermittent fever.
Paresthesia or anesthesia of the lip.
Clearly identifiable cause.
No loosening of the teeth, no fistulas and no or minimal swelling.
169
If is not controlled within 10-14 days after onset then subacute
suppurative ostemyelitis is established.
Pus travels through haversian canals and accumulates under the
periosteum which penetrates to spread through to soft tissues.
Deep pain, malaise fever and anorexia are present. Teeth are sensitive to
percussion and become loose. Pus may be seen around the sulcus of the teeth or
through skin fistulas and is associated with fetid odor.
The skin overlying the effected bone is warm, erythematous, tender to
touch; firm cellulites with expansion of bone from periosteal activity and
paresthesia of mental nerve, regional lumphadenopathy are usually present.
Trismus may not be present and patient’s temperature may reach 101-102
degrees F along with signs of dehydration.
Mild leukocytosis with a left shift and mildly elevated ESR are present
but are not valid indicators of the course or the extent of the disease.
If inadequately treated the progression to subacute or chronic form is warranted.
Findings are limited to fistulas, induration of soft tissues with a thickened or
wooden character to the affected area with pain and tenderness.
Primary chronic form is not preceded by an episode of acute symptoms, is
insidious in onset with onset of mild pain, slow increase of jaw size and gradual
development of sequestra, often without fistulas.
Imaging Studies:
Plain films: Plain radiographs are relatively inexpensive, may be used to
make the diagnosis, help in interpreting and choosing other studies, and allow
one to exclude other conditions (eg, gas in the soft tissues). In uncomplicated
acute infection, the triad of soft tissue swelling, bone destruction, and periosteal
reaction is fairly specific for osteomyelitis and is sufficient to warrant a course
of therapy (empiric until the microbiologic diagnosis has been established).
Plain films are generally insensitive for the diagnosis of acute
osteomyelitis. This is in part due to the 2-3 weeks required for bone changes to
be evident on plain films, although changes may be seen at this time on the other
imaging modalities. Furthermore, in complicated situations, bone changes may
not be distinguishable from those due to another process, such as a Charcot
joint, fractures, or cancer. Thus, the diagnosis of acute osteomyelitis cannot be
excluded if the plain film findings are negative. Further testing should be
performed because early therapy is essential to reduce the formation of necrotic
bone and the development of chronic osteomyelitis.
The primary findings are different in chronic osteomyelitis, which is
characterized by bone sclerosis, periosteal new bone formation, and sequestra. It
is difficult to distinguish active from inactive infection.
CT scan: This modality is used to evaluate an area in which focal findings
are present on examination and plain films findings are negative. The CT scan
(with and without contrast) is very accurate for detecting cortical destruction,
intraosseous gas, periosteal reaction, and soft tissue extension.
MRI: This study is an alternative to CT scan and is especially useful in
evaluating a patient for osteomyelitis in the vertebrae and in the infected foot. In
170
vertebral osteomyelitis, findings on T1-weighted images include decreased signal intensity in the disk and adjacent vertebral bodies and loss of endplate definition. Findings on T2-weighted images include increased signal intensity in the
disk and adjacent vertebral bodies. With gadolinium, there is enhancement of
the disk, adjacent vertebral bodies, and involved paraspinal and epidural soft
tissue.
MRI provides useful anatomic detail in planning for surgical debridement,
since it may show abscesses that need drainage, and can reduce the risk of
operating on bland cellulitis. MRI also can be used to delineate soft
tissue/epidural involvement and spinal cord impingement that cannot be seen on
nuclear medicine images.
When evaluating the foot for osteomyelitis, MRI is as specific or more
specific and more sensitive than technetium bone scan. In one series in which
bone biopsy was used as the criterion standard, the sensitivity was 72% for MRI,
68% for bone scan, and 45% for indium white blood cell scan.
MRI cannot be used in patients with certain metal implants. In addition,
false-positive results can occur with bone infarct or fracture or in healed
osteomyelitis. Differentiating cancer from osteomyelitis may be difficult with
MRI.
Scintigraphy: Multiple different nuclear medicine imaging procedures are
available to evaluate for osteomyelitis, including bone scan, indium-labeled
leukocyte scan, and bone marrow scan.
Three-phase bone scan
The 3-phase bone scan uses technetium Tc 99m bound to phosphorus as
the tracer, which accumulates in areas of increased osteoblast activity (reactive
new bone formation and increased blood flow). The images obtained are immediate (flow), 15 minute (blood pooling), and 4 hour (bone imaging). The scan
findings are different in cellulitis and osteomyelitis. Cellulitis results in increased activity in the first 2 phases and normal or diffusely increased activity in the
third phase.In comparison,osteomyelitis results in intense uptake in all 3phases.
The 3-phase technetium bone scan is the test of choice in evaluating for
acute osteomyelitis if the plain film findings are normal. In this situation, it has
an estimated sensitivity and specificity of almost 95% and findings generally are
positive in 2-3 days of infection. However, any process that results in increased
bone turnover appears as a hot spot that is indistinguishable from osteomyelitis.
These false-positive findings can occur with posttraumatic injury, following
surgery, diabetic feet, septic arthritis, noninfectious inflammatory bone disease,
cancer, healed osteomyelitis, and Paget disease.
Indium-labeled leukocyte scan: Indium-labeled leukocyte scanning uses
white blood cells labeled with radioactive indium as the tracer. It accumulates at
sites of inflammation or infection and in the bone marrow. It is not specific for
bone. Since it accumulates in marrow, it is less sensitive for imaging those areas
with red marrow (eg, the axial skeleton). The indium scan also can be used for
the diagnosis of osteomyelitis at sites of fracture nonunion. Two prospective
studies found a sensitivity and specificity of 91% and 97%, respectively, in this
171
setting. Indium scans have better sensitivity and specificity than bone scans in
diabetic feet, but the specificity is poor, especially in the hindfoot.
Bone marrow scan: The bone marrow scan uses technetium Tc 99m–labeled sulfur colloid as the tracer. It is taken up by the reticuloendothelial system,
including the bone marrow, spleen, and liver. It allows one to image the marrow
instead of the bone. Its main use is in conjunction with the indium-tagged white
blood cell scan to evaluate for suspected osteomyelitis in the axial skeleton,
where the presence of marrow decreases the accuracy of the indium scan.
Gallium citrate scanning: Gallium citrate scanning uses radioactive
gallium citrate as the tracer. It acts as an analog of calcium and iron and attaches
to transferrin to accumulate at sites of inflammation. Gallium imaging is the
most sensitive and specific radionuclide scanning technique for vertebral
osteomyelitis. A typical positive test result reveals intense uptake in 2 adjacent
vertebrae with loss of the intervening disk space. In one study of 41 patients
with suspected vertebral osteomyelitis, increased gallium uptake was detected in
all of the 39 patients with biopsy-proven osteomyelitis.
Dual tracer scans: Dual tracer examinations combine an inflammation
imaging tracer (indium or gallium) with an "anatomic" tracer (either technetium
bone scan or technetium sulfur colloid marrow scan), with images being
collected either sequentially or simultaneously. Sequential studies combine the
sensitivity of the bone scan (if findings are negative, no further imaging is done)
with the specificity of the indium scan. Simultaneous studies combine the
sensitivity and specificity of the indium scan and the bone scan to provide the
anatomic detail needed to localize the infection to bone or soft tissue.
Other: Radiolabeled antigranulocyte antibodies are being investigated in
an attempt to find a more accurate tracer for localizing infection.
Treatment: Medical and surgical treatments are usually required although
in some rare occasions sole antibiotic treatment may be successful.
Principles of treatment are:
Evaluation and correction of compromised host defenses.
Gram staining and culture and sensitivity testing.
Imaging of the region to determine the extend of the lesion and to rule out
the presence of tumors.
Empirical administration of Gram stain –guided antibiotics.
Removal of loose teeth and sequestra.
Prescription of culture-guided antibiotic therapy.
Possible placement of irrigating drains / polymethylmethacrylate –
antibiotic beads.
Sequestrectomy, debridement, decortication, resection or reconstruction
as indicated.
Acute Suppurative ostemyelitis:
The initial management usually is aided by hospitalization to administer
high doses of antibiotic therapy, identify and correct host compromise factors
and teat the cause.
172
Since many organisms are responsible for ostemyelitis and are resistant to
penicillin, a drug effective against those should be added to the regiment.
Examples are: Penicillin + Metronidazole, Amoxicillin + Metronidazole,
Amoxicillin + Clavulanate potassium and Ampicillin + Sulbactam sodium.
Other effective regiments are Clindamycin, Clindamycin + metronidazole and
Cephalosporines.
Chronic Suppurative Osteomyelitis:
Requires surgical procedures in addition to antibiotic treatment.
Antibiotic therapy should be initiated with intravenous administration of
medications usually Ampicillin +Sulbactam.for 2 weeks or until patient is
showing improvement for 48-72 hours. Oral therapy should be continued for 4-6
weeks after the patient has no symptoms or from the date of the last
debridement.
Clindamycin can be used as an alternative is Unasyn is not effective.
Antibiotic Therapy in Osteomyelitis of long bones:
Organism First-Choice Antibiotics Alternative Antibiotics
S aureus
Nafcillin or cloxacillin100-200 mg/kg/d q6h
Cefazolin
(methicillin sensitive)
S aureus (methicillin resistant) Vancomycin 1 g q12h Trimethoprimsulfamethoxazoleor minocycline plus rifampin
Coagulase-negative organisms Vancomycin 1 g q12h ornafcillin 2 g q6h (if
sensitive) Cefazolin, clindamycin
Group A or Bstreptococci
Clindamycin 900 mg q8h
Benzylpenicillin,
cefazolin
Enterococci Ampicillin 50-100 mg/kg/d q6h+ gentamicin 5 mg/kg/d q8h
Vancomycin
E coli,P mirabilis Ampicillin 2 g q6hCefazolin, gentamicin,levofloxacin
Local Antibiotic Therapy:
Closed wound irrigation-suction:
Tubes are placed against the bone to allow for drainage of pus and serum
and for irrigation in order to reduce the number of accumulated microorganisms.
This type of therapy is especially helpful when determination of the extend of
chronic infection of residual bone cannot be determined.
Irrigation though, prior debridement is unlikely to be effective and it
might actually prolong the process.
Technique: After debridement saucerization or decortication, small
pediatric nasogastric feeding tubes, French catheters or polyethylene irrigation
tubes3-4mm in diameter and 6-10 inches in length are perforated along a
distance 3-4cm from the tip. The tubes are placed into the bone bed through
separate skin incisions along the lateral bony surface and are affixed to the bone
with sutures through holes drilled into the bone.
Two drains are usually placed, one serving for antibiotic irrigation while
the second one serves for suction. The skin is closed water tight over the tubes.
Multiple ways of irrigation-suction system exist;
173
2lt of antibiotic solution can be instilled and then suctioned every 24h,the
solution can be instilled on a 12 h cycle and left in place for 3h then suctioned
for 9h via a low intermittent suction.
Important points: the wound should not be overfilled and the volume of
the irrigation antibiotic solution should be gradually decreased to allow for
closure of the wound and healing.
Systemic antibiotics should be used throughout the irrigation period and at
least for 2 months after the cessation of clinical evidence of disease.
Antibiotic – Impregnated beads:
The beads are used to deliver high concentrations of antibiotics into the
wound bed and in immediate proximity to the infected bone.
The beads release high local concentrations, but low systemic
concentrations thus reducing the risk of toxicity.
Tobramycin or gentamycin or clindamycin contained in acrylic resin bone
cement beads. Usually a chain of beads is applied against bleeding bone after
decortication and a drain is inserted and the wound is closed. The system is left
in place for 10-14 days and removed. Systemic antibiotics are administered
simultaneously.
Hyperbaric oxygen therapy: it has been used to promote healing in refracttory chronic ostemyelitis. In situations in which osteomyelitis is associated with
decreased systemic blood flow (eg, in diabetes, vasculitis) or segmental blood
flow (eg, in trauma), hyperbaric oxygen therapy may be used as adjunctive
therapy. Reduced oxygen tensions in infected bone have been shown to interfere
with normal polymorphonuclear leukocyte activity. Hyperbaric oxygen therapy
has been shown to increase the oxygen tensions within infected bone, thereby
augmenting polymorphonuclear leukocyte and macrophage activity.
Wound healing is a dynamic process that requires an adequate oxygen
tension to proceed. In the ischemic or infected wound, hyperbaric oxygen
therapy provides oxygen to promote collagen production, angiogenesis, and
ultimately wound healing. Referral for hyperbaric oxygen is made whenever
refractory osteomyelitis occurs or a soft tissue infection develops that is not
amenable to a local or microvascular flap.
Surgical Treatment:
Surgical treatment as an adjunct to medical management is usually
necessary.
In the acute stage it should be limited to removal of severely loose teeth
and bone fragments and incision and drainage of fluctuant areas,
osteoperforation. This may proceed to sequestrectomy with or without
saucerization, decortication resection and then reconstruction.
Application of heat is highly discouraged because of the risk of extention
o fth einfection through bone.
Treatment of systemic conditions and supportive therapy consisting of
high –protein, high vitamin diet with adequate hydration should be instituted
simultaneously.
174
Surgical therapy: Surgical management of contiguous focus osteomyelitis
can be very challenging. The principles of treating any infection are equally
applicable to the treatment of infection in bone. These include adequate
drainage, extensive debridement of all necrotic tissue, obliteration of dead
spaces, stabilization, adequate soft tissue coverage and restoration of an
effective blood supply.
The number and nature of the required surgical procedures increases with
the severity of the infection, which can be divided into 4 categories, as follows:
Category 1 – Removal of necrotic tissue by extensive debridement
Category 2 – Dead space obliteration with flaps, antibiotic beads, and bone
grafts
Category 3 – Provision of soft tissue coverage of the bone
Category 4 – Stabilization of bone by external or open reduction and internal
fixation
Category 1
Debridement surgery is the foundation of osteomyelitis treatment. It is the
most commonly performed procedure and may need to be repeated multiple
times. The goal of debridement is to reach healthy, viable tissue, but even when
all necrotic tissue has been adequately debrided, the remaining bed of tissue
must be considered contaminated with the responsible organism. Debridement
should be direct, atraumatic and executed with reconstruction in mind. All dead
or ischemic hard and soft tissue is excised unless a noncurative procedure has
been chosen. Surgical excision of bone is carried down to uniform haversian or
cancellous bleeding, known as the paprika sign.
Sequestrectomy:
Sequestra which can be cortical or cortico-cancelous are usually seen after
2 weeks from the onset of the infection and once fully formed can persist for
several months. Sequastra are avascular therefore poorly penetrated by
antibiotics. Sequastra can be removed with minimal trauma, but the risk of
subjecting the patient to a chronic infection and prolong antibiotic treatment
should be bared in mind.
Sequestectomy and Saucerization:
Saucerization is the unroofing of the bone to expose the medullary cavity.
This is ususfull in chronic osteomyelitis, since it permits excision of sequestra
either formed or forming ones.It should be performed as soonas the acure stage
has resolved, so to decompress the bone allow for extrusion of pus, debris and
avascular bone.
Procedure:
1. A full thickness mucoperiosteal flap is reflected usually buccaly, to exposed
infected bone. Reflection should not comprise blood supply.
2. Loose teeth and bony sequestra are removed.
3. The lateral cortex of the mandible is reduced, until bleeding bone is
encountered, producing a saucerlike defect.
4. All granulation tissue and loose bony fragments are removed, irrigation
follows and hemostasis is achieved.
175
5. The wound is packed lightly with gauze covered with triple antibiotic
ointment and the flap is loosely sutured over. The packing is removed 3-6 days
and is replaced several times until the surface of the bone is epithelialized.There
is minimal or no risk of fracture in the jaws with this type of procedure unlike
long bones. In long bones primary closure and immediate bone graft may be
required.Saucerization is rarely required for the maxilla.
Decortication:
First introduced in 1917 and further described by Mowlem, decortication
involves removal of the chronically infected cortex, usually the buccal and the
inferior border are removed 1-2cm beyond the affected area. It can be used as
initial treatment of primary or secondary chronic ostemyelitis or more
commonly when initial conservative treatment has failed.
Procedure:
1. Full thickness mucoperiosteal flap is reflected buccaly and extends to the
inferior border.
2. Involved teeth are removed.
3. The buccal cortex and the inferior borders are removed until bleeding bone is
encountered.
4 Primary closure is achieved and pressure dressing is applied to maintain close
contact of the bone bed to the vascular soft tissue. Irrigation tubes and or
antibiotic beads may be placed.
If extensive debridement is required and the remaining bone is suspected
to be prone to fracture, appropriate stabilization and reconstruction should be
performed.
Resection and reconstruction:
May be required for low- grade persistent or chronic ostemyelitis. It is
especially used in cases of persisted infection after decortication, marked disease
involving both buccal and lingual cortices and in cases of pathologic fractures.
Category 2
Adequate debridement may leave a large bony defect (dead space).
Appropriate management of dead space created by debridement surgery is
mandatory in order to arrest the disease and to maintain the integrity of the
skeletal part. The goal of dead space management is to replace dead bone and
scar tissue with durable vascularized tissue. Local tissue flaps or free flaps may
be used to fill dead space. An alternative technique is to place cancellous bone
grafts beneath local or transferred tissues where structural augmentation is
necessary. Careful preoperative planning is critical for conservation of the
patient's limited cancellous bone reserves. Open cancellous grafts without soft
tissue coverage are useful when a free tissue transfer is not a treatment option
and local tissue flaps are inadequate.
Complete primary or delayed primary wound closure should be performed
whenever possible. Suction irrigation systems are not recommended because of
the high incidence of associated nosocomial infections and the unreliability of
the apparatus. Healing by secondary intent is also discouraged since the scar
tissue that fills the defect may later become avascular. Antibiotic-impregnated
176
acrylic beads can be used to sterilize and temporarily maintain dead space. The
beads are usually removed within 2-4 weeks and replaced with a cancellous
bone graft. The most commonly used antibiotics in beads are vancomycin,
tobramycin and gentamicin. Local delivery of antibiotics (amikacin,
clindamycin) into dead space also can be achieved with an implantable pump.
Category 3
Adequate coverage of the bone by soft tissue is necessary to arrest
osteomyelitis. Most soft tissue defects are closed primarily, but small soft tissue
defects may be covered with a split thickness skin graft. In the presence of a
large soft tissue defect or an inadequate soft tissue envelope, local muscle flaps
and free vascularized muscle flaps may be placed in a 1- or 2-stage procedure.
Local and free muscle flaps, when combined with antibiotics and surgical
debridement of all nonviable osseous and soft tissue for chronic osteomyelitis,
have a success rate ranging from 65% to 100%. Local muscle flaps and free
vascularized muscle transfers improve the local environment by supplying blood
vessels, which are critical for host defense, antibiotic delivery, and osseous and
soft tissue healing.
Category 4
If movement is present at the site of infection, measures must be taken to
achieve permanent stability of the skeletal unit. Stability can be achieved with
plates, screws, rods, and/or an external fixator. One type of external fixation
allows bone reconstruction of segmental defects and difficult infected
nonunions. The Ilizarov external fixation method uses the theory of distraction
histogenesis, in which bone is fractured in the metaphyseal region and slowly
lengthened. The growth of new bone in the metaphyseal region pushes a
segment of healthy bone into the defect left by surgery. The Ilizarov technique is
used for difficult cases of osteomyelitis when stabilization and bone lengthening
are necessary. It also can be used to compress nonunions and correct malunions,
and in a small group of patients for reconstruction of difficult deformities that
result from osteomyelitis.
However, this technique is labor intensive and requires an extended period
of treatment, averaging 9 months in the device. The Ilizarov pins usually
become infected, and the device is painful. Infected pseudoarthrosis with
segmental osseous defects can be treated by debridement and microvascular
bone transfers. Vascularized bone transfer is also useful for the treatment of
infected segmental osseous defects of long bones that are more than 3 cm in
length. Vascularized bone transfers can be placed after 1 month without clinical
evidence of infection.
Loss of bone stability, bone necrosis, and soft tissue damage frequently
occur in contiguous focus osteomyelitis. Surgical debridement of infected bone
and soft tissue provides specimens for culture and hastens eradication of the
infection. Other steps in the surgical management of contiguous focus
osteomyelitis should be tailored to the specific anatomy of the bone infection.
When osteomyelitis is characterized by a full thickness, cortical sequestration,
patients usually can be treated with removal of the dead infected bone (bone
177
saucerization). Bone grafting may be necessary to augment structural support.
These patients may require external fixation for structural support while the
bone graft incorporates. Complex reconstruction of both bone and soft tissue is
frequently necessary.
In some cases, osteomyelitis progresses to an infection involving the
entire diameter of the bone. These patients often require an intercalary resection
of the bone in order to arrest the disease process. Since this advanced stage of
osteomyelitis involves an entire through-and-through section of bone, a loss of
bony stability occurs either before or after debridement surgery. As a result,
treatment often must be directed toward establishing structural stability and
obliterating debridement gaps by means of cancellous bone grafts or the Ilizarov
technique. Vascularized bone grafting is the other possible treatment modality.
Other types of ostemyelitis of the jaws:
Ostemyelitis associated with fractures:
Either failure to achieve effective fixation or overzealous use of bone
plates, wires and screws that compromise the vascularity of the fragments may
lead to ostemyelitis. Immediate IMF should be employed in order to offer
comfort and decrease the ingress of microorganisms and debris caused by
movement. High doses of antibiotics along with removal of loose teeth
fragments and foreign bodies (plates, screws, wires) are necessary
measurements to facilitate healing.
Infantile Ostemyelitis:
Although uncommon disease for the jaws it merrist special attention since
it involves risks with ocular, intracranial spread and facial deformities.
It is believed to occur by hematogenous route or from perinatal trauma, it
occurs few weeks after birth and usually involves the maxilla. Before the
antibiotic era a 30% mortality rate was approached.
Clinical findings involve facial cellulites centered about the orbit and is
associated with inner and outer canthal swelling, palpebral edema, closure of the
eye and proptosis. Purulent discharge from the nose and the medial canthus may
be evident. Generalized symptoms include fever, irritability, malaise,
anorexia,dehydration and even convulsions and vomiting.
Intraoraly involvement of the maxilla buccaly and palataly is evident with
fistulas occasionally present.
Little radiographic change is noted early, but CT scans may be used to
demonstrate orbital abscess, possible dural extension or involvement of the
sinus. Leukocytosis with left shift is usually present. The usual offending
microorganism is Staph. Aureus. Penicillin G or Ampicillin –Sulbactam, or
Clindamycin should be administered early. If there are areas of fluctuance,
incision and drainage should be utilized to facilitate decompression possibly
prevent further spead and in order to obtain speciment for cultures and
sensitivety. Supportive treatment, hydration, fever control and very close
monitoring should be employed. Antibiotic treatment should continue for 2-4
weeks after all signs of infection have subsided.
178
Chronic recurrent multifocal ostemyelitis and its mandibular
manifestation diffuse sclerosing ostemyelitis and the most recently introduced
clinical term “ primary chronic ostemyelitis”. The term describes a disease with
an insidious onset that lacks an acute stage. It shows periodic episodes with
differing intensity, lasting from few days to several weeks. Common
presentation pain, swelling, limited mouth opening and regional
lymphadenopathy. No pus formation no fistulas and no sequestration are
present. It is not limited to a certain group, but most data reported involves adult
patients.
Association with the heterogenic SAPHO syndrome is recently been
suggested.
SAPHO stands for Synovitis, Acne, Pustulosis, Hyperkeratosis and Osteitis.
Radiographicaly there is evidence of extensive involvement of the
mandible b/l, with mixed pattern of sclerosis and osteolysis, with extragnathic
involvement evident by scintigraphic imaging. Usually treatment requires more
than one surgical procedures, hyperbaric oxygen and need for long follow ups
due to high risk of recurrence.
Furuncle
Boil or furuncle is a skin disease caused by the inflammation of hair
follicles, thus resulting in the localized accumulation of pus and dead tissues.
Individual boils can cluster together and form an interconnected network of
boils called carbuncles. In severe cases, boils may develop to form abscesses.
Symptoms. The symptoms of boils are red, pus-filled lumps that are
tender, warm, and/or painful. A yellow or white point at the center of the lump
can be seen when the boil is ready to drain or discharge pus. In a severe
infection, multiple boils may develop and the patient may experience fever and
swollen lymph nodes. A recurring boil is called chronic furunculosis.
In some people, itching may develop before the lumps begin to develop.
Boils are most often found on the back, underarms, shoulders, face, lip, thighs
and buttocks, but may be found elsewhere. Boils on the ear tend to be more
painful, and can create shooting pain in the entire area when touched.
Sometimes boils will emit an unpleasant smell, particularly when drained
or when discharge is present, due to the presence of bacteria in the discharge.
Symptoms
Skin lesion is a papule or nodule
Usually is pea-sized, may occasionally be as large as a golf ball
Is usually swollen
Is pink or red
May grow rapidly
May develop white or yellow centers
May weep, ooze, or crust
May be located with hair follicles
Is tender, mildly to moderately painful
179
May be single or multiple
May run together (coalesce) or spread to other skin areas
May increase in painfulness as pus and dead tissue fills the area
May decrease in painfulness as the area drains
Skin redness or inflammation around the boil
Fever (occasionally)
Fatigue (occasionally)
General discomfort, uneasiness, or ill feeling (occasionally)
Itching of the skin may occur before the skin lesions develop.
Signs and tests. Diagnosis is primarily based on the appearance of the
skin. A skin biopsy and bacterial culture of the lesion may help to make the
diagnosis or determine the exact type of bacteria involved.
Causes. Boils are generally caused by an infection of the hair follicles by
Staphylococcus aureus or staph, a strain of bacteria that normally lives on the
skin surface. It is thought that a tiny cut of the skin allows this bacterium to
enter the follicles and cause an infection. This can happen during bathing or
while using a razor.
People with immune system disorders, diabetes, poor hygiene or
malnutrition (Vitamin A or E deficiency) are particularly susceptible to getting
boils; however, they also occur in healthy, hygienic individuals. Hidradenitis
suppurativa causes frequent boils. Boils in the armpits can sometimes be caused
by anti-perspirant deodorants.
Treatments. Most boils run their course within 4 to 10 days. For most
people, self-care by applying a warm compress or soaking the boil in warm
water can help alleviate the pain and hasten draining of the pus (colloquially
referred to as "bringing the boil to a head"). Once the boil drains, the area should
be washed with antibacterial soap and bandaged well. For recurring cases,
sufferers may benefit from diet supplements of Vitamin A and E.
In serious cases, prescription oral antibiotics such as dicloxacillin
(Dynapen) or cephalexin (Keflex), or topical antibiotics, are commonly used.
For patients allergic to penicillin-based drugs, erythromycin (E-base, Erycin)
may also be used.
However, some boils are caused by a "Antibiotic resistance" superbug
known as community-acquired Methicillin-resistant Staphylococcus aureus, or
CA-MRSA. Bactrim or other sulfa drugs must be prescribed relatively soon
after boil has started to form. MRSA tends to increase the speed of growth of the
infection. Magnesium sulfate paste applied to the affected area can prevent the
growth of bacteria and reduce boils by absorbing pus and drying up the lesion.
Complications
Spread of infection to other skin surfaces
Abscess formation
Sepsis (general internal infection)
Abscess of kidneys or other internal organs
Osteomyelitis
180
Endocarditis
Brain infection
Brain abscess
Spinal cord infection
Spinal cord abscess
Permanent scarring of the skin
Carbuncle
Definition. A carbuncle is a local, but deep, staphylococcal skin infection.
Causes, incidence, and risk factors. A carbuncle consists of several
furuncles that develop close together. They expand and join together to form a
larger mass (aggregation of cells) with multiple drainage points. This mass may
be deeper beneath the skin surface than simple furuncles. They develop slowly,
and may be so deep that they do not drain on their own. Carbuncles may develop
anywhere, but they are most common on the back and the nape of the neck.
Carbuncles are less common than boils. Men are more prone to carbuncles than
women.
Staph skin infections are contagious. They may spread to other areas of
the body, and may spread to other people. It is not uncommon for several family
members to be affected at the same time. Poor hygiene, run-down physical
condition, friction from clothing or shaving, and similar factors may make these
infections more likely. Diabetics and people with suppressed immune systems
are more prone to development of staph skin infections, as are people with
dermatitis (skin inflammations). Often, however, no direct cause is found for
furunculosis or carbunculosis.
Symptoms
Skin lesion nodule
Usually is pea-sized, may occasionally be as large as a golf ball
Is usually swollen
Is pink or red
May grow rapidly
May develop white or yellow centers May weep, ooze, or crust
May be located with hair follicles
Is tender, mildly to moderately painful
May be single or multiple
May run together (coalesce) or spread to other skin areas
May increase in painfulness as pus and dead tissue fills the area
May decrease in painfulness as the area drains
Skin redness or inflammation around the boil
Fever (occasionally)
Fatigue (occasionally)
General discomfort, uneasiness, or ill feeling (occasionally)
Itching of the skin may occur before the skin lesions develop.
181
Signs and tests. Diagnosis is primarily based on the appearance of the
skin. A skin biopsy and bacterial culture of the lesion may help to make the
diagnosis or determine the exact type of bacteria involved.
Treatment. Carbuncles usually must drain before they will heal. This
most often occurs in less than 2 weeks. Carbuncles that persist longer than 2
weeks, recur, are located on the spine or the middle of the face, or that are
accompanied by fever or other symptoms require treatment by a health care
provider because of the risk of complications from the spread of infection.
Antibacterial soaps, topical (applied to a localized area of the skin)
antibiotics, and systemic antibiotics may help to control infection. Warm moist
compresses encourage carbuncles to drain, which speeds healing. Gently soak
the area with a warm, moist cloth several times each day. Never squeeze a boil
or attempt to lance it at home because this can spread the infection and make it
worse.
Deep or large lesions may need to be drained surgically by the health care
provider.
Meticulous hygiene is vital to prevent the spread of infection. Draining
lesions should be cleaned frequently. The hands should be washed thoroughly
after touching a boil. Do not re-use or share washcloths or towels. Clothing,
washcloths, towels, and sheets or other items that contact infected areas should
be washed in very hot (preferably boiling) water. Dressings should be changed
frequently and discarded in a manner that contains the drainage, such as by
placing them in a bag that can be closed tightly before discarding.
Expectations (prognosis)
Carbunculosis may heal spontaneously and usually responds well to
treatment. It often recurs for months or years following an initial infection.
Complications
Spread of infection to other skin surfaces
Abscess formation
Sepsis (general internal infection)
Abscess of kidneys or other internal organs
Osteomyelitis
Endocarditis
Brain infection
Brain abscess
Spinal cord infection
Spinal cord abscess
Permanent scarring of the skin
Phlegmon
Phlegmon
Definition. Phlegmon is a spreading diffuse inflammatory process with
formation of suppurative/purulent exudates or pus.
Etiology. Commonly by bacteria - staphylococci, streptococci,
pneumococci, spore and non-spore forming anaerobes, etc. Factors affecting the
182
development of phlegmons are virulence of bacteria and human's immunity
strength defenses.
Classifications
1)By clinical course:
a)acute
b)subacute
2)By severity of condition:
a)mild
b)average
c)severe (with spreading to other location(s))
3)By location:
I)Superficial
a)cutaneous
b)subcutaneous
c)interstitial tissue
d)intramuscular
II)Deep
e)mediastinal
f)retroperitoneal
4)By etiology:
a)single
b)mix (eg:spore and non-spore forming anaerobs)
5)By pathogenesis:
a)per continuitatem (through neighbouring tissues)
b)hematogenous (through non-valvular veins like venous plexus of face eg: v.
pterygoideus plexus → inflamation of veins (phlebitis) → thrombus formation
in veins → embolization of thrombus into sinus venousus systems)
c)odontogenous
6)By exudative character:
a)purulent phlegmon
b)purulent-hemorrhagic phlegmon
c)putrefactive phlegmon
7)By presence of complications:
a)with complications (disturbance of mastication, ingestion, speech,
cardiovascular and respiratory system, peritonitis, lymphadenitis, loss of
conscious if very severe, etc)
b)without complication
Clinical Pictures.
1) Symptom of intxoxication like increase body temperature (up to 38-40°C),
general fatigue, chills, sweatings, headache, loss of appetite).
2)Inflammatory signs - dolor(localized pain), calor(increase local tissue
temperature), rubor (skin redness/hyperemia), tumor(either clear or non-clear
bordered tissue swelling), functio laesa(diminish affected function).
183
NB: severity of patient condition with phlegmons is directly proportional
to the degree of intoxication level i.e the severe the condition, the higher degree
of intoxication level.
Diagnostics. 1)Complaints and clinical appearances 2)Anamnesis
3)Visual and Palpations 4)Blood test - leucocytosis (up to 10-12x109/L),
decrease or absence eosinophils level, shift of leucocyte formula to the left
(neutrophilos), increase ESR (up to 35-40 mm/hr). 5)Urine test - presence of
bacteria in urine, increase urinary leucocyte counts. 6)X-ray test 7)Ultrasound
test
Treatments. The main goal treatment remove the cause of phlegmonous
process in order to achieve effective treatment and prevention of residives.
If patients condition is mild and the signs of inflammatory process is
presence without signs of infiltrates, then conservative treatment with antibiotics
is sufficient.
In severe condition, immediate operation is necessary with application of
drainage system. All of these are done under general anaesthesia. During
operation, the cavity or place of phlegmonous process are wash with antiseptic,
antibiotic solutions and proteolyic ferments.
In post-operative period, i/v drips of detoxification, antibiotics,
haemosorbtion, vitaminotherapy. Additionally, the use of i/v or i/m
antistaphylococci γ-globulin or anatoxin can be taken as immunotherapy.
During operation of phlegmon dissection at any location, it is important:
1)to avoid spreading of pus during operation 2)take into account the cosmetic
value of operating site especially phlegmmonous process of face. 3)during
dissection, avoid damaging nerves especially facial nerves. Use the correct
incision line.
Treatment of Pyoinflammatory Diseases: Soft tissue pyoinflammatory
diseases – phlegmon, abscess, pyodermia, pyomyositis – after incision and
tissue removal is processed by sodium solution and irrigated by bacteriophage.
Phage impregnated tampons can be put in the wound. The permanent putting of
phages in the wound by tampon impregnation or by drainage is preferable. The
optimal drainage of the exudates presents the significant condition – under
conditions of pus stasis the penetration of phage into the focus of infection is
hampered, the liquid phage is impregnated and looses the concentration.
The phage therapy effect is increased by use of proteolytic medications
locally, which increases the phages tissue permeability and decreases the
potential of microbe resistance.
Putting of phages in the tissues is especially effective by ultra-low
frequency cavitation – the bacteriophage and proteolytic enzyme are added to
the special solution.
Processing of Purulent Cavities:
Pleural, abdominal and abscess cavities. After preliminary rinsing of
drainages placed in the cavities, the cavity is rinsed by liquid phage medication.
At the end of the procedure the 3-5 ml of medication is left in the cavity.
The permanent flowing irrigation of cavities (wounds):
184
In the cavities (wounds) must be the excretive drainage from low point and
micro irrigator at higher level. The permanent irrigation – lavage is carried out
by permanent or fractional flushing.
Creating the Depo-Focus of bacteriophage:
Is achieved by using the immobilized bacteriophage, antibiotic, and
proteolytic enzyme on the biodegradable polymer. This medication
“PhagoBioDerm” is manufactured in two forms – perforated plates and in the
form of powder. It is used:
for preventive measures: leaving in the surgical wound (in this case the
powder is preferable)
for therapy: after reaching of the good lavage of purulent wound and
ensuring the perfect drainage by liquid medication, the wound needs
dressing. In this case the “PhagoBioDerm” plate or powder is placed in
the wound for 2-5 days and the wound is not dressed during indicated
period.
in deep cavities – abdominal cavity, liver abscess, soft tissues, maxillary
sinus – the permanent leaving of “PhagoBioDerm” is possible. It’s full
lysis and resorption is done within 2-4 months. In this period of time the
antibacterial concentration of bacteriophage permanently exists.
the predetermination of micro flora sensitivity to phage especially
increases the effect of phage therapy.
Abscess
An abscess is a localized collection of pus in any part of the body that is
surrounded by swelling (inflammation).
An abscess is a collection of pus that has accumulated in a cavity formed
by the tissue on the basis of an infectious process (usually caused by bacteria or
parasites) or other foreign materials (e.g. splinters or bullet wounds). It is a
defensive reaction of the tissue to prevent the spread of infectious materials to
other parts of the body.
The organisms or foreign materials that have gained access to a part of
tissue kill the cells, resulting in the release of toxins. The toxins trigger an
inflammatory response, which 1) draws huge amounts of white blood cells to
the area and 2) increases the regional blood flow.
The final structure of the abscess is an abscess wall that is formed by the
adjacent healthy cells in an attempt to build a barrier around the pus that limits
the infected material from neighboring structures and also limits immune cells
from attacking the bacteria.
Abscesses must be differentiated from
empyemas, which are
accumulations of pus in a preexisting rather than a newly formed anatomical
cavity.
Causes, incidence, and risk factors. Abscesses occur when an area of
tissue becomes infected and the body's immune system tries to fight it. White
blood cells move through the walls of the blood vessels into the area of the
185
infection and collect within the damaged tissue. During this process, pus forms.
Pus is the build up of fluid, living and dead white blood cells, dead tissue, and
bacteria or other foreign substances.
Abscesses can form in almost every part of the body and may be caused
by infectious organisms, parasites, and foreign substances. Abscesses in the skin
can be easily seen, and are red, raised, and painful. Abscesses in other areas of
the body may not be obvious, but if they may cause significant organ damage.
Manifestations
The cardinal symptoms and signs of any kind of inflammatory process are
redness, heat, swelling, pain and loss of function. Abscesses may occur in any
kind of solid tissue but most frequently on skin surface (where they may be
superficial pustules (boils) or deep skin abscesses), in the lungs, brain, kidneys
and tonsils. Major complications are spreading of the abscess material to
adjacent or remote tissues and extensive regional tissue death (gangrene).
Abscesses in most parts of the body rarely heal themselves, so prompt medical
attention is indicated at the first suspicion of an abscess.
Treatment. The abscess should be inspected to identify if foreign objects
are a cause, requiring surgical removal. Surgical drainage of the abscess (e.g.
(Surgical Procedure)" lancing) is usually indicated once the abscess has
developed from a harder serous inflammation to a softer pus stage. This is
expressed in the Latin medical aphorism Ubi pus, ibi evacua.
As Staphylococcus aureus bacteria is a common cause, an antiStaphylococcus antibiotic such as Flucloxacillin or dicloxacillin is used. It is
important to note that antibiotic therapy alone without surgical drainage of the
abscess is seldom effective. If foreign objects are not the cause, surgical removal
is not needed, but for a normal infection a doctor will prescribe antibiotics and
painkillers to treat the abscess.
In critical areas where surgery presents a high risk, surgery may be
delayed or used as a last resort. The drainage of a lung abscess may be
performed by positioning the patient in a way that enables the contents to be
discharged via the respiratory tract. Warm compresses and elevation of the limb
may be beneficial for skin abscess.
Recurrent infections
For recurrent infections due to [Staphylococcus], consider the following
measures:
Topical mupirocin applied to the nares. In this randomized controlled
trial, patients used nasal mupirocin twice daily 5 days a month for 1 year.
Chlorhexidine baths. In a randomized controlled trial, nasal recolonization with
S. aureus occurred at 12 weeks in 24% of nursing home residents receiving
mupirocin ointment alone (6/25) and in 15% of residents receiving mupirocin
ointment plus chlorhexidine baths daily for the first three days of mupirocin
treatment (4/27). Although these results did not reach statistical significance,
the baths are an easy treatment.
186
Prevention of abscesses depends on where they may develop. For
example, good hygiene can help prevent skin abscesses. Dental hygiene and
routine care will prevent dental abscesses
Pericoronitis
The mandibular retromolar pad or operculum is often hyperplastic,
pushing against or even overlapping the last molar in the arch. Food debris and
bacteria may become entrapped between this pad and the tooth, resulting in
acute infection and extreme pain. This pericoronitis was first reported by Gunnel
in 1844 as "painful affection."
Discussion
Pericoronitis is a special type of acute periodontal abscess that occurs
when ginngival tissue (operculum) overlies an erupting tooth (usually a third
molar, also known as a wisdom tooth). Recurring acute symptoms are usually
initiated by trauma from the opposing tooth or by impaction of food or debris
under the flap of tissue that partially covers the erupting tooth.
When dental referral is not readily available, one procedure for releiving
the pain is surgical removal of the operculum. inject local anesthetic directly
into the overlying tissue and then cut it away using the outline of the tooth as a
guide for the incision. Sutures are not required.
Clinical Features
Pericoronitis typically occurs in teenagers and young adults, presenting
shortly after the eruption of the second or third mandibular molars. It presents as
an erythematous, tender, sessile swelling of the retromolar pad, sometimes with
surface ulceration from continuous trauma from the opposing maxillary molars.
Pus may be expressed from the tissue/tooth interface, and a foul taste may be
present. Pain may be mild but is usually quite intense and may radiate to the
external neck, the throat, the ear, or the oral floor. The patient often cannot close
the jaw because of tenderness and extreme pain may, conversely, result in the
inability to open the jaws more than a few millimeters (trismus or "lock jaw").
Cervical lymphadenopathy, fever, leukocytosis, and malaise are common signs
and symptoms, and the malady may be associated with an ipsilateral tonsillitis
or upper respiratory infection.
Pathology and Differential Diagnosis
Pericoronitis is usually surgically removed after a course of antibiotic
therapy in order to prevent future painful episodes, hence, active pus production
is seldom seen in biopsy samples. The retromolar mass is comprised of an
admixture of moderately dense collagenic tissue and edematous granulation
tissue, with moderate to large numbers of mixed chronic inflammatory cells
throughout. The superior mucosa may be ulcerated with an ulcer bed of fibrinoid
necrotic debris. The epithelium immediately adjacent to the offending tooth
typically presents with a combination of rete process hyperplasia, degeneration
and necrosis, perhaps with associated neutrophils. Bacterial colonies, dental
plaque and necrotic food debris may be attached to the epithelium. The
187
pathologist should distinguish this lesion from pyogenic granuloma and routine
gingivitis, and this often requires correlation with clinical features.
Treatment and Prognosis
Acute pericoronitis is treated by local antiseptic lavage and gentle
curettage under the flap, with or without systemic antibiotics. Once the acute
phase is controlled, the offending molar is extracted or a wedge of hyperplastic
pad tissue is removed surgically. Recurrence is unlikely with either of these
treatments
DISEASES OF TEMPOROMANDIBULAR JOINT
Arthritis
Arthritis (from Greek arthro-, joint + -itis, inflammation; plural:
arthritides) is a group of conditions where there is damage caused to the joints
of the body. Arthritis is the leading cause of disability in people over the age of
65.
There are many forms of arthritis, each of which has a different cause.
Rheumatoid arthritis and psoriatic arthritis are autoimmune diseases in which
the body is attacking itself. Septic arthritis is caused by joint infection. Gouty
arthritis is caused by deposition of uric acid crystals in the joint that results in
subsequent inflammation. Additionally, there is a less common form of gout that
is caused by the formation of needle shaped crystals of calcium pyrophosphate.
This form of gout is known as pseudogout. The most common form of arthritis,
osteoarthritis is also known as degenerative joint disease and occurs following
trauma to the joint, following an infection of the joint or simply as a result of
aging. There is emerging evidence that abnormal anatomy may contribute to
early development of osteoarthritis.
Causes, incidence, and risk factors. Arthritis involves the breakdown of
cartilage. Cartilage normally protects the joint, allowing for smooth movement.
Cartilage also absorbs shock when pressure is placed on the joint, like when you
walk. Without the usual amount of cartilage, the bones rub together, causing
pain, swelling (inflammation), and stiffness.
You may have joint inflammation for a variety of reasons, including:
Broken bone
Infection (usually caused by bacteria or viruses)
An autoimmune disease (the body attacks itself because the immune
system believes a body part is foreign)
General "wear and tear" on joints
Often, the inflammation goes away after the injury has healed, the disease
is treated, or the infection has been cleared.
With some injuries and diseases, the inflammation does not go away or
destruction results in long-term pain and deformity. When this happens, you
have chronic arthritis. Osteoarthritis is the most common type and is more likely
to occur as you age. You may feel it in any of your joints, but most commonly in
your hips, knees or fingers. Risk factors for osteoarthritis include:
188
Being overweight
Previously injuring the affected joint
Using the affected joint in a repetitive action that puts stress on the joint
(baseball players, ballet dancers, and construction workers are all at risk)
History and physical examination. All arthritides feature pain. Patterns of
pain differ among the arthritides and the location. Osteoarthritis is classically
worse at night or following rest. Rheumatoid arthritis is generally worse in the
morning; in the early stages, patients often do not have symptoms following
their morning shower. In elderly people and children, pain may not be the main
feature, and the patient simply moves less (elderly) or refuses to use the affected
limb (children).
Elements of the history of the pain (onset, number of joints and which
involved, duration, aggravating and relieving factors) all guide diagnosis.
Physical examination typically confirms diagnosis. Radiographs are often used
to follow progression or assess severity in a more quantitative manner.
Blood tests and X-rays of the affected joints often are performed to make
the diagnosis.
Screening blood tests may be indicated if certain arthritides are suspected.
This may include: rheumatoid factor, antinuclear factor (ANF), extractable
nuclear antigen and specific antibodies.
Many people associate cracking joints with arthritis; however, there is no
evidence to support such an association. A joint is an area where two or more
bones meet. This area is surrounded by joint fluid to protect the bones from
rubbing against each other. When a joint is cracking, the fluid is pushed out and
the "cracking" sound is the result of a high pressure of fluid. Rheumatoid
arthritis is what happens when there is a loss of fluid in the joints causing
damage to the lining of the joint itself. There is no evidence that cracking your
knuckles causes such damage.
Types of arthritis
Primary forms of arthritis:
Osteoarthritis
Rheumatoid arthritis
Septic arthritis
Gout and pseudogout
Juvenile arthritis
Still's disease
Ankylosing spondylitis
Secondary to other diseases:
Systemic lupus erythematosus (SLE)
Henoch-Schunlein purpura
Psoriatic arthritis
Reactive arthritis (Reiter's syndrome)
Hemochromatosis
Hepatitis
189
Wegener's granulomatosis (and many other vasculitis syndromes)
Familial Mediterranean fever (FMF), HIDS (hyperimmunoglobulinemia
D and periodic fever syndrome) and TRAPS (TNF-alpha receptor
associated periodic fever syndrome).
Inflammatory Bowel Disease (Including Crohn's Disease and Ulcerative
Colitis)
Diseases that can mimic arthritis include:
Pierre
Marie-Bamberger
syndrome
(hypertrophic
pulmonary
osteoarthropathy, a paraneoplastic phenomenon of lung cancer)
multiple myeloma
osteoporosis
Treatment
Treatment options vary depending on the type of arthritis and include
physical and occupational therapy, and medications (symptomatic or targeted at
the disease process causing the arthritis). Arthroplasty (joint replacement
surgery) may be required in eroding forms of arthritis.
Treatment of arthritis depends on the particular cause, which joints are
affected, severity, and how the condition affects your daily activities. Your age
and occupation will also be taken into consideration when your doctor works
with you to create a treatment plan.
If possible, treatment will focus on eliminating the underlying cause of the
arthritis. However, the cause is NOT necessarily curable, as with osteoarthritis
and rheumatoid arthritis. Treatment, therefore, aims at reducing your pain and
discomfort and preventing further disability.
It is possible to greatly improve your symptoms from osteoarthritis and
other long-term types of arthritis without medications. In fact, making lifestyle
changes without medications is preferable for osteoarthritis and other forms of
joint inflammation. If needed, medications should be used in addition to lifestyle
changes.
Exercise for arthritis is necessary to maintain healthy joints, relieve
stiffness, reduce pain and fatigue, and improve muscle and bone strength. Your
exercise program should be tailored to you as an individual. Work with a
physical therapist to design an individualized program, which should include:
Range of motion exercises for flexibility
Strength training for muscle tone
Low-impact aerobic activity (also called endurance exercise)
A physical therapist can apply heat and cold treatments as needed and fit
you for splints or orthotic (straightening) devices to support and align joints.
This may be particularly necessary for rheumatoid arthritis. Your physical
therapist may also consider water therapy, ice massage, or transcutaneous nerve
stimulation (TENS).
Rest is just as important as exercise. Sleeping 8 to 10 hours per night and
taking naps during the day can help you recover from a flare-up more quickly
and may even help prevent exacerbations. You should also:
190
Avoid positions or movements that place extra stress on your affected
joints.
Avoid holding one position for too long.
Reduce stress, which can aggravate your symptoms. Try meditation or
guided imagery. And talk to your physical therapist about yoga or tai chi.
Modify your home to make activities easier. For example, have grab bars
in the shower, the tub, and near the toilet.
Other measures to try include:
Taking glucosamine and chondroitin - these form the building blocks of
cartilage, the substance that lines joints. These supplements are available at
health food stores or supermarkets. Early studies indicate that these compounds
are safe and may improve your arthritis symptoms. More research is underway.
Eat a diet rich in vitamins and minerals, especially antioxidants like
vitamin E. These are found in fruits and vegetables. Get selenium from Brewer's
yeast, wheat germ, garlic, whole grains, sunflower seeds, and Brazil nuts. Get
omega-3 fatty acids from cold water fish (like salmon, mackerel, and herring),
flaxseed, rapeseed (canola) oil, soybeans, soybean oil, pumpkin seeds, and
walnuts.
Apply capsaicin cream (derived from hot chili peppers) to the skin over
your painful joints. You may feel improvement after applying the cream for 3-7
days.
MEDICATIONS. Your doctor will choose from a variety of medications
as needed. Generally, the first drugs to try are available without a prescription.
These include:
Acetaminophen (Tylenol) -- recommended by the American College of
Rheumatology and the American Geriatrics Society as first-line treatment for
osteoarthritis. Take up to 4 grams a day (2 extra-strength Tylenol every 6 hours).
This can provide significant relief of arthritis pain without many of the side
effects of prescription drugs. DO NOT exceed the recommended doses of
acetaminophen or take the drug in combination with large amounts of alcohol.
These actions may damage your liver.
Aspirin, ibuprofen, or naproxen - these nonsteroidal anti-inflammatory
(NSAID) drugs are often effective in combating arthritis pain. However, they
have many potential risks, especially if used for a long time. They should not be
taken in any amount without consulting your doctor. Potential side effects
include heart attack, stroke, stomach ulcers, bleeding from the digestive tract,
and kidney damage. In April 2005, the FDA asked drug manufacturers of
NSAIDs to include a warning label on their product that alerts users of an
increased risk for heart attack, stroke, and gastrointestinal bleeding. If you have
kidney or liver disease, or a history of gastrointestinal bleeding, you should not
take these medicines unless your doctor specifically recommends them.
Prescription medicines include:
Cyclo-oxygenase-2 (COX-2) inhibitors - These drugs block an
inflammation-promoting enzyme called COX-2. This class of drugs was initially
believed to work as well as traditional NSAIDs, but with fewer stomach
191
problems. However, numerous reports of heart attacks and stroke have prompted
the FDA to re-evaluate the risks and benefits of the COX-2s. Rofecoxib (Vioxx)
and valdecoxib (Bextra) have been withdrawn from the U.S. market following
reports of heart attacks in patients taking the drugs. Celecoxib (Celebrex) is still
available, but labeled with strong warnings and a recommendation that it be
prescribed at the lowest possible dose for the shortest duration possible. Talk to
your doctor about whether COX-2s are right for you.
Corticosteroids ("steroids") - these are medications that suppress the immune system and symptoms of inflammation. They are commonly used in severe cases of osteoarthritis, and they can be given orally or by injection. Steroids
are used to treat autoimmune forms of arthritis but should be avoided in infectious arthritis. Steroids have multiple side effects, including upset stomach and
gastrointestinal bleeding, high blood pressure, thinning of bones, cataracts, and
increased infections. The risks are most pronounced when steroids are taken for
long periods of time or at high doses. Close supervision by a physician is
essential.
Disease-modifying anti-rheumatic drugs - these have been used
traditionally to treat rheumatoid arthritis and other autoimmune causes of
arthritis. These drugs include gold salts, penicillamine, sulfasalazine, and
hydroxychloroquine. More recently, methotrexate has been shown to slow the
progression of rheumatoid arthritis and improve your quality of life.
Methotrexate itself can be highly toxic and requires frequent blood tests for
patients on the medication.
Anti-biologics - these are the most recent breakthrough for the treatment
of rheumatoid arthritis. Such medications, including etanercept (Enbrel),
infliximab (Remicade) and adalimumab (Humira), are administered by injection
and can dramatically improve your quality of life.
Immunosuppressants
these
drugs,
like
azathioprine
or
cyclophosphamide, are used for serious cases of rheumatoid arthritis when other
medications have failed.
It is very important to take your medications as directed by your doctor. If
you are having difficulty doing so (for example, due to intolerable side effects),
you should talk to your doctor.
SURGERY AND OTHER APPROACHES. In some cases, surgery to
rebuild the joint (arthroplasty) or to replace the joint may help maintain a more
normal lifestyle. The decision to perform joint replacement surgery is normally
made when other alternatives, such as lifestyle changes and medications, are no
longer effective.
Normal joints contain a lubricant called "synovial fluid." In joints with
arthritis, this fluid is not produced in adequate amounts. One other treatment
approach is to inject arthritic joints with a manmade version of joint fluid such
as hylan G-F 20 (Synvisc) or other hyaluronic acid preparations. This synthetic
fluid may postpone the need for surgery at least temporarily and improve the
quality of life for arthritis patients. Many studies are evaluating the effectiveness
of this type of therapy.
192
Expectations (prognosis)
A few arthritis-related disorders can be completely cured with treatment.
Most are chronic (long-term) conditions, however, and the goal of treatment is
to control the pain and minimize joint damage. Chronic arthritis frequently goes
in and out of remission.
Complications
Chronic pain
Lifestyle restrictions or disability
Temporomandibular Joint (TMJ) Disorders
The temporomandibular joint is the connection between the temporal bone
of the skull and the lower jawbone (mandible). There are two
temporomandibular joints, one on each side of the face just in front of the ears.
Ligaments, tendons, and muscles support the joints and are responsible for jaw
movement.
Temporomandibular
disorders,
often
called
TMJ
disorders
(temporomandibular joint disorders), are most common in women in their early
20s and between the ages of 40 and 50 (in rare cases, babies are born with
temporomandibular joint abnormalities). Temporomandibular disorders include
problems with the joints, the muscles surrounding them, or both.
Causes. Most often, the cause of a temporomandibular disorder is a
combination of muscle tension and anatomic problems within the joints.
Sometimes, there is a psychologic component as well. Specific causes include
muscle pain and tightness, internal joint derangement, arthritis, ankylosis, and
hypermobility.
Muscle Pain and Tightness: Muscle pain and tightness around the jaw
(myofascial pain syndrome) come mainly from muscle overuse, often brought
on by problems of misalignment of the upper and lower sets of teeth, missing
teeth, injury to the head or neck, or even toothache. Pain is also produced by
trying to open the jaw too widely. Muscle pain and tightness can also result from
clenching or grinding the teeth (bruxism) at night due to psychologic or sleeprelated stress. Clenching and grinding while asleep exert far more force than
clenching and grinding while awake.
Internal Joint Derangement: In internal joint derangement, the disk inside
the joint lies in front of its normal position. Internal joint derangement can occur
with or without reduction. In internal joint derangement with reduction, which is
the more common type (occurring in about one third of the adult population), the
disk lies in front of its normal position only when the mouth is closed. As the
mouth opens and the jaw slides forward, the disk slips back into its normal
position. As the mouth closes, the disk slips forward again. In internal joint
derangement without reduction, the disk never slips back into its normal
position, and the degree to which the mouth can be opened is limited.
Arthritis: Arthritis in a temporomandibular joint may result from
osteoarthritis, rheumatoid arthritis, infectious arthritis, or injury, particularly
193
injury that causes bleeding into the joint. Such injuries are fairly common in
children who are struck on the side of the chin.
Osteoarthritis, a type of arthritis in which the cartilage of the joints
degenerates (Osteoarthritis (OA)), is most common in older people. The
cartilage in the temporomandibular joints is not as strong as the cartilage in
other joints. Osteoarthritis occurs mainly when the disk is missing or has
developed holes.
Rheumatoid arthritis, a disease in which the body attacks its own cells (an
autoimmune disease), causing inflammation, affects the temporomandibular
joint in only about 17% of people with this type of arthritis. The
temporomandibular joint generally is the last joint to be affected by rheumatoid
arthritis.
Infectious arthritis is caused by an infection that has spread from an
adjoining area of the head or neck or that has been carried by the bloodstream
from another part of the body.
Ankylosis: Ankylosis is loss of joint movement resulting from fusion of
bones within the joint or calcification (the deposit of calcium into body tissues)
of the ligaments around it.
Hypermobility: Hypermobility (looseness of the jaw) results when the
ligaments that hold the joint together become stretched. In hypermobility,
dislocation is usually caused by the shape of the joints, ligament looseness
(laxity), and muscle tension. It may be caused by trying to open the mouth too
wide or by being struck on the jaw.
Symptoms. Symptoms of temporomandibular disorders include
headaches, tenderness of the chewing muscles, and clicking or locking of the
joints. Sometimes the pain seems to occur near the joint rather than in it.
Temporomandibular disorders may be the reason for recurring headaches that do
not respond to usual medical treatment. Other symptoms include pain or
stiffness in the neck radiating to the arms, dizziness, earaches or stuffiness in the
ears, and disrupted sleep.
People with temporomandibular disorders have difficulty opening their
mouth wide. For example, most people without temporomandibular disorders
can place the tips of their index, middle, and ring fingers held vertically in the
space between the upper and lower front teeth without forcing. For people with
temporomandibular disorders (with the exception of hypermobility), this space
usually is markedly smaller.
Muscle Pain and Tightness: People with muscle pain usually have very
little pain in the joint itself. Rather, they feel pain and tightness on the sides of
the face upon awakening or after stressful periods during the day. Nighttime
clenching and grinding of the teeth may cause a person to awaken with a
headache, which may slowly diminish over the day. As the jaw opens, it may
move slightly (deviate) to one side or the other. The chewing muscles are
typically tender to the touch.
Internal Joint Derangement: Internal joint derangement with reduction
usually causes a clicking or popping sound in the joint when the mouth opens
194
wide or the jaw shifts from side to side. In many people, these joint sounds are
the only symptoms. However, some people experience pain, particularly when
chewing hard foods. In a small percentage of people who have missing teeth and
who grind their teeth, these sounds progress to locking of the joints.
Internal joint derangement without reduction usually produces symptoms
of pain and makes it difficult for people to open their mouth wide, as is typical
of most temporomandibular disorders. After 6 to 12 months, the pain may decrease, but the limited degree to which the mouth can be opened generally persists.
Arthritis: With osteoarthritis, because it occurs mainly when the disk is
missing or has developed holes, the person feels a grating sensation in the
temporomandibular joints when opening and closing the mouth. When
osteoarthritis is severe, the top of the jawbone flattens out, and the person cannot
open the mouth wide. The jaw may also shift toward the affected side, and the
person may be unable to move it back.
Rheumatoid arthritis usually affects both temporomandibular joints about
equally, which is rarely the case in other types of temporomandibular disorders.
When rheumatoid arthritis is severe, especially in young people, the top of the
jawbone may degenerate and shorten. This damage can lead to sudden
misalignment of many or all of the upper and lower teeth. If the damage is
severe, the jawbone may eventually fuse to the skull (ankylosis).
Ankylosis: Typically, calcification (the deposit of calcium into body
tissues) of the ligaments around the joint (extraarticular ankylosis) is not painful,
but the mouth can open only about 1 inch or less. Fusion of bones within the
joint (intraarticular ankylosis) causes pain and more severely limits jaw
movement.
Hypermobility: In a person with hypermobility, the jaw may slip forward
completely out of its socket (dislocate), causing pain and an inability to close the
mouth. Dislocation may occur suddenly and repeatedly.
What are TMJ disorders and how are they caused? TMJ disorders are a
group of complex problems related to the jaw joint. Other names include
myofacial pain dysfunction and Costen's syndrome. Because muscles and joints
work together, a problem with either one can lead to stiffness, headaches, ear
pain, bite problems (malocclusion), clicking sounds, or locked jaws. The
following are behaviors or conditions that can lead to TMJ disorders:
Teeth grinding and teeth clenching (bruxism) increase the wear on the
cartilage lining of the TMJ. Patients may be unaware of this behavior unless
they are told by someone observing this pattern while sleeping or by a dental
professional noticing telltale signs of wear and tear on the teeth. Many patients
awaken in the morning with jaw or ear pain.
Habitual gum chewing or fingernail biting.
Dental problems and misalignment of the teeth (malocclusion). Patients
may complain that it is difficult to find a comfortable bite, or that the way their
teeth fit together has changed. Chewing on only one side of the jaw can lead to
or be a result of TMJ problems.
195
Trauma to the jaws. Previous history of broken jaw or fractured facial
bones. Stress frequently leads to unreleased nervous energy. It is very common
for people under stress to release this nervous energy by either consciously or
unconsciously grinding and clenching their teeth.
Occupational tasks such as holding the telephone between the head and
shoulder.
What are the common symptoms of TMJ disorders?
TMJ
pain
disorders usually occur because of unbalanced activity of the jaw muscles and/or
jaw muscle spasm and overuse. Symptoms tend to be chronic, and treatment is
aimed at eliminating precipitating factors. Many symptoms may not appear
related to the TMJ itself. Common symptoms include:
Headache: 80% of patients with a TMJ disorder complain of headache,
and 40% report facial pain. Pain is often made worse while opening and
closing the jaw. Exposure to cold weather or air- conditioned air may
increase muscle contraction and facial pain.
Ear pain: 50% of patients with a TMJ disorder notice ear pain but do not
have signs of infection. The ear pain is usually described as being in front
of or below the ear. Often, patients are treated multiple times for a
presumed ear infection, which can often be distinguished from TMJ by an
associated hearing loss or ear drainage (which would be expected if there
really was an ear infection). Because ear pain occurs so commonly, ear
specialists are frequently called on to make the diagnosis of a TMJ
disorder.
Sounds: Grinding, crunching, or popping sounds, medically termed
crepitus, are common for patients with a TMJ disorder. These sounds may
or may not be accompanied by increased pain.
Dizziness: 40% of patients with a TMJ disorder report a vague dizziness
or imbalance (usually not a spinning type vertigo). The cause of this type
of dizziness is not well understood.
Fullness of the Ear: 33% of patients with a TMJ disorder describe
muffled, clogged, or full ears. They may notice ear fullness and pain
during airplane takeoffs and landings. These symptoms are usually caused
by Eustachian tube dysfunction, the structure responsible for the
regulation of pressure in the middle ear. It is thought that patients with
TMJ disorders have hyperactivity (spasms) of the muscles responsible for
regulating the opening and closing of the Eustachian tube.
Ringing in the Ear - Tinnitus: For unknown reasons, 33% of patients with
a TMJ disorder experience noise or ringing (tinnitus). Of those patients,
half will have resolution of their tinnitus after successful treatment of their
TMJ.
Diagnosis. A dentist or doctor almost always diagnoses a
temporomandibular disorder based solely on a person's medical history and on a
physical examination. Part of the examination involves gently pressing on the
side of the face or placing the little finger in the person's ear and gently pressing
196
forward while the person opens and closes the jaw. Also, the doctor gently
presses on the chewing muscles to detect pain or tenderness and notes whether
the jaw slides when the person bites.
When a doctor suspects internal joint derangement, further tests can be
done. Magnetic resonance imaging (MRI) is now the goldSome Trade Names
MYOCHRYSINE standard with which doctors assess whether internal joint
derangement has occurred or to find out why a person is not responding to
treatment. Doctors occasionally use electromyography, which analyzes muscle
activity, to monitor treatment and, less commonly, to make a diagnosis.
Laboratory tests are rarely useful.
A doctor suspects osteoarthritis when a creaking sound is heard when the
person opens his mouth (crepitus). X-rays and a computed tomography (CT)
scan can confirm the diagnosis. Infectious arthritis may be suspected when the
area over and around the temporomandibular joint is inflamed and when
movement of the joint is painful and limited. Infection in another part of the
body serves as a clue as well. To confirm the diagnosis of infectious arthritis, the
doctor may insert a needle into the temporomandibular joint and withdraw fluid
(aspiration), which is then analyzed for bacteria.
If hypermobility is the cause, the person generally can open the mouth
wider than the breadth of three fingers; the jaw may be chronically dislocated. If
ankylosis is the cause, the jaw's range of motion tends to be markedly reduced.
Treatment. Treatment varies considerably according to the cause. Two
common treatments are splint therapy and analgesics to relieve pain.
Muscle Pain and Tightness: Splint therapy usually is the main treatment
for jaw muscle pain and tightness. For people who realize that they clench or
grind their teeth, splint therapy can help them break the habit. A thin plastic
splint is made to fit over either the upper or the lower set of teeth and is adjusted
to give the person an even bite. The splint, usually worn at night (a nightguard),
reduces grinding, allowing the jaw muscles to rest and recover. For pain during
the day, a splint allows the jaw muscles to remain relaxed and the bite to be
stable, thereby reducing discomfort. The splint can also prevent damage to teeth
that are under exceptional stress from the grinding. Day splints are worn only
until symptoms subside, usually fewer than 8 weeks. Longer use may be
warranted depending on the severity of symptoms.
Physical therapy may also be prescribed. Physical therapy may involve
ultrasound treatment, electromyographic biofeedback (in which the person
learns to relax the muscles), spray and stretch exercises (in which the jaw is
stretched open with a passive jaw motion device after the skin over the painful
area has been sprayed with a skin refrigerant or numbed with ice), or friction
massage. Transcutaneous electrical nerve stimulation (TENS) also may help.
Stress management, sometimes along with electromyographic biofeedback,
often brings dramatic improvement.
Drug therapy may also be helpful. For instance, muscle-relaxing drugs,
such as cyclobenzaprineSome Trade Names FLEXERIL, may be prescribed to
ease tightness and pain, particularly while the person waits for a splint to be
197
made. However, these drugs are not a cure, generally are not recommended for
older people, and are prescribed for only a short time, usually for a month or
less. Analgesics such as aspirinSome Trade Names ECOTRINASPERGUM or
other nonsteroidal anti-inflammatory drugs (NSAIDs) also relieve pain. A
prescription for opioid analgesics is usually not given because treatment may be
needed for some time and these drugs can be addictive. Sleep aids (sedatives)
may be used occasionally and for a short time to help people who have trouble
sleeping because of the pain.
Regardless of the type of treatment, most people experience significant
relief within about 3 months. If the symptoms are not severe, many people
recover without treatment within 2 to 3 years.
Internal Joint Derangement: In internal joint derangement with or without
reduction, treatment is needed only if a person has jaw pain or trouble moving
the jaw. If a person seeks treatment right after symptoms develop, a dentist or
doctor may be able to manually move the disk back into its normal position. If a
person has had the disorder for fewer than 3 months, a splint may be applied to
hold the lower jaw forward. This splint keeps the disk in position, permitting the
supporting ligaments to tighten. Over 2 to 4 months, the splint is adjusted to
allow the jaw to return back to its normal position, with the expectation that the
disk will remain in place.
A person with internal joint derangement with or without reduction should
avoid opening the mouth wide—for instance, when yawning or biting into a
thick sandwich—because injured joints are not as protected in these activities as
would be a normal jaw. People with this disorder are advised to cut food into
small pieces and to eat food that is easy to chew.
Sometimes the slipped disk becomes stuck in front of the temporomandibular joint, preventing the jaw from opening fully. The disk must then be manually moved out of position to allow the joint to move fully. Passive jaw motion devices, which stretch the jaw, have been used to slowly increase jaw motion.
These devices are used several times a day.One such device is a threaded screwtype instrument that is placed between the front teeth and turned, much like a car
jack, to gradually create a wider opening. If such a device is not available, then a
doctor may use a stack of tongue depressors placed between the front teeth, with
an additional tongue depressor being added to the middle of the stack.
If internal joint derangement cannot be treated by nonsurgical means, an
oral-maxillofacial surgeon may need to reshape the disk and sew it back into
place. However, the need for traditional surgery is relatively rare since the
introduction of procedures such as arthroscopy. All surgical procedures are used
in combination with splint therapy.
Arthritis: A person with osteoarthritis in a temporomandibular joint needs
to rest the jaw as much as possible, use a splint or other device to control muscle
tightness, and take an analgesic (such as aspirin Some Trade Names ECOTRIN
ASPERGUM, acetaminophen. Some Trade Names TYLENOL, or another
nonsteroidal anti-inflammatory drug) for pain. The pain usually goes away in 6
months with or without treatment. Even without treatment, most of the
198
symptoms subside, probably because the band of tissue behind the disk becomes
scarred and functions like the original disk. Usually, jaw movement is sufficient
for normal activities, though the jaw may not open as wide as it used to.
Rheumatoid arthritis of the temporomandibular joint is treated with the
drugs used for rheumatoid arthritis of any joint. Maintaining joint mobility and
preventing fusion of the joint are particularly important. Usually, the best way to
accomplish these goals is by exercising the jaw under a physical therapist's
direction. To relieve symptoms, particularly muscle tightness, the person wears
a splint at night that does not restrict jaw movement. If joint fusion freezes the
jaw, the person may need surgery and, in rare cases, an artificial joint to restore
jaw mobility.
Infectious arthritis is treated with antibiotics. Penicillin is usually the
antibiotic used initially, until test results determine the type of bacteria present
and thus the best antibiotic to use. Pus in the joint, if present, may be removed
with a needle.
Ankylosis: Occasionally, stretching exercises help people with
calcification, but people with calcification or bone fusion usually need surgery
to restore jaw movement.
Hypermobility: Prevention and treatment of dislocation resulting from
hypermobility are the same as those for other causes of a dislocated jaw. When
dislocation occurs, a helper is sometimes needed to snap the jaw back into
position. Many people who experience repeated dislocations, however, learn
how to maneuver the joint back into place themselves by consciously relaxing
the muscles and lightly shifting the lower jaw until it pops back into place.
Surgery to tighten the ligaments of the temporomandibular joint is sometimes
necessary to prevent recurrent dislocations.
How can TMJ be treated?
The mainstay of treatment for acute TMJ pain is
heat & ice, soft diet, and anti-inflammatory medications.
1. Jaw Rest: It can be beneficial to keep the teeth apart as much as possible. It is
also important to recognize when tooth grinding is occurring and devise
methods to cease this activity. Patients are advised to avoid chewing gum or
eating hard, chewy, or crunchy foods such as raw vegetables, candy, or nuts.
Foods that require opening the mouth widely, such as a big hamburger, are not
recommended.
2. Heat & Ice Therapy: Assists in reducing muscle tension and spasm. However,
immediately after an injury to the TMJ, treatment with cold applications is best.
Cold packs can be helpful for relieving pain.
3. Medications: Anti-inflammatory medications such as aspirin, ibuprofen
(Advil, and others), naproxen (Aleve, and others), or steroids can help control
inflammation. Muscle relaxants, such as diazepam (Valium), aid in decreasing
muscle spasms.
4. Physical Therapy: Passively opening and closing the jaw, massage, and
electrical stimulation help to decrease pain and increase the range of motion and
strength of the joint.
199
5. Stress Management: Stress support groups, psychological counseling, and
medications can also assist in reducing muscle tension. Biofeedback helps
patients recognize times of increased muscle activity and spasm and provides
methods to help control them.
6. Occlusal Therapy: A custom made acrylic appliance which fits over the teeth
is commonly prescribed for night, but may be required throughout the day. It
acts to balance the bite and reduce or eliminate teeth grinding or clenching
(bruxism).
7. Correction of Bite Abnormalities: Corrective dental therapy, such as
orthodontics, may be required to correct an abnormal bite. Dental restorations
assist in creating a more stable bite. Adjustments of bridges or crowns act to
ensure proper alignment of the teeth.
8. Surgery: Surgery is indicated in those situations where medical therapy has
failed. It is done as a last resort. TMJ arthroscopy, ligament tightening, joint
restructuring, and joint replacement are considered in the most severe cases of
joint damage or deterioration.
TRAUMATIC DISEASES OF MAXILLOFACIAL REGION
Classification of damages of gnatholofacial region
I. Mechanical damages of upper, middle, lower and lateral parts of the
face.
1. On localization:
A.Traumas of soft tissues with damage of:
Tongue
Salivary glands
Large nerves
Large vessels.
B. Dental injuries
C. Fractures of bones:
Mandible
Maxilla
Zygomatic bones
Nasal bones
Two bones and more.
2. On type of wounds: stab, blind, bite, flesh, lacerated, incised,
chopped, penetrating into the oral cavity, not
penetrating
into the oral cavity; penetrating into maxillary
sinus and
nasal cavity .
3. On the mechanism of damage:
Knife wounds
Missile wounds (bullet).
II. The Combined damades
III. Burns
200
IV. Cold injury (Frostbite)
Dental injuries
Dental trauma (Fig. 28). Teeth can be traumatized due to a number of
causes including accidental falls, motor accidents, blow during fights, sports
activities etc. Children and more so boys during the growing period are more
prone for such injuries.
Fig. 28 Examples of some dental injuries.
Dental fractures are commonly seen with other oral injuries. Early
recognition and management can improve tooth survival and functionality.
Approximately 82% of traumatized teeth are maxillary teeth. Fractures to the
maxillary teeth are distributed among the central incisors (64%), lateral incisors
(15%), and canines (3%).
Different types of injuries to teeth and surrounding tissues. Trauma to the
oral structures can range from fractures involving the tooth crown, root or both
to injury affecting the supporting structures or surrounding tissues or even
complete dislodgment of the tooth from its socket. They may also be
accompanied by injury of the tissue surrounding the teeth including the gums,
bone supporting the teeth and the jaw bones.
Uncomplicated fractures and Complicated fractures. Not all fractures of
the teeth may be considered as a dental emergency. Fractures can occur in a
number of places in the teeth. Fractures that involve only the enamel or enamel
and dentin may not pose much problems for the health and recovery of the teeth.
These are called uncomplicated fractures. Complicated fractures of the teeth are
those where the pulp tissue is exposed to the exterior. These fractures are to be
treated as an emergency and may require endodontic treatment
Treatment of uncomplicated crown fracture. Very minor chipping
involving only the tip of the crown can be treated by reshaping the teeth
followed by polishing. In case of slightly larger fractures involving the enamel
and dentin composite fillings can be given to replace the broken part.
Treatment of complicated crown fracture. In case the pulp exposure is
small and the area is clean, then medicated cements can be placed over the
exposed pulp to allow the pulp tissue to heal. Larger pulp exposures may require
partial pulpotomy procedures where a part of the pulp tissue is removed and
medicated dressings are placed over the remaining pulp for healing. Some
complicated fractures may require comprehensive endodontic treatment.
201
In case the entire crown comes off can the tooth. Fractures can occur at
the neck of the tooth leading to the entire crown being lost. In these types of
fractures the remaining root can be treated endodontically and a post inserted
into it which can support an artificial crown
When traumatized teeth become mobile. Trauma to the teeth can be
transmitted to the supporting structures, which get damaged. This can cause
mobility of the teeth. Such mobile teeth may require splinting for a specified
period of time till the supporting tissues heal and the tooth becomes stable.
Splinting. Splinting is a procedure where the teeth are supported in its
position for a period of time. This is done to teeth that are traumatized or teeth
whose supporting structures are affected by disease, which prevents them from
supporting the teeth. Splinting involves binding a group of teeth together so that
the biting forces are shared by a large number of teeth instead of being born by
the affected tooth.
Displacement of traumatized tooth. Teeth that are traumatized can be displaced from their position. This displacement can be in any direction depen-ding
upon the type and direction of trauma. The displacement can be into the bone (i.e. the tooth is driven into the bone) or can be displaced partially out of the socket. Such displaced teeth may have to be repositioned in the socket and spli-nted
for a specified period of time. These teeth may also require endodontic therapy.
Fractured pieces of the crown can be reattached using composite cements.
So it is advisable to retain the fractured piece and take it with you when you
consult the dentist.
If the tooth is intact it can be placed back into the socket. However, the
outcome of the treatment depends on how quickly the tooth is put back in its
place. Prolonged time out of the mouth and drying of the tooth will haves poor
prognosis. Until a dentist is approached the tooth should be carried in the
patient's mouth or externally in a cup of milk or sterile water or normal saline.
The tooth should never be allowed to dry. In these emergencies the success of
treatment depends on how quickly the tooth is put back inside. So it is advisable
to contact your dentist immediately.
Complications:
Tooth loss
Cosmetic deformity
Other treatment includes Analgesics and antibiotic and NSAIDs
Fractures of alveolar process are more often on the maxilla, and are
quite often accompanied by fractures or dislocations of teeth. Follicles of
permanent teeth can be damaged or infected in children.
Clinic: disorder of bite, raptures (breaks) of mucous membrane on the
line of fracture, haemorrhage in the vestibule of the oral cavity, pathological
mobility of part of alveolar process, difficulty at chewing and speech, fractures
and dislocations of teeth. Radiological research helps the diagnosis of fracture.
Treatment. At the level of roots of the damaged teeth it is necessary to
extract fragments of alveolar process together with teeth because their
engraftment is impossible. Sharp bone edges are smoothed out and covered by
202
flap of mucous membrane. When the line of fracture passes above roots of teeth,
reposition and fixation of fragments are made to the dental arch with the
aluminium or steel splint, or splint made from quickly curing (harden) plastic.
Fractures of the maxilla make about 7 % of all fractures of bones of the
face. Fragments are displaced depending on direction of injuring force, weight
of fragments and from contraction of muscles of mastication and facial
expression attached to maxilla.
There are 5 types of fractures of the upper part of the face according to
Limberg. The first type is the fracture of zygomatic bone; the second is fracture
of bones of nose; the third is lower transverse fracture of maxilla from piriform
aperture to pterygoid process of sphenoid bone; the fourth is fracture in the
suborbital region, total separation of maxilla with nasal bones; the fifth is
subbasal fracture on the junction of maxilla and zygomatic bone with other
bones of skull or full craniofacial separation. Fractures of the fourth and fifth
type, as a rule, are accompanied by combined craniocereberal damages
(fractures of base of skull, concussion and bruises of brain). According to
classification of Lefor, fractures of the maxilla are divided into three types,
Lefor 1 corresponds to the third, Lefor II to the fourth, and Lefor III - to the fifth
type (of classification on Limberg)(Fig. 29).
Fig. 29 Le Fort classification of fractures to the maxilla.
Almost all fractures of maxilla are with rapture of mucous membrane of oral
cavity, nose, or maxillary sinus.
Fractures of the mandible (Fig. 30) make about 70 % of all fractures of
bones of the face. Fractures of body of mandible, including the central and
lateral parts, angle, are observed almost at 80 % of patients. Fractures of ramus
are divided into proper fractures of ramus,fractures of coronary and condylar
processes. It is necessary to distinguish single, double (unilateral and bilateral),
threefold and multiple fractures of the mandible, without displacement and with
displacement of fragments, linear, splintered, with presence or absence of teeth
in the line of fracture. At the formulation of the diagnosis it is necessary to
specify precisely anatomical location of fracture (at fracture of body of
mandible the formula of teeth is indicated). Character of fracture, displacement
203
of fragments depends on size and direction of injuring force, contraction of the
masticational muscles attached to the mandible. Fractures in the region of dental
arch usually opened. More often lines of fracture pass in area of the least
resistance of mandible (« a line of weakness »): neck of condylar process,
angle, alveoli of 8th teeth, area of canine, area of mental foramen, midline.
Fractures of bones of nose make about 10 % of all fr. of face, can be
accompanied by fr. of orbit, air nasal sinuses, ethmoid labyrinth. Almost at 40 %
of patients the combined craniocereberal trauma takes place.
Fractures of zygomatic bone and zygomatic arch make about 10 % of all
fr. of bones of the face. It can be as a result of direct impact or at squeezing of
face. Displacement of fragments depends on direction of injuring force and is
rare from contraction of muscles. Almost at half of patients they are
accompanied with combined damages of the maxilla (maxillary sinus); bones of
orbit and nose. At 25 % of patients craniocereberal damages (concussion and
bruises of brain, fr. of the base of skull) are observed.
Fig. 30 Common sites of fracture in the mandible.
Fractures of the alveolar process of the mandible are uncommon.
Unfortunately, their treatment is often fraught with problems, and uninitiated
surgeons tend to underestimate these types of fractures and their problems.
History of the Procedure: Mandible fractures are described in early
Egyptian writings. Hippocrates advocated the use of bandages and interdental
wiring for the treatment of mandibular fractures. In a 3-part article published
during the Civil War, Gunning wrote of using dental splints attached to
elaborate external appliances. In 1881, Gilmer first described the use of bars on
both arches, fixed to the teeth and each other with fine wire ligatures.
Schede is credited for the first mandibular bone plating. He is said to have
used a steel plate screwed to the mandible in the late 1880s. In 1934, Vorschutz
described external fixation using transdermal bone screws and plaster. The
Morris biphase is a refinement of that technique.
Problem: Simply stated, the approach to treating these injuries is to do
what is necessary to reduce them and then do what is necessary to hold them in
reduction until they are healed. The treatment options are somewhat limited by
the lack of room for drill holes and associated hardware when the fracture
204
involves a dentulous segment. Even with an edentulous segment, hardware is
rarely practical because it usually ends up under a dental prosthesis and cannot
be tolerated.
Etiology: Alveolar process fractures are the result of blunt or penetrating
trauma. The 2 most common etiologies of such blunt trauma in adults are fist
fights and motor vehicle accidents. Other sources of facial trauma include
athletic injuries, falls, and industrial accidents.Penetrating trauma to the
mandible is most commonly in the form of a gunshot wound.
Pathophysiology: For blunt trauma to produce an isolated fracture of the
alveolar process, the blow must be concentrated on a segment of the alveolar
process; however, this is an uncommon event. Alveolar process fractures that
share fracture lines with other mandibular fractures are more common, usually
representing a comminution of a body of mandible fractures.
Clinical: The usual presenting reports with any fracture of the mandible as
a result of trauma are localized tenderness, swelling, and malocclusion.
Medical therapy: Medical therapies for alveolar process fractures are for
patient comfort and to prevent complications, namely infection.
Mild-to-moderate analgesics may be required, taking into consideration
any associated injuries that may contraindicate their use or limit their dose.
Acetaminophen in liquid or tablet form may be sufficient. For an isolated
alveolar process fracture, nothing stronger than acetaminophen with codeine
should be required. Requests for stronger analgesia should prompt the treating
surgeon to consider unrecognized injuries, complications, or substance abuse.
Antibiotic therapy reduces the prevalence of infections with mandibular
fractures. Penicillin, prescribed at the appropriate dose for age, is an excellent
choice. For the patient who is allergic to penicillin, clindamycin is a good
alternative.
Surgical therapy: Reduction and immobilization of the fracture is
mandated for alveolar process fractures. The specific approach depends on the
specifics of the injury. Previously, no classification of these fractures has been
available to guide decision making. The authors offer the following
classification:
Class I fracture of the alveolar process: This involves a fracture of the
edentulous segment.
Class II fracture of the alveolar process: The fracture involves dentulous
segment with little, if any, displacement.
Class III fracture of the alveolar process: The fracture involves dentulous
segment with moderate-to-severe displacement.
Class IV fracture of the alveolar process: The alveolar process fracture shares
one or more fracture lines with other fractures of the tooth-bearing facial
skeleton.
Complications
Malunion and malocclusion. Malunion and malocclusion are the most
common major complications. They result from inadequate reduction and/or loss
of reduction during the healing process.
205
Infection. Infection is usually localized and typically responds to
antibiotics. Collections of pus should be drained. If present, hardware may
require removal.
Exposure of implanted hardware. This complication is uncommon
because hardware is rarely used for these fractures. However, if this
complication occurs, it requires removal of hardware.
Nonunion. Nonunion is an uncommon complication. It requires that the
fracture lines be exposed and freshened with reapplication of fixation. Nonunion
may require a bone graft in extreme cases.
LeFort Fractures
The most common mechanism producing facial fractures is auto
accidents. About 70 % of auto accidents produce some type of facial injury,
although most are limited to soft tissue. The face seems to be a favorite target in
fights or assaults, which are the next most common mechanism. The remainder
of facial fractures are produced by falls, sports, industrial accidents and gunshot
wounds. Less than 10 % of all facial fractures occur in children, perhaps because
of the increased resiliency of a child's facial skeleton. The most common
patterns of midfacial fractures are summarized in the table below.
The Lefort Classification. The next set of fractures in this rogue's gallery
of common facial fractures are the LeFort complexes. These are complex
bilateral fractures associated with a large unstable fragment ("floating face") and
invariably involve the pterygoid plates. Legend has it that LeFort dropped skulls
off of a French tavern roof and analyzed the resulting fracture patterns. This
certainly sounds like the kind of study that we would all like to do, even without
NIH funding. In reality, LeFort studied fracture patterns produced in cadavers.
He found three main planes of "weakness" in the face, which correspond to
where fractures often occur: the transmaxillary plane, the subzygomatic or
pyramidal plane (this is really two planes with an apex up at the bridge of the
nose), and a craniofacial plane.
The Lefort I fracture, or transverse fracture, extends through the base of
the maxillary sinuses above the teeth apices essentially separating the alveolar
processes, palate, and pterygoid processes from the facial structures above. This
transverse fracture across the entire lower maxilla separates the alveolus as a
mobile unit from the rest of the midface. Fracture dislocations of segments of
the alveolus may be associated with this fracture. With high-energy injuries, the
palate may be split in the midline in addition to the LeFort I fracture.
A pyramidal fracture of the maxilla is synonymous with a LeFort II
fracture. This fracture pattern begins laterally, similar to a LeFort I, but medially
diverges in a superior direction to include part of the medial orbit as well as the
nose. The fracture extending across the nose may be variable, involving only the
nasal cartilage or as extensive as to separate the nasofrontal suture. The fracture
extends diagonally from the pterygoid plates through the maxilla to the inferior
orbital rim and up the medial wall of the orbit to the nose. This separates the
maxillary alveolus, medial wall of the orbit and nose as a separate piece.
206
A LeFort III fracture or craniofacial dysjunction denotes a complete separation of the midface or facial bones from the cranium. This fracture transverses
the zygomaticofrontal suture, continues through the floor of the orbit, and finally
through the nasofrontal suture. The bones of the orbit are separated through the
lateral wall, floor, and medial wall. It is unusual to have this fracture as a single
segment of bone; more commonly, it comminutes with varying combinations of
zygomatic, nasoethmoid, and orbital fractures. The fractures may not be
symmetric on both sides and minimal mobility may be present.
Pathophysiology: The maxilla has 4 processes: zygomatic, frontal,
palatine, and alveolar. The maxillary sinus is housed within the maxilla and
varies in size depending on the degree of pneumatization.
The midface can be thought of as a grid of horizontal and vertical
buttresses that provide support for the face. The 3 paired vertical buttresses of
the midface are the nasomaxillary, zygomaticomaxillary, and pterygomaxillary
structures. The nasomaxillary buttress is formed by the lower maxilla, the
frontal process of the maxilla, the lacrimal bone, and the nasal process of the
frontal bone. The zygomaticomaxillary buttress is formed from the lateral
portion of the maxilla, zygoma, and lateral portion of the frontal bone. The final
buttress extends along the pterygoid plates to the skull base. The lone unpaired,
vertical support mechanism is the nasal septum/ethmoid complex.
The horizontal buttresses are composed of the alveolus, hard palate,
inferior orbital rim, and frontal bar. Horizontal buttresses have coronal and
sagittal components. The sagittal buttresses are vital for facial projection. The
midface is relatively deficient in sagittal buttresses. The skull base is at a 45°
angle relative to the occlusal plane of the maxilla and can act as an axial buttress
as well
Mortality/Morbidity: Because of the degree of force required to produce
midface fractures, they are often associated with a high incidence of serious
intracranial and ophthalmologic injury. Le Fort fractures are often comminuted
and associated with frontal or mandible fractures.
Sex: Midface fractures predominantly affect males, who outnumber
females by 5 to 1. Typically, these fractures affect younger males.
Age: Compared with adults, children have a far lower incidence of
midface fractures because of anatomic differences and the overall elasticity of
their tissues.
Maxillary fractures today are often the result of motor vehicle accidents.
These high-velocity injuries many times produce fracture patterns not classified
by the standard LeFort system, but are described simply by the anatomic
structure fractured and the degree of comminution present. Computerized
tomographic (CT) scans and the more recent development of three-dimensional
reconstructions have aided greatly in the diagnosis, classification, and
preoperative planning of these complex maxillary fractures.
With the exception of the LeFort I injury, "pure" LeFort injuries are not
commonly seen. More commonly seen are variants of the LeFort classification.
One of the most common of these is the LeFort II - tripod fracture complex. This
207
complex is usually due to the large forces encountered in a motor vehicle
accident. LeFort was probably unable to apply this much force to the cadaver
faces in his study, and it is therefore not too mysterious why he didn't describe
these more complex injuries. When describing these injuries, one should
probably give a separate diagnosis to each half of the face. Even more complex
patterns may be encountered, such as a mixed LeFort II/LeFort III complex or a
LeFort III/LeFort II/tripod complex.
Besides the classic LeFort patterns and the mixed LeFort variants, there is
another common pattern which is called, for obvious reasons, a "smash"
fracture. In these injuries, severe comminution of the face is present, and
underlying skull injury is likely. These patients are often in unstable condition
with associated axial and appendicular skeletal injuries as well. This category
includes several varieties of otherwise unclassifiable fractures, which are named
for the portion of the face primarily involved. Subclassifications of smash
fractures include the frontal, naso-frontal (naso-ethmoid) or central facial smash
syndromes. CT is mandatory for adequately displaying all of the bony and soft
tissue components of these injuries.
Evaluation and Imaging
The approach to evaluating a patient with a suspected maxillary or
periorbital fracture should begin with the same “ABC’s” (airway, breathing,
circulation) as other victims of trauma. Due to the previously discussed high
rates of associated injuries, a high index of suspicion for other injuries should be
maintained throughout the process of evaluation. Cervical spine injury should
be suspected until examination and/or x-rays are negative.
A thorough history is taken including mechanism of injury, time of injury,
associated symptoms, and a complete medical history. Especially important to
note are any visual disturbances, malocclusion, or parathesias. Physical
examination begins with an inspection of the face for asymmetry, contusions,
lacerations, or swelling. Palpation of the entire facial skeleton is then performed
noting step-offs or areas of mobility. The mobility of the midface can be tested
by grasping the maxillary alveolus and attemping movement. The LeFort I
fracture may have mobility at the inferior portion of the maxilla, the LeFort II
may be mobile at the level of the inferior orbital rims, while the LeFort III
fracture may produce mobility of the entire midface. Examination of facial
sensation is tested to determine any deficits, especially in the infraorbital nerve
distribution. The oral cavity is inspected and any malocclusion should be
documented. A thorough eye exam should be performed including visual
acuity, inspection of the anterior chamber and the retina, pupillary reflexes, and
extraoccular movements. Due to the rate of associated ocular injury previously
discussed, ophthalmologic consultation may be indicated in many situations.
Plain film xrays have largely been replaced by computed tomography in
the evaluation of patients with maxillary and ZMC fractures. CT of the face
with axial and coronal cuts allows more precise diagnosis of these facial
fractures. If other injuries will not allow coronal cuts to be obtained, coronal
reconstructions may be made from an axial scan. Evaluation of the CT scan
208
includes noting location of fractures, displacement, comminution, status of
vertical and horizontal buttresses, and condition of the orbit.
Radiographic signs of facial fractures
Direct Signs
nonanatomic linear lucencies
cortical defect or diastatic suture
bone fragments overlapping causing a "double-density"
asymmetry of face
Indirect Signs
soft tissue swelling
periorbital or intracranial air fluid in a paranasal sinus
Clinical Details:
Because of the accompanying injuries to the entire body, the standard
trauma protocol of ABCs must be strictly followed prior to any intervention.
Often, the midface fracture assumes a less important role because of the severity
of intracranial injury and associated body injuries. Since about one half of
midface fractures are associated with significant cerebral edema, a low Glasgow
Coma Scale score (<5), and a poor prognosis, it is important to understand the
goals of the family and the other medical teams involved in the care of the
trauma patient.
First of all, it is important to evaluate the airway early to rule out intraoral
hemorrhage, edema, loose teeth, and posteroinferior displacement of the
maxilla. Establishment of a safe airway is a priority, and a tracheostomy may be
needed if intubation proves to be not possible or unsafe for the patient.
Bleeding may complicate midface fractures. If the bleeding is severe
enough, packing of the midface vessels and temporary reduction of the fracture
may be necessary. Angiography may be necessary to locate arterial bleeding
from the internal maxillary prior to embolization.
Obvious clinical signs of facial skeleton compromise include
malocclusion, subcutaneous emphysema, abnormally mobile skeletal structures,
and palpable step-offs. Crepitus can be a result of paranasal sinus air leaking
into the soft tissues of the face. Palpable step-offs are especially seen with
zygomatic fractures. Associated facial fractures must be evaluated and ruled out.
The patient's visual status, before and after traumatic insult, is vital in the
treatment algorithm of midface fracture. There is a high incidence of visual
problems associated with midface fractures, including enophthalmos, diplopia,
entrapment, and epiphora. Epiphora occurs in 4% of Le Fort II or III fractures.
CSF leakage is also seen, especially in Le Fort III fractures. Any
persistent clear rhinorrhea should be tested appropriately for CSF fluid leak
(CSF Otorrhea). Patients may complain of paresthesias of the upper jaw due to
damage to the superior alveolar nerve.
As in all facial fractures, malocclusion is important to assess. Patients may
present with trismus and mouth pain. Palatal fractures often include a lip
209
laceration and/or lacerations of the gingival and palatal mucosa. Patients with a
palatal fracture may have an anterior open-bite deformity.
Facial edema may obscure the facial examination, and step-offs may not
be palpable. It is important to assess fracture mobility by palpating the anterior
maxilla between the thumb and forefinger. Motion at the level of the anterior
nasal spine without simultaneous motion is a sign of a Le Fort I fracture. Le Fort
I fractures may be associated with gingival crepitation.
Le Fort II fractures result in motion of the nasal pyramid along the medial
orbit rims. The patient may have midface flattening and elongation. Le Fort II
fractures often are associated with infraorbital paresthesias.
Le Fort III fractures have motion at the zygomaticofrontal suture (craniofacial dysjunction). The patient may have anosmia due to fracture at the cribriform plate, severe edema, or lengthening; this is known as a dish-face deformity.
Midface fractures are usually not confused with other phenomena. The
main concern is whether associated fractures are present. Examples include
nasoethmoidal and orbitozygomatic fractures. These associated fractures are
typically evident on examination or CT scanning. A history of trauma to the face
and proper suspicion of imaging results should lead to the proper diagnosis.
Treatment
All patients with midface fractures are given antibiotics, because these
fractures are considered open or compound. Violation of the paranasal sinus or
alveolus and open soft-tissue wounds are inevitable sequelae of midface
fractures. Antibiotics have been shown to decrease the incidence of infection
after midface fractures.
Typically, the earlier the repair of a midface fracture, the better the
surgical result. This creates a dilemma for the midface reconstructive surgeon in
that most patients with a midface fracture also have serious bodily injury. On the
other hand, early repair prevents soft tissue scarring and memory from insetting,
as well as fibrous malunion between the bony fragments.
A long surgical procedure in a terminal patient is not desirable for the
patient, the patient's family, or the surgeon. Also, an additional procedure in a
patient in unstable condition may not be in the patient's best interests. Piotrowski
and Brandt have elucidated some parameters for reconstructive surgeons to
allow for safe early repair. If the intracranial pressure is less than 15 mm Hg,
midface repair—early, intermediate, or late—does not negatively affect the
patient's recovery.
The radiologist and the reconstructive surgeon must communicate about
the specific location of the fracture. Exposure is crucial in repair of the midface
fracture. Generally speaking, a Le Fort I fracture is approached from a sublabial
exposure, a Le Fort II fracture is approached with a combination of sublabial
and periorbital exposure, and a Le Fort III fracture requires a combination of
sublabial and bicoronal fracture for adequate exposure.
The surgical approaches to fractures of the midface have changed
radically in the past 20 years. The technology has now evolved to allow for
miniplate fixation to the midface instead of bulky external hardware. Complex
210
internal wiring used to be the standard of care 10 years ago, but due to poor
cosmetic results and extended periods of IMF, newer technologies have replaced
it. Miniplate technology is the placement of strong titanium plates to bridge the
fractured areas. The principle is similar to that of bridge making: Stable areas
are fixed to unstable areas until the overall stability of the area has been secured.
If large deficiencies are present, bone grafting may be necessary.
Large, displaced segment fractures may require the displaced segment to
be pulled forward with a hook or index finger. If the fracture is impacted into
adjacent bone and is immobile, a Rowe forceps may be useful. Nondisplaced
midface fractures require little intervention. Usually, a short period of IMF is all
that is needed. With any displacement, an open approach is typically required. A
variety of midface fractures can be addressed effectively with a closed
technique. Patients with nondisplaced, noncomminuted fractures represent ideal
candidates for a closed approach.
Mandible Fractures
Fractures of the mandibular body may be classified by anatomic location,
condition, and position of teeth relative to the fracture, favorableness, or type.
Angle fractures occur in a triangular region between the anterior border of
the masseter and the posterosuperior insertion of the masseter. These fractures
are distal to the third molar.
Mandible fractures are also described by the relationship between the
direction of the fracture line and the effect of muscle distraction on fracture
fragments. Mandible fractures are favorable when muscles tend to draw bony
fragments together and unfavorable when bony fragments are displaced by
muscle forces. Vertically unfavorable fractures allow distraction of fracture
segments in a horizontal direction. These fractures tend to occur in the body or
symphysis-parasymphysis area. Horizontally unfavorable fractures allow
displacement of segments in the vertical plane. Angle fractures are often
unfavorable because of the actions of the masseter, temporalis, and medial
pterygoid muscles, which distract the proximal segment superomedially. depicts
the vertical and horizontal forces acting on the mandible, as well as the
relationship of muscle pull to fracture angulation.
Problem: The angle of the mandible is the triangular region bounded by
the anterior border of the masseter muscle to the posterior and superior
attachment of the masseter muscle (usually distal to the third molar). This area
may become fractured secondary to vehicular accidents, assaults, falls, sporting
accidents, and other miscellaneous causes.
Frequency: In general, incidences of fractures of the mandibular body,
condyle, and angle are relatively similar, while fractures of the ramus and
coronoid process are rare. The literature suggests the following mean frequency
percentages based on location.
Body - 29%
Condyle - 26%
Angle - 25%
211
Symphysis - 17%
Ramus - 4%
Coronoid process - 1%
The mandible is involved in 70% of patients with facial fractures. The
number of mandible fractures per patient ranges from 1.5-1.8. Approximately
50% of patients with a mandible fracture have more than 1 fracture. In a series
of 136 patients with angle fractures, 40% had fractures exclusive to the angle,
while 60% had multiple fractures that included an angle fracture. The mandible
fracture patterns of a suburban trauma center found that violent crimes such as
assault and gunshot wounds accounted for the majority of fractures (50%), while
motor vehicle accidents were less likely (29%).
Etiology: Vehicular accidents and assaults are the primary causes of
mandibular fractures throughout the world.
Data from industrialized nations suggest that mandible fractures have
various causes as follows:
Vehicular accidents - 43%
Assaults - 34%
Work-related causes - 7%
Falls - 7%
Sporting accidents - 4%
Miscellaneous causes - 5%
Assault most often causes mandible angle fractures.
Pathophysiology: Optimal mandible function requires maintenance of
normal anatomic shape and stiffness (ie, resistance to deformation under load).
Normal occlusion can be defined when the mesiolabial cusp of the maxillary
first molar approximates the buccal groove of the mandibular first molar.
Fractures result secondary to mechanical overload. Torque results in spiral
fractures; avulsion, in transverse fractures; bending, in short oblique fractures;
and compression, in impaction and comminution.Degree of fragmentation
depends upon energy transfer as a result of overload. Therefore, wedge and
multifragmentary fractures are associated with higher energy release. An
evidence based study involving 3002 patients with mandibular fractures found
that the presence of a lower third molar may double the risk of an angle fracture
of the mandible. Another study compared fractures with wisdom teeth to those
without and found an increased infectious risk (16.6%) in fractures with wisdom
teeth compared with 9.5% risk in fractures without wisdom teeth.
Clinical:
History Obtain a thorough history specific to preexisting systemic bone
disease, neoplasia, arthritis, collagen vascular disorders, and temporomandibular
joint (TMJ) dysfunction.
Knowledge of the type and direction of the causative traumatic force helps
determine the nature of injury. For example, motor vehicle accidents (MVAs)
have a larger associated magnitude of force than assaults. As a result, a patient
who has experienced an MVA most often sustains multiple, compound,
comminuted mandibular fractures, whereas a patient hit by a fist may sustain a
212
single, simple, nondisplaced fracture. Knowing the direction of force and the
object associated with the fracture also assists the clinician in suspecting and
diagnosing additional fractures.
Physical examination. Pertinent physical findings are limited to the injury
site. Change in occlusion may be evident on physical examination. Any change
is highly suggestive of mandibular fracture. Ask the patient to compare
postinjury and preinjury occlusion. Posttraumatic premature posterior dental
contact (anterior open bite) and retrognathic occlusion may result from a
mandibular angle fracture. Unilateral open bite deformity is associated with a
unilateral angle fracture. Anesthesia, paresthesia, or dysesthesia of the lower lip
may be evident. Most nondisplaced mandible fractures are not associated with
changes in lower lip sensation; however, displaced fractures distal to the
mandibular foramen (in the distribution of the inferior alveolar nerve) may
exhibit these findings.
Change in facial contour or loss of external mandibular form may indicate
mandibular fracture. An angle fracture may cause the lateral aspect of the face to
appear flattened. Loss of the mandibular angle on palpation may be because of
an unfavorable angle fracture in which the proximal segment rotates superiorly.
The anterior face may be displaced forward, causing elongation. Lacerations,
hematoma, and ecchymosis may be associated with mandibular fractures. Their
presence should alert the clinician that thorough investigation is necessary to
exclude fracture. Do not close facial lacerations before treating underlying
fractures except in the case of life-threatening hemorrhage.
Pain, swelling, redness, and localized calor are signs of inflammation
evident in primary trauma.
Indications. Use the simplest means possible to reduce and fixate a mandibular
fracture. Because open reduction can carry an increased morbidity risk, use
closed techniques for the following conditions:
Nondisplaced favorable fractures
Grossly comminuted fractures
Edentulous fractures (using a mandibular prosthesis)
Fractures in children with developing dentition
Coronoid and condylar fractures
Indications for open reduction include the following:
Displaced unfavorable angle, body, or parasymphyseal fractures
Multiple facial fractures
Bilateral displaced condylar fractures
Fractures of an edentulous mandible (with severe displacement of fracture
fragments in an effort to reestablish mandible continuity)
Relevant Anatomy: The angle of the mandible is the triangular region
bounded by the anterior border of the masseter muscle to the posterior and
superior attachment of the masseter muscle (usually distal to the third molar).
Contraindications: Evaluate and monitor the patient's general physical
condition prior to treating mandibular fractures. Any force capable of causing a
213
mandibular fracture may also injure other organ systems. Case reports have
documented concurrent posttraumatic thrombotic occlusion of the internal carotid artery and basilar skull fractures. Bilateral cervical subcutaneous emphysema, pneumothorax, pneumomediastinum, and spleen lacerations have also
been associated with mandible fractures after trauma. Patients should not undergo surgical reduction of mandible fractures until these issues are addressed.
Imaging Studies:
The single most informative radiologic study used in diagnosing
mandibular fractures is the panoramic radiograph.
Panorex provides the ability to view the entire mandible in one
radiograph.
Panorex requires an upright patient, and it lacks fine detail in the TMJ,
symphysis, and dental/alveolar process regions.
Plain films, including lateral-oblique, occlusal, posteroanterior, and
periapical views, may be helpful.
The lateral-oblique view helps in diagnosing ramus, angle, or posterior
body fractures. The condyle, bicuspid, and symphysis regions often are
unclear.
Mandibular occlusal views show discrepancies in the medial and lateral
position of the body fractures.
Caldwell posteroanterior views demonstrate any medial or lateral
displacement of ramus, angle, body, or symphysis fractures.
CT scanning may also be helpful. CT scanning allows physicians to
survey for facial fractures in other areas, including the frontal bone, nasoethmoid-orbital complex, orbits, and the entire craniofacial horizontal and
vertical buttress systems. Reconstruction of the facial skeleton is often helpful to
conceptualize the injury. CT scanning is also ideal for condylar fractures, which
are difficult to visualize.
Medical therapy: Patients with isolated nondisplaced or minimally
displaced condylar fractures may be treated with analgesics, soft diet, and close
observation. Patients with coronoid process fractures may be similarly treated.
Additionally, these patients may require mandibular exercises to prevent
trismus. If the fractured coronoid restricts mandible movement, medical therapy
is contraindicated. Administer prophylactic antibiotics for compound fractures.
Penicillin remains the antibiotic of choice.
The techniques for closed reduction and fixation of the dentulous
mandible vary. Placement of Ivy loops using 24-gauge wire between 2 stable
teeth, with use of a smaller-gauge wire to provide maxillomandibular fixation
(MMF) between Ivy loops, has been successful. Arch bars with 24- and 26gauge wires are versatile and frequently are used. In an edentulous mandible,
dentures can be wired to the jaw with circummandibular wires. The maxillary
denture can be screwed to the palate. (Any screw from the maxillofacial set can
be used as a lag screw.) Arch bars can be placed and intermaxillary fixation
(IMF) achieved. Gunning splints have also been used in this scenario because
214
they provide fixation and yet permit food intake. In cases of comminuted
fractures, a mandibular reconstruction plate may be required to restore anatomic
position and function.
Surgical therapy: Multiple approaches for open reduction and internal
fixation (ORIF) exist. Consider fracture location, nerve position, and skin-crease
lines when choosing the appropriate approach.
Intraoral approach. The intraoral approach is usually used in fractures that
are nondisplaced or only slightly displaced. The mandible base may require additional stab incisions to place screws for plate fixation.Intraoral lacerations may
be used for access in fixation of mandible fractures. Local anesthesia may be
sufficient for simple or nondislocated fractures when 1-plate fixation is required.
Extraoral approach. External incisions are usually necessary with
fractures that have a high degree of dislocation or with comminuted fractures,
since placing longer and stronger plates is difficult via the intraoral approach.
Although not impossible, reducing and securing angle and ramus fractures are
difficult; therefore, these fractures usually require an extraoral approach.
General anesthesia is indicated in the extraoral approach. Give careful attention
to the marginal mandibular branch of the facial nerve.
Transverse fracture line without displacement. Semirigid fixation using
miniplates and monocortical screws may be used in a transverse fracture line
with limited exposure. Although 1-plate fixation is possible using a 2.0 miniplate, the forces that occur during function are usually too great to be neutralized
by a single plate. This fracture is better managed using two 2.0 miniplates (4-6
holes, 2-3 screws on each side), the first in the area of the oblique line and the
second at the inferior border. Fixation is also possible using a single lag screw in
the anteroposterior-oblique approach in nonosteoporotic bones.
Transverse fracture line with displacement. With dislocation, the medial
pterygoid and masseter muscles cause vertically and horizontally unfavorable
vector forces, which make reduction more difficult. For a minimally displaced
fracture, achieve reduction by fixing a 2.0 miniplate of suitable length to the
proximal fragment on the medial aspect of the anterior border of the ramus using
2-3 screws. Reduce the fracture using the plate as a handle to complete IMF.
Bend the free end of the plate to conform to the distal oblique ridge and fix with
monocortical screws.
A widely displaced fracture may require stabilization by using a
reconstruction plate. Following IMF, widely displaced or comminuted angle
fractures can be reduced with clamps and stabilized by splinting with a
reconstruction plate (ie, 2.4 low-contact dynamic compression plate [LCDCP] at
the inferior border, well anchored with 3 screws on each side of the fracture). A
2.0 miniplate may be placed at the oblique line.
No difference in short-term complication rates can be found when
comparing 2.0 mm locking plates with 2.0 mm monocortical plates. In a
prospective randomized clinical study at Harborview Medical Center, 90
patients with 122 fractures were stratified to 64 treatment sites that received
215
locking plates and 58 sites that received standard plates. No statistically
significant difference was found between the plates used.
Angular fractures with basal triangle. As with displaced fractures, use an
angulated 2.4 reconstruction plate with 6-8 holes at the base of the mandible
after IMF. The triangle can be fixed to the plate, or lag screws (2.0, 2.4) can be
placed. Use a 2.0 miniplate along the oblique line.
Comminuted angular fractures. These often occur in association with
other mandibular and maxillary fractures. After temporary IMF, reduction and
fixation of fragments within simpler fractures can be accomplished using 1.5 or
2.0 miniplates and lag screws and then bridging with a 2.4 reconstruction plate.
Miniplates are often used to reduce large fragments of a comminuted angular
fracture. However, miniplates may not be strong enough to bridge severely
comminuted fractures.
Comminuted fractures of the ascending mandibular ramus. In the case of
concurrent fractures of the ascending ramus, a combined submandibular and
preauricular approach may be warranted. Simplify the fracture using 2.0
miniplates and subsequent bridging and then stabilize it with a 2.4 universal
fracture plate or reconstruction plate.
Preoperative details: Approach mandibular fractures methodically.
Patients rarely die solely from mandibular fractures. Diagnose and treat in an
efficient manner. As with all trauma patients, strictly adhere to advanced trauma
life support (ATLS) protocols. Particular attention to the airway is of critical
importance to any patient with craniofacial trauma. Use prophylactic antibiotics
for compound fractures. Penicillin remains the antibiotic of choice. Evaluate
nutritional needs.
Intraoperative details: The goal of treatment is to reestablish occlusion.
Function is compromised with malunion. Most mandibular fractures can be
treated by closed reduction. Nondisplaced favorable fractures can be managed
with closed reduction and IMF alone. Arch bars or Ernst ligatures may be placed
and supplemented with an autopolymerizing resin.
The 3 separate techniques for rigid fixation of the mandible that have been
developed are (1) the bicortical Luhr system, using vitallium plates, (2) the Arbeitsgemeinschaft fOr Osteosynthesefragen/Association for the Study of Internal
Fixation (AO/ASIF) system of stainless steel compression or reconstruction
plates with bicortical screws, and (3) the Champy miniplate technique placed
along the line of ideal osteosynthesis, using monocortical screws.
A prospective randomized clinical trial comparing 2.0-mm locking plates
to 2.0-mm standard plates in the treatment of mandible fractures found no
statistically significant difference between the plates. In addition, mandible
fractures treated with 2.0-mm locking plates and 2.0-mm standard plates present
similar short-term complication rates.
With multiple facial fractures, usually treat mandibular fractures first,
since the mandible is the foundation on which other facial bones may be
repaired to restore form and function. Perform intraoral surgery prior to an
extraoral approach. IMF time varies according to type, location, number, and
216
severity of fracture(s). Generally, 6 weeks of IMF are prescribed, although this
is only an empiric approximation.
Treat dental injuries concurrently with the fracture. Fractured teeth may
become infected or jeopardize bone union and should be removed in
consultation with a dentist. Mandibular cuspids help determine occlusion and
should be preserved if possible.
Respect the third molar in angle fractures. Removal of the third molar is
associated with conversion of a closed fracture to an open fracture, loss of the
bony buttress on the tension side, and loss of the possibility for a tension band
plate. Extract the third molar only when the apex is open to the fracture line, the
root is fractured, or the molar is partially erupted.
Postoperative details: Administer analgesic medications in the
postoperative period. Administer antibiotic therapy covering gram-positive
organisms to patients with open fractures. Keep wire cutters at the bedside in
case of emesis. Reevaluate nutritional needs.
Follow-up care: Maintain IMF for 4-6 weeks. Tighten wires every 2
weeks. After wires are removed, a Panorex radiograph is usually taken to ensure
complete fracture union.
Complications following repair of a mandibular fracture are rare.
The most common complication is infection or osteomyelitis. Malunion
and nonunion of the mandible occur because of failure to observe treatment
goals as previously outlined. Malunion is described as delayed, incomplete, or
faulty union following a fracture. More specifically, delayed union is
characterized as no clinical evidence of bone union after 8 weeks.
Several factors contribute to malunion, including infection (the greatest
factor), injury severity, inadequate reduction, lack of fracture stability, lack of
compliance, alcoholism, and metabolic and nutritional deficiencies.
Nonunion describes improperly healed fractures. Nonunion may be due to
delay in treatment, inadequate immobilization, and osteomyelitis of the fracture
before and after surgery.
Contributing factors include oral sepsis, teeth in the fracture line, alcohol
abuse and chronic disease, prolonged time prior to treatment, poor patient
compliance, and displacement of fracture fragments.
In addition, plate fracture has been identified as a complication. Material
analysis of AO plates used in mandible fractures revealed titanium plate fracture
in 4 out of 110 mandibular reconstructions. The plate fracture was most
common in angle-type plates due to constriction on the internal side of the plate.
Both closed and open reductions of mandibular fractures cause favorable
results for bony union. In a study of 922 mandibular body and angle fractures
that were repaired using an intraoral approach without IMF, solid bony union
was achieved in more than 99% of patients.
Fractures of nose
Although nasal fractures are the most common facial fracture, they often
go unnoticed by both physicians and patients. Patients with nasal fractures
usually present with some combination of deformity, tenderness, hemorrhage,
217
edema, ecchymosis, instability, and crepitation; however, these features may not
be present or may be transient (Tremolet de Villers, 1975). To further
complicate the matter, edema can mask underlying nasal deformity, crepitation,
and instability; thus, many physicians and patients fail to pursue further
diagnosis and appropriate treatment. If untreated, nasal fractures can result both
in unfavorable appearance and in unfavorable function, especially when the
underlying structural integrity of bone and cartilage is lost.
Untreated nasal fractures account for the high percentage of rhinoplasty
and septoplasty procedures performed months to years after the initial trauma
occurs. Thus, appropriate treatment is best rendered in a timely manner, before
scarring and soft tissue changes occur. As always, thorough history taking and
physical examination should precede radiographic evaluation. If radiographic
evaluation is warranted, it is best used when other facial fractures are suspected
in combination with a nasal fracture, because isolated nasal fractures are treated
on the basis of the physical examination alone. The fact that patients may have
displaced nasal fractures and normal-appearing plain radiographic findings
should be emphasized.
Pathophysiology:The nasal bones and underlying cartilage are susceptible
to fractures because the nose maintains a prominent position and central location
on the face and because it has a low breaking strength. Patterns of fracture are
known to vary with the momentum of the striking object and the density of the
underlying bone (Murray, 1984). As with other facial bones, younger patients
tend to have larger nasoseptal fracture segments, whereas older patients are more likely to present with more-comminuted fracture patterns (Cummings, 1998).
Weak areas are noted in the cartilage framework and the junctions of the
upper lateral cartilages with the nasal bones and the septal cartilage at the
maxillary crest. The weak areas account for an increase in the rate of
fracture/dislocation after nasal trauma. A lateral force of only 16-66 kPa and a
greater frontal force of 114-312 kPa can displace the bony dorsum (Murray,
1984). A large force in any direction can cause comminution of the nasal bones
with an associated C-shaped deformity of the nasal septum. The C-shaped
deformity usually begins under the dorsum of the nose and extends posteriorly
and inferiorly through the perpendicular plate of the ethmoid and ends with an
anterior curve in the cartilaginous septum approximately 1 cm above the
maxillary crest (Murray, 1984).
Murray et al reported that almost any deviation of the fractured nasal
bones involves a concomitant fracture of the septal cartilage. Cartilage fracture
lines are often oriented vertically in the caudal septum and horizontally in the
posterior portions (Murray, 1984).
Lateral impact injuries are the most common type of nasal injury leading
to fracture (Illum,1983).Lateral injury produces a depression of the ipsilateral
nasal bone that usually involves the lower one half of the bone,the nasal process
of the maxilla, and a variable portion of the pyriform margin. Nasal fracture and
displacement without septal fracture usually occur with weaker applied forces;
however, with increased force, displacement of the bilateral nasal bones can be
218
noted, and the septum is usually dislocated and fractured as well (Cummings,
1998).
Other injuries that are commonly associated with nasal fractures include
midface injuries involving the frontal, ethmoid, and lacrimal bones; nasoorbital
ethmoid fractures; orbital wall fractures; cribriform plate fractures; frontal sinus
fractures; and maxillary Le Fort I, II, and III fractures.
Frequency:
Fracture of nasal bones is the most common site-specific bone injury of
the facial skeleton. Nasal fractures account for 39-45% of all facial fractures
(Hussain, 1994).
Mortality/Morbidity:
The morbidity of nasal fractures includes nasal airway obstruction due to dorsal
nasal collapse, septal deviation, valvular collapse, epistaxis, or a poor cosmetic
outcome.
Perhaps the worst morbidity results from septal hematoma, leading to septal
perforation and necrosis, which causes severe nasal collapse and deformation.
Sex: The male-to-female ratio in nasal fractures is greater than 2:1.
Age:
The incidence is increased in patients aged 15-30 years (Muraoka, 1991).
A small but significant increase in the number of nasal fractures is noted in the
elderly population because of a higher rate of falls.
Most nasal bone fractures in young adults are related more to altercations
and sporting injuries and less to motor vehicle accidents; however, these rates
vary according to the location of the conducted study and the association with
alcohol (Muraoka, 1991; Hussain, 1994; Scherer, 1989; Logan, 1994).
Anatomy: The nasal skin is thin and loosely adherent over the superior
two thirds of the nose. The skin thickens and adheres more tightly over the
caudal one third of the nose. Sensory innervation of the nose is supplied by the
supratrochlear, infratrochlear, anterior ethmoid, and infraorbital nerves. The
blood supply of the external nose is supplied by the dorsal nasal, external nasal,
lateral nasal, and septal arteries.
The paired, rectangular, flat nasal bones project from the frontal processes
of the maxilla, joining in the midline and articulating with the nasal process of
the frontal bone at the nasion. The thinner caudal portion of the nasal bones
articulate with the upper lateral cartilages; this area is vulnerable to dislocation
as a result of trauma. The nasion is more stable than the midline scaffolding
provided by the cartilaginous septum. The ethmoid air cells are situated
posterior to the nasal bones. Approximately 80% of fractures occur at the lower
one third to one half of the nasal bones (Murray, 1984). This area represents a
transition zone between the thicker proximal and thinner distal segments.
The cartilaginous septum is a quadrangle-shaped cartilage set between 2
bony structures: the vomer and the perpendicular plate of the ethmoid. The
septovomerine angle is the center of growth for the cartilaginous septum. The
hard palate represents the floor of the nasal cavity, and the cribriform plate
constitutes the roof.
219
Clinical Details: Patients with nasal fractures usually present with some
combination of dorsal or septal deformity, tenderness, hemorrhage, hematoma,
edema, ecchymosis, instability, and crepitation. However, these features may not
be present, or they may be transient.
Preferred Examination: Although the use of plain images is not suggested
(see Limitations of Techniques below), the preferred examination includes the
acquisition of Waters and lateral nasal views.
Limitations of Techniques:
Controversy regarding radiologic techniques
The use of plain images and, recently, CT scans for the diagnosis and management of nasal fractures has been controversial; however, several small studies have shown that use of these modalities is neither cost-effective nor beneficial to the patient or physician. Nasal fractures are usually evident and can be
eliciting by means of careful history taking and physical examination. Rarely is
the radiologic confirmation of these injuries needed (Jones, 1991). However, some clinicians still use plain images and CT scans, and the radiologist must understand some of the diagnostic pitfalls to reduce the rate of erroneous readings.
de Lacey et al evaluated 100 consecutive patients presenting to the
emergency department with a history of trauma to the nose (de Lacey, 1977).
Nasal radiographs were obtained in each patient, including both the Waters and
lateral views. No radiographic findings of fracture were depicted in 65 of the
100 patients. Nasal fractures were depicted in 45 patients, yet only 3 patients
required reduction, and 31 of 45 patients were discharged without the need for
treatment. The authors then compared the lateral radiographs in 50 control
subjects and 50 persons with dry skulls.
When images from control subjects were compared with images persons
with dry skulls, misreads were identified and classified as midline defects, high
lateral-wall defects, and low lateral-wall defects. In 50 control subjects, 33
cortical defects were observed. After close inspection, the misreads were found
to be the result of the midline nasal suture, the nasomaxillary suture (low defect)
and thinning of the nasal wall (high defect), respectively. de Lacey et al
concluded that the lateral view was unreliable for the evaluation of nasal
fractures because of the high incidence of similar defects found in noses from
control subjects and in patients with dry skulls when evaluated using plain
radiography (de Lacey, 1977).
Clayton and Lesser prospectively evaluated 54 patients clinically,
radiologically, and under anesthesia within 19 days of nasal injury (Clayton,
1986). At each stage, these examinations were evaluated to assess the
contribution of each study to the care of the patient. Occipitomental (Waters)
views and lateral views were obtained in all patients. External examination and
nasal rhinoscopy were performed to evaluate the patients clinically. The patients
were grouped into 3 categories: patients not needing manipulation, patients
requiring manipulation, and patients requiring later review because of edema.
Manipulation was required in 24 patients, and 19 patients underwent further
examination with anesthesia at the time of repair.
220
Six patients had no clinical evidence of a fracture despite radiographic
evidence of a fracture. None of these 6 patients required manipulation.
Examination under anesthesia changed the type of repair necessary in 5 patients:
1 patient was found not to have a septal fracture and 4 were found to have
bilateral fractures. The authors concluded that examination under anesthesia
provided more accurate information than radiography or clinical examination
alone or together. The authors also found that the Waters view, the lateral view,
or the 2 views in combination did not provide useful information, as compared
with physical examination alone. Standard radiographs were not helpful in
deciding whether to perform manipulation or when and how to perform
manipulation for repair (Clayton, 1986).
Findings:
Waters view
The Waters (occipitomental) view is perhaps the best overall view for
observing facial fractures in general. The radiograph is obtained in the
posteroanterior position with the canthomeatal line at an angle of approximately
37° relative to the surface of the film. The patient's dentures and oral prosthetic
devices, if any, should be removed because these structures may cause
interference.
The Waters view demonstrates the orbits, maxillae, zygomatic arches,
dorsal pyramid, lateral nasal walls, and septum. The radiologist should look for
abnormalities of the nasal septum and arch, keeping in mind the areas of relative
weakness. Marked deviation, displacement with sharp angulation, and soft tissue
swelling are all signs of possible fracture. Soft tissue edema can be sufficient to
obscure the extent of a fracture. Other structures, such as the frontal, maxillary,
and ethmoid sinuses, may also be involved. Any such involvement should alert
the physician to the possibility of concomitant fractures. Some have found that
coronal sutures can mimic nasal fractures because the sutures can become
superimposed over the nasal bones (Keats, 2001).
Lateral view
The lateral view (profilogram) is obtained with the infraorbitomeatal line
parallel to the transverse axis of the film and the intrapupillary line
perpendicular to the plate. This orientation provides a true lateral projection that
is neither tilted nor rotated; therefore, paired structures are superimposed. Many
prefer to include the full profile from the forehead to the chin with a technique
that uses a Bucky grid (Ruenes, 2001).
Fractures of the nasal bones are frequently transverse. The lateral view
obtained by using a soft tissue technique is probably best for depicting both old
and new fractures of the nasal bones. The profilogram provides no information
regarding a possible laterally displaced nasal bone (Ruenes, 2001; Cummings,
1998). Short lucent lines that reach the anterior cortex of the nasal bone, with or
without displacement, should be regarded as a fracture (de Lacey, 1977).
Evaluation of air zones by profilogram can provide important information, since
the air zones commonly are lost after trauma. Alterations of air zone shapes may
indicate cartilage volume increases or septal hematoma (Ruenes, 2001).
221
Other lines, such as normal sutures or longitudinally oriented nasociliary
grooves, can be mistaken for longitudinal fractures. However, a nasociliary
groove should never cross the plane of the nasal bridge; if this is demonstrated,
the line is a fracture. Fortunately, fractures usually demonstrate a sharpened
delineation, with greater lucency than normal sutures and grooves (Redman,
1993). The radiologist must closely look for marked deviation, displacement
with sharp angulation, and soft tissue swelling. It is important to remember that
only approximately 15% of old fractures heal by ossification; therefore, old
fractures are easily mistaken for new fractures and increase the rate of falsepositive readings (Logan, 1994).
Degree of Confidence: Radiographic findings consistent with nasal
fracture may be identified in 53-90% of patients with isolated nasal fractures
(Illum, 1991). Because of this and other concerns, Logan et al questioned the
reliability of nasal bone radiographs (Logan, 1994).
False Positives/Negatives: Logan et al believed that the high percentage of
false-negative and false-positive results with nasal bone radiographs had a number of causes. Old fractures, vascular markings, cartilage fractures, midline nasal
sutures, nasomaxillary sutures, and thinning of the nasal wall represent a few of
the many features that may mislead even an experienced radiologist. Logan et al
reported a true-positive rate of 86% and a false-positive rate of 8%. de Lacey et
al conducted a similar study that showed that 66% of control subjects had a
false-positive reading using Waters view radiographs (de Lacey, 1977).
Unfortunately, an accurate depiction of the rate of false-positive and falsenegative results from injured patients cannot be obtained by using their data
Findings: CT scans are usually obtained when another traumatic facial or
skull fracture is suspected. Many fractures are also demonstrated on routine head
CT scans in patients with trauma. Although CT scans can be used to
demonstrate the extent of nasal injury, they are rarely required. Contiguous, thin
(2-3 mm), axial and coronal sections with bone windows must be obtained;
however, axial images can be used to reconstruct coronal planes. These scans
are helpful when associated injuries are suspected in combination with nasal
fractures.
CT scans depict important structures, such as the orbital walls, zygomatic
arches, frontozygomatic sutures, maxillary buttresses, ethmoid air cells, nasal
bones, dorsal pyramid, and floor of the frontal sinuses with the associated nasofrontal ducts. Recent nasal fractures usually are easily recognized on CT scans;
Intervention: Fractures of the nasal bones are the most site-specific bone
injury of the facial skeleton. If left uncorrected, the loss of structural integrity
and the soft tissue changes that follow may lead to both unfavorable appearance
and function. Thus, appropriate and timely treatment is warranted to decrease
morbidity in patients with these fractures. The management of nasal fractures is
based solely on the clinical assessment of function and appearance; therefore, a
thorough physical examination of a decongested nose is paramount.
Many patients who sustain nasal trauma present with profuse bleeding,
which can usually be controlled with clot removal and the application of a
222
topical vasoconstrictor. If this approach is unsuccessful, gauze packing, balloon
catheterization, or other procedures may be required, but the ligation of vessels
is rarely necessary. Nasal packing is the most common procedure performed to
control bleeding after topical vasoconstrictors fail. Packing material is placed
directly at the area of hemorrhage to supply pressure; however, bilateral packing
is occasionally necessary to control bleeding from several areas. The packing is
commonly removed in 2-5 days, and nasal reduction can be performed at the
same time.
Profuse edema of the soft tissues surrounding the nose is a common
phenomenon, one which may slow early treatment. The fixation of the fractured
nasal bones is usually observed after 2-3 weeks, but adherence to surrounding
soft tissues can occur in as few as 5-10 days. Elevation of the head and locally
applied ice packs can quickly decrease the edema and pain associated with many
nasal fractures, allowing more timely and proper reduction.
Fracture reduction should be accomplished when accurate evaluation and
manipulation of the mobile nasal bones can be performed; this is usually within
5-10days in adults and3-7days in children(Cummings,1998).In patients with minimal swelling, immediate reduction can be performed, especially if the pati-ent
is scheduled for other operative procedures; however, nasal fracture reducti-on
is a lower priority compared with most other emergency surgical intervenetions.
Closed reduction is the initial plan of treatment preferred by most facial
plastic surgeons. If the reduction is not maintained or if poor cosmesis occurs,
open septorhinoplasty can be accomplished in a controlled fashion months later.
Medical/Legal Pitfalls:
Wexler opined that nasal radiographs were a medicolegal necessity and
considered them an important adjunct in acute injuries to the nasal bones
(Wexler, 1975). Unfortunately, this opinion was not founded on solid
experimental data. The practice of ordering unnecessary radiographs encourages
poor patient care and devalues the importance of thorough history taking and
physical examination. This practice also leads to needless irradiation, expense,
and wasted time (Fielding, 1990). Several other authors neither share Wexler's
opinion nor present data to support such a belief (de Lacey, 1977; Clayton,
1986; Illum, 1991; Logan, 1994).
Clayton and Lesser published information from one of the leading
medical defense organizations in Britain (Clayton, 1986). The organization
stated that radiographs of the nasal bones are indicated only if it was in the best
interests of the individual patient to help in surgical management. The same
organization stated that radiographs are not a medicolegal requirement. Logan et
al supported this dictum by demonstrating that nasal radiograph radiography has
a high incidence of false-positive and false-negative results; thus, the degree of
accuracy is low. This low degree of accuracy greatly reduces the medicolegal
value of radiographs (Clayton, 1986). It should be noted that these results are
observer dependent, and some radiologists have a greater knowledge and
aptitude for reading such images.
223
De Lacey et al found that occipitomental views are of no help in the
diagnosis of nasal fractures. The authors found that lateral views were helpful
for diagnosing nasal fractures in only some patients. Lateral radiographs offer no
information regarding lateral nasal displacement. de Lacey et al believed that, in
patients in whom isolated nasal bone fractures are suspected, radiographs are
unnecessary. They stated, "It requires stating emphatically that there is no
medico-legal reason for taking x-rays of the nasal bone, as treatment of a
fracture will depend solely on the function or cosmetic effect and this depends
only on the clinical findings" (de Lacey, 1977).
History taking and physical examination are the best diagnostic tools for
diagnosing nasal fractures and for determining which nasal fractures require
treatment by a specialist. Unfortunately, despite a large body of evidence against
the use of radiographs for isolated nasal trauma, the practice of requesting
routine nasal radiographs appears to be undiminished. The legal value of an
examination depends on the degree of medical findings supported by the
examination results (Illum, 1991). In isolated cases of nasal trauma, radiographs
have a high number of false-negative results and a large but unknown number of
false-positive results. Thus, the legal value is low because of the uncertain
degree of confidence in the findings. Radiographic examinations of the nose
have been known to fail in the assessment of nasal fractures (Illum, 1991).
Surgical exploration and fracture repair should be initiated in the event of
(1) a displaced or comminuted fracture, (2) trismus, or (3) significant aesthetic
deformity.
Emergent surgical repair and decompression are necessary when
exophthalmos or signs and symptoms of orbital apex syndrome are present, but
these are rarely observed with isolated arch disruption
Contraindications: Surgical correction is contraindicated in patients who
are medically unstable and unable to tolerate anesthesia.
Medical therapy: If surgical correction is undertaken, initiate prophylactic
antimicrobial therapy if a history of endocarditis or other conditions requiring
antibiotics is known.
Surgical therapy: Reconstruction of the zygomatic arch following injury is
necessary for restoration of malar symmetry and support for the maxilla and
masticatory loads. Repair of the zygomatic arch is usually performed in concert
with repair of ZMC fracture stabilization. In 1999, Turk et al found that direct
repair and plating of the zygomatic arch was not indicated in more than 1500
patients, secondary to spontaneous reduction with repair of other ZMC fracture
components. If an aesthetic deformity is the product of an arch fracture or if
trismus is present, direct repair and fixation are indicated.
As with any surgical endeavor, successful outcomes are the result of a
planned approach that affords excellent exposure of the operative site and of the
use of meticulous surgical technique. More specifically, repair of zygomatic
arch fractures requires a precise reduction and definitive stabilization to ensure
positive outcomes
224
Zygomatic fractures
The zygomaticomaxillary complex (ZMC) is both a functional and
aesthetic unit of the facial skeleton. This complex serves as a bony barrier,
separating the orbital constituents from the maxillary sinus and temporal fossa.
The zygoma has 4 bony attachments to the skull, and ZMC fractures
should be referred to as tetrapod fractures. Trauma to the ZMC usually results in
multiple fractures (ie, tetrapod), but solitary bony disruption may occur, as with
an isolated zygomatic arch fracture, which is the focus of this article.
History of the Procedure: In 1751, Dupuytren detailed an intraoral and
external technique to realign a medial displaced zygomatic arch. He also
discovered a crucial relationship between the temporalis muscle and fascia as a
plane to realigning zygomatic arch fractures.
In 1844, Stroymeyer described the percutaneous traction technique, which
is still used today for repair of zygomatic arch breaks.
In 1927, Gillies was first to institute the masking of incisions within the
hairline.
Frequency: The prominent zygoma is the second most commonly
fractured facial bone, and these fractures are eclipsed in number only by nasal
fractures. The vast majority of zygomatic fractures occur in men in their third
decade of life.
In 1994, Covington et al reviewed 259 patients with zygoma fractures and
found that ZMC fractures occurred in 78.8% of patients, while isolated orbital
rim and isolated arch fractures occurred in 10.8% and 10.4% of patients,
respectively. Of note, displaced or comminuted fractures were found in 59.3%
of patients with isolated zygomatic arch fractures.
Etiology: Zygoma fractures usually result from high-impact trauma.
Leading causes of fractures include assault, motor vehicle or motorcycle
accidents, sports injuries, and falls.
Clinical: Patients with zygomatic arch fractures are usually evaluated
following traumatic injury to the face. These fractures may result in trismus and
flattening of the midface. Patients report asymmetry between the malar regions
or difficulty with increasing their oral aperture, and these reports may prompt a
patient to seek consultation
Injuries of soft tissues of the face
No other part of the body is as conspicuous, unique, or aesthetically
significant as the face. Because an individual's self-image and self-esteem are
often derived from his or her own facial appearance, any injury affecting these
features requires particular attention.
Historically, severe facial trauma often resulted in cosmetic and
functional defects; however, recent advances in the science of reconstructive
surgery and in the management of trauma patients have significantly improved
the morbidity and mortality of patients with facial traumatic injuries. This article
focuses on facial soft tissue trauma.
Frequency
225
In the United States, approximately 3 million people present to emergency
departments for treatment of traumatic facial injuries each year. Most of these
injuries are relatively minor soft tissue injuries that simply require first-aid care
or primary closures. A small percentage of facial traumas (0.04-0.09%) require
major repair with possible bony reconstruction.
Etiology
In the United States, motor vehicle accidents (MVAs) were the most
frequent cause of facial injuries before 1970. In recent years, with the institution
of state seat belt laws, the number of deaths from MVAs has declined along with
the incidence of facial injuries, although the prevalence of facial trauma has
remained fairly constant. This is due to the growing population and other human
factors, such as on-the-job accidents, sports-related injuries, domestic
interpersonal violence, self-inflicted wounds, and animal bites.
The mechanism of injury for facial trauma varies widely from one locality
to the next, depending significantly upon the degree of urbanization,
socioeconomic status of the population, and cultural background of each region.
MVAs continue to be a primary contributor to significant facial injuries in rural
areas. In contrast, in inner metropolitan areas, domestic violence is the leading
cause of facial trauma despite a denser population, a difference that may be due
to stricter enforcement of traffic laws.
Initial survey. Although patients with traumatic facial injuries often
present with extremely disfigured appearances, their injuries are seldom life
threatening. Treat each patient who presents with significant traumatic facial
injuries as a patient with general trauma, and strictly adhere to American
Trauma Life Support (ATLS) protocols.
After obtaining the patient's pertinent history and mechanism of injury on
initial assessment, follow the ATLS ABCDE mnemonic (ie, airway, breathing,
circulation, disability, exposure), and address the most life-threatening problems
first. Evaluate the patient's facial injuries only after establishing a definitive
airway, stabilizing hemodynamics, and assessing other associated lifethreatening injuries.
Airway. The foremost priority in treating any trauma patient is establishing a definitive airway. In a conscious patient who is alert, awake, talking, and
in no obvious respiratory distress, it can be assumed that the patient has a patent
airway, and the physical assessment of other areas may be continued.
Consider airway obstruction in a conscious patient who demonstrates any
degree of respiratory distress. Blood, vomitus, facial bone fragments, dentures,
or other foreign bodies may cause either partial or complete airway obstruction.
Perform a quick finger sweep of the oral cavity if any physical obstruction to the
airway is suggested; this frequently suffices to dislodge any matter and to relieve
any obstructions in the upper airway. Consider nasal intubation if respiratory
distress continues; however, due to possible intracranial contamination, do not
intubate if the patient presents with a considerably distorted nasal anatomy,
extensive nasopharyngeal hemorrhaging, leakage of cerebrospinal fluid, or
226
possible fracture of the cribriform plate. Sedate the patient and perform an
orotracheal intubation.
If a conscious patient is uncooperative and combative (often seen in those
with alcohol intoxication), insert an endotracheal or nasotracheal tube after
administering sedation. In an unconscious patient with poor vital reflexes (ie,
gag, cough, swallow) or in a patient with a Glasgow Coma Scale (GCS) score of
8 or less, perform orotracheal intubation to prevent aspiration and to protect the
airway. Patients with massive soft tissue damage, such as that caused by shotgun
injuries to the face, must be sedated and intubated at once. Aspiration from
hemorrhaging becomes a primary concern.
If attempts at intubation are unsuccessful, emergency cricothyroidotomy
or a formal tracheostomy may be performed. As these are invasive procedures
with their own complications, use them only as a last resort to ensure an
adequate airway.
Hemorrhage and shock. Hemorrhage resulting in systemic shock from
facial trauma alone rarely occurs, except in cases of extensive penetrating
injuries such as gunshot wounds to the face. Bleeding from the facial artery, the
superficial temporal artery, the angular artery, or a combination of these is most
commonly encountered and can usually be controlled, at least temporarily, by
applying direct pressure to the wound.
Closely monitor the airway at all times as blood from facial hemorrhage
may obstruct the upper airway or may result in emesis and aspiration that can
further compromise the airway. If a patient with facial trauma presents with
shock, promptly assess other associated injuries.
Definitive evaluation and physical examination
Systematically examine the face by visual inspection and by palpation,
starting superiorly with the scalp and the frontal bones and proceeding inferiorly
and laterally. Inspect and note any obvious swellings, depressions, or
ecchymosis. These indicate possible underlying bone fracture or hematoma. Any
gross soft tissue asymmetry may signify underlying nerve damage. With
palpation, determine the presence and location of any fractured bone fragments
and dislodged or dislocated bony prominences. Determining the presence of
crepitus, tenderness, or step-offs is essential. If possible, assess sensorimotor
functions of the face.
General anatomy
Scalp injuries
Due to the extensive blood supply of the scalp, hemorrhaging of the scalp
often appears profuse and always heightens suspicion of intracranial damage.
On the other hand, it is not uncommon for minor scalp injures to be missed due
to an inadequate examination. To avoid missing any scalp injuries, examine
patients thoroughly during the secondary survey. Search for any possible
underlying skull fractures. Though shaving of hair is usually unnecessary, some
shaving may be needed to avoid missing additional lacerations if obvious
foreign body fragments are lodged in the hair or if the patient has long hair.
227
All injuries need to be copiously irrigated and have all foreign bodies
removed. Simple linear lacerations with good hemostasis can be closed with
staples. Close more extensive lacerations, lacerations with profuse bleeding, or
large avulsions of the scalp flap with continuous nonabsorbable sutures
encompassing all layers of the scalp. This method usually achieves good
hemostasis. If lacerations are jagged or macerated, obtain clean edges by
trimming the macerated areas, and bevel the incisions parallel to the hair
follicles to avoid secondary alopecia.
Eyebrow injuries. Eyebrow injuries should focus attention toward any
underlying fractures of the supraorbital ridge or frontal sinuses. These fractures
are often not well depicted in radiographs. If present, consider their management
prior to any surgical repair of the overlying soft tissues.
Eyebrows are never shaved. Superficial linear lacerations across the
eyebrow are meticulously closed with nonabsorbable sutures and careful
alignment of the margins. The resulting scar can be anticipated and concealed in
the hairs of the eyebrow. Subcuticular sutures may also be placed, provided that
the strength is adequate for the wound. Close deeper lacerations in layers.
Approximate lacerations involving divided muscles to minimize surface
contractures and functional defects. Neatly trim and debride jagged or macerated
tissue, following the line of the eyebrows to avoid additional hair loss.
Eyelid injuries. Patients presenting with eyelid injuries must be examined
thoroughly for any associated ocular and nasolacrimal duct injuries. Any
evidence of lens displacement, hyphema, retinal detachment, visual impairment,
global disruption, or foreign body presence warrants an ophthalmologic
consultation. Presence of enophthalmos or exophthalmos must be established, as
these conditions indicate either an orbital floor fracture or a blow-in fracture,
respectively. Extraocular muscle functions must be assessed with voluntary eye
movements. A visual acuity test must also be performed.
The eyelid is perhaps the most delicate structure of the face and consists
of several layers of fine musculature. Improper repair may result in ptosis or a
retracted eyelid. Lacerations of the eyelid are characterized as superficial or deep and horizontal (parallel to the lid margins) or vertical (perpendicular to the lid
margins). Superficial horizontal lacerations require only simple sutures or SteriStrips. Close superficial vertical lacerations in layers, as they often traverse normal skin tension lines and the underlying musculature. The key suture is placed
at the ciliary margin. Close the subcutaneous tissue and muscles with absorbable
sutures first and then the skin with 6-0 interrupted nonabsorbable sutures.
Deep and through-and-through lacerations of the eyelid warrant a careful
search for retained foreign bodies. Wound margins must be aligned carefully,
and key sutures must be placed first at the ciliary margin and at the tarsus. The
remainder of the eyelid is then apposed and repaired. Conjunctival lacerations
may be disregarded, as they generally heal well without any intervention. Skin
sutures may be removed after 48 hours.
Ear injuries. Though deceptively simple, the ears consist of unique
arches and contours that are symmetrical to each other, which makes their repair
228
and reconstruction often difficult and challenging for the plastic surgeon. If the
repaired ear is slightly uneven compared to the nonaffected ear, the aesthetic
symmetry of the patient is grossly affected.
Carefully clean and debride ear injuries. If the wound is a linear
laceration, it usually requires only primary closure with careful approximation
of the cartilage perichondrium and skin and closure in 3 layers, using 5-0
nonabsorbable sutures for the skin layer. For lacerations involving the helix, key
sutures are placed at the outer rim to preserve its contour and to prevent
subsequent notching.
If the injury is an avulsion, the wound is thoroughly cleansed and
debrided, and the margins are minimally trimmed and closed in layers. Small
avulsed ear fragments can be reattached similarly. As the ear has a highly
vascular pedicle, avulsions of the ear or even amputations, if properly treated,
tend to heal quite well. Venous congestion can be troublesome.
If a small area of the ear is peripherally jagged or missing, a wedge resection may be performed and the skin closed primarily. In addition, if the defect
requires skin grafts, they should be grafted only onto regions where underlying
perichondrium is present. However, if the wound is a large and grossly
noticeable defect, leave the wound open and plan reconstruction for a future
date. Frequently clean the wound and change dressings to avoid desiccation.
Nasal injuries. Visual inspection of the nose usually provides ample
information as to the underlying injury. Gross midline deviation of the nose
usually indicates underlying fractured nasal bones or cartilages. Soft tissue
swelling of the nose indicates hematoma, fractured nasal bones and/or cartilages,
or both. Intranasal inspection with a nasal speculum may reveal a deviated
septum, a septal hematoma, or cerebrospinal fluid leakage. If a septal hematoma
is suspected, aspirate it using an 18- or 20-gauge needle; perform an incision and
drainage procedure if the hematoma is confirmed. If the hematoma is missed
and untreated, chondromalacia of the nasal cartilages may develop and become
what is known as a saddle-nose deformity.
Superficial lacerations through the skin of the nose require only simple
nonabsorbable skin sutures to close the wound. Deeper bites that include the
cartilages may be used if the laceration extends down to the cartilages and if the
cartilages are aligned easily with no significant deviation.
For full-thickness lacerations of the nose, perform wound closure in
layers, ie, through the skin, cartilage, and mucous membrane. First, carefully
align and close the divided mucous membranes with 4-0 to 6-0 absorbable
sutures. Then, accurately align and close the skin and cartilage with
nonabsorbable interrupted sutures.
For lacerations that involve distinct nasal landmarks, such as the nasal
rim, nostril border, or the alar rim, first place key sutures at those regions to
ensure smooth, continuous contours without notching.
Nasal packing after surgical closure of the wound is at the surgeon's
discretion. In general, packing is unnecessary if the underlying supporting
elements are intact and in good alignment. Petrolatum-impregnated gauze may
229
be used to pack the nose to provide support if unstable underlying cartilaginous
or bony fragments are suspected. Note that nasal packing, in addition to causing
discomfort, obstructs air circulation and drainage and may cause additional
bleeding when removed from the delicate mucous membrane.
Lip injuries. Laceration of the lip is always repaired with reference to the
cutaneous-vermilion border or the white roll. Identify, carefully align, and mark
distinct landmarks, such as the white roll or the philtral column, prior to local
anesthesia injection. This is especially important if the injury extends through
the mid line of the lip at the Cupid's bow or the philtral tubercle. If not properly
treated as described, such regions may become distorted or obliterated when
local edema occurs after injection, thus causing improper suture placement and
necessitating a subsequent secondary repair.
After proper alignment and anesthetizing of the tissue, the first anchoring
suture should approximate the 2 sides of the laceration at the white roll, forming
a smooth and continuous line throughout the border. If the injury extends deep
to or through the orbicularis oris muscle, the musculature is closed first with
buried absorbable sutures. Proper alignment must be achieved for muscular
continuity. The mucous membrane is then closed with absorbable sutures, again
with attention to alignment. The skin layer is closed last with 5-0 or 6-0
nonabsorbable interrupted sutures. Instruct patients to minimize movement and
strain on the mouth.
Special areas
Nerve injuries. The facial nerve, because of its predominant and superficial distribution, is most susceptible to facial injuries. Injury to the nerve causes
significant cosmetic and functional defects. Any facial injury demands a complete functional evaluation of the main facial nerve trunk and its branches before
any treatment. If transection has occurred, obvious signs of motor deficit will be
present. Injuries to the temporal and eyebrow regions affect the temporal and
zygomatic branches, causing inability to raise the eyebrows or close the eyelids.
Injuries to the mandibular area margins affect the marginal mandibular, causing
inability to frown. Buccal branch injuries cause inability to smile and loss of the
nasolabial crease. Infraorbital nerve injury creates wrinkles in the cheek.
Repair transection of the facial nerve as soon as possible after the injury,
ideally within 72 hours. If repair is delayed, the distal severed ends will contract,
rendering identification of the severed ends using a nerve stimulator difficult or
impossible. Carry out nerve anastomosis under a microscope, using 8-0
nonabsorbable sutures in 3-4 positions circumferentially under minimal tension
to prevent fibrosis. If the nerve ends cannot be delineated clearly (ie, if the ends
are macerated or jagged), trim them off prior to anastomosis. If significant nerve
loss makes direct anastomosis impossible, find and tag the nerve ends for future
nerve grafting.
Parotid duct injuries. As the parotid gland is situated superficially in the
cheek, it is vulnerable to any trauma to the face. Any injury along an imaginary
line drawn from the tragus of the ear to the mid portion of the upper lip should
alert practitioners to the possibility of parotid injury.
230
Consider injury to the gland if there is clear discharge from the cheek
wound. Similarly, a sagging upper lip indicates possible injury to the parotid
duct, since the buccal branches of the facial nerve often run along with the
parotid duct.
If parotid duct transection is suspected, probe and cannulate it in the operating room. A small catheter is inserted into the parotid duct orifice, which
opens on the oral mucosa directly opposite the second maxillary molar tooth. If
no transection is present, the catheter passes freely and meets resistance. If transection has occurred, either partial or complete, the catheter will pass through
the distal open end of the transected duct and become visible. The proximal
severed end of the duct can be identified by massaging the gland to express
saliva. The catheter then should be cannulated through the proximal end of the
duct until it meets resistance. Under magnification, the duct can be anastomosed
over the catheter using 7-0 or 8-0 monofilament sutures. After repair, the
catheter can be removed if the duct is only partially transected and if there are
minimal associated injuries. However, if the duct is completely transected or
other significant associated damage to the area is present, leave the catheter in
place 5-7 days to ensure duct patency and to minimize fistula formation.
If the parotid duct is damaged such that the distal end cannot be identified
or if the duct orifice is obliterated, a new orifice can be constructed more
proximally to maintain parotid gland function. An alternative is duct ligation,
which causes the parotid gland to atrophy and cease functioning.
Lacrimal duct injuries. Injuries to the medial canthal region must be
inspected for lacrimal duct injury. Both upper and lower canaliculi must be
examined thoroughly to determine the extent of injury. With complete
transection, if the severed ends of the duct can be identified easily, align the
ends, cannulate with a fine catheter, and repair with fine sutures. In cases in
which the duct is partially transected, the canaliculus can simply be
approximated and observed.
With more severe injuries involving other excretory components of the
lacrimal system, such as the lacrimal sac and nasolacrimal duct, repair of the
lacrimal duct may be deferred until the main components have returned to
function unless epiphora and obstructive dacryocystitis occurs.
Animal bites. Animal bites to the face are generally the results of dog
attacks. The facial soft tissue injuries sustained are usually lacerations and tears
of the scalp, cheek, or neck. As animal saliva harbors numerous virulent
microorganisms, the main concern from such injury is wound infection. Human
bites, though appearing to be more innocuous, are actually more destructive in
terms of infection. The human oral florae are unique from those of animals and
are more virulent. The treatment, however, is similar to that for animal bites.
Copiously irrigate facial wounds from animal bites with isotonic sodium
chloride solution, and excise any macerated or destroyed tissue. If the wound is
less than 6 hours old and if the margins can be clearly delineated, the wound
may be approximated and closed with fine interrupted sutures. If the wound is
more than 6 hours old, depending on the degree of penetration and size of bite,
231
closure of the wound is at the surgeon's discretion. Animal bite wounds of this
duration are extremely prone to infection and, if closed, have a higher rate of
wound dehiscence.
Administer antibiotics in all cases of animal bites regardless of duration.
Although antibiotics do not usually prevent local infection, they may avert
fulminant sepsis.
The decision whether to administer rabies vaccine depends on the status
of the animal. Whether the animal is a domesticated, immunized pet or a wild
animal must be determined. Ideally, the animal should be caught, confined, and
observed because the incubation period of the rabies virus is about 10-14 days in
animals and 2-8 weeks in humans. If the animal shows signs of rabies, the patient can be treated within the incubation period. If the animal is found dead or is
killed, a microscopic examination of the brain for Negri bodies or the fluorescein antibody test is mandatory to determine whether the animal was rabid. If
the results are positive, the patient must undergo the rabies vaccination protocol.
Gunshot wounds to the face. The first priorities with any gunshot wound
are to establish a definitive airway, control any hemorrhage, and stabilize the
patient.
Civilian gunshot wounds to the face generally result from recreational
accidents, domestic violence, or suicide attempts. Gunshot wounds to the face
range from small-caliber recreational BB gun pellets to full-scale shotgun blasts
in which the facial soft tissue is obliterated.
Although the entry wound may appear trivial for small-caliber, lowvelocity missile injury, the blast effect produced along the path of the missile
can be devastating. Patients with this type of injury must be observed closely. If
the bullet is lodged within the soft tissue with no functional deficit or major
aesthetic defect, it may be left in place. If the wound becomes grossly infected
or begins to cause significant discomfort, initiate surgical intervention with
removal of the bullet and incision and drainage of the wound.
Through-and-through gunshot injuries or close-range shotgun wounds
often produce associated maxillofacial bony injuries. These must be evaluated
fully prior to any soft tissue repair. If facial fractures are present, consider rigid
fixation first. Carefully debride unsalvageable soft tissue, heavily damaged bony
fragments, and foreign bodies, and preserve and replace displaced viable soft
tissues in their corresponding anatomic locations.
If the patient survives the initial injury, complete facial reconstruction
procedures can often encompass a period of many years, depending on the
extent of injury, the degree of infection, and the health of the patient.
Although most soft tissue facial trauma consists of contusions, abrasions,
lacerations, or a combination of these that require only a careful physical
examination, more complex wounds benefit from radiologic studies that may
reveal any foreign body implanted within the soft tissue or any underlying
fractures that may complicate management. Order diagnostic studies only after
determining that the patient is clinically stable. Defer studies if they may
interfere with or delay clinical treatment.
232
Radiographs
As in any trauma patient, first obtain cervical spine radiographs to exclude
neck injury. (Include patients who are unconscious or alcohol intoxicated.)
Skull radiographs may also help to assess the degree of suspected skull
fractures, although current recommendations lean toward head CT scanning for
a more localized diagnosis.
Facial radiographs, including anteroposterior, lateral, submentovertex,
Panorex, mandibular, Towne, and Waters views, are required if significant facial
fractures are suspected.
Though quick and relatively inexpensive, radiographs lack 3-dimensional
representation. Physicians must extrapolate the information presented to form a
diagnosis.
CT scanning is an excellent modality in diagnosing the more complex
facial traumatic injuries and is invaluable in its ability to provide 3-dimensional
relationships among the structures in question. Obtain both axial and coronal
cuts of the facial skeleton.
In delicate and complex areas such as the eyes, obtain lateral oblique cuts
through the globe to evaluate the bony architecture surrounding the orbit.
If necessary, 3-dimensional reconstructions can be made for a more
detailed analysis.
The main disadvantage of CT scanning is the higher dosage of radiation
exposure to the patient.
Magnetic resonance imaging
As MRI has become more widely accessible and established in medical
centers, it has proven beneficial in assessing traumatic soft tissue injuries.
In addition to its main advantage of operation without radiation, MRI also
provides 3-dimensional information similar to CT scanning.
Because the device uses very powerful magnets, its disadvantages are in
its danger of operation for patients with metallic implants (eg, pacemakers,
mechanical heart valves) and its cost.
Arteriography: In patients in whom extensive hemorrhage occurs with
questionable source, arteriography serves as an excellent study to evaluate and
isolate the source of hemorrhage or to exclude major vascular injuries.
Medical therapy
Antibiotics
The use of antibiotics depends on the mechanism of the facial injury (eg,
animal or human bite, assaults, MVA), the degree of injury (superficial or
extensive), and the immune status of the patient. Because infection of the face
secondary to trauma can become devastating and can cause significant cosmetic
and functional deformities, 24-hour prophylactic coverage with a cephalosporin
is usually necessary. Approximately 10% of patients who are allergic to
penicillin have an adverse reaction to cephalosporins. If this occurs, an
aminoglycoside may be used.
Anesthesia
233
For facial lacerations that can be closed primarily, local anesthetic agents
such as lidocaine (Xylocaine) 1% or 2% with epinephrine (1:100,000) are used.
The vasoconstrictive effects of epinephrine provide for hemostasis and prolong
the effect of anesthesia. Avoid epinephrine in areas with end arteries, such as the
tip of the nose or the ear lobe, as it may induce irreversible vasoconstriction leading to necrosis. For injuries involving the nares, topical anesthetic agents applied to the nasal mucous membranes may be used. Cocaine (5%) is the agent of
choice in this case because it is fast acting, has an intermediate duration of action, and can be introduced easily via cotton-tipped applicators or cotton gauze.
Surgical therapy
Timing
If possible, repair facial injuries within the first 8 hours of the initial
insult. Tissues are less vulnerable to infection and the wound healing process is
at its optimum during that time. Repair may be postponed for up to 72 hours if
the patient is unstable, provided that he or she receives antibiotic coverage and
that the wound is cleansed and dressed. If it is still not possible to repair injuries
after 3 days, then healing by secondary intention becomes necessary, and
subsequent scar revision might be indicated after secondary wound closure.
Location
Unless the injury is superficial and toward the periphery of the face, repair
extensive facial soft tissue injuries in the operating room and not in the
emergency department. Frequently, adult patients sustaining significant facial
trauma from assault are intoxicated and combative, which complicates the
delicate procedures required on the face. Treat such patients under general
anesthesia. Similarly, for younger children, who are usually uncooperative,
conduct operative treatment under general anesthesia.
Wound preparation
All forms of facial injuries, such as abrasions, lacerations, and avulsions,
should be well irrigated with isotonic sodium chloride solution prior to any
handling of tissue. This serves to cleanse and to provide better visualization of
the wound. Carefully remove any lodged foreign body fragments to minimize
disturbance to surrounding tissue. If any macerated or friable tissue is present,
meticulous debridement of the affected areas may be carried out, provided that
subsequent possible cosmetic deformities are considered and minimized.
If the injury extends through hirsute regions, such as the scalp, mustache,
or beard, the hair may be shaved around the wound to facilitate suturing. The
eyebrow, however, is never shaved. (Once shaved, the eyebrow may not grow
back.) In addition, the form and contour of the eyebrow also serve as crucial
positions for aesthetic symmetry and as important landmarks for repair.
Mishandling of the eyebrow may result in difficult to correct defects of improper
alignment, disproportionate growth, or both.
Wound closure
Close most facial wounds with fine sutures. If the wound requires closure
in layers, fine absorbable 4-0 or 5-0 sutures may be used on the mucosa or
muscles. Close the skin with nonabsorbable monofilament sutures. Subcuticular
234
sutures may be used on conspicuous areas. Trim macerated or jagged wound
margins before any closure.
Suture removal
The face has a very rich vasculature that promotes quicker healing. In areas where the skin is thin, as in the eyelids, sutures are removed in 3-4 days; elsewhere on the face, they are left 4-6 days. Sutures in children can be removed
earlier due to their ability to heal quickly. Sutures in the ears are often left in
place 10-14 days, especially with underlying cartilage injury, as scars over divided ear cartilage tend to thicken and spread when sutures are removed too early.
Frostbite
Frostbite is the medical condition whereby damage is caused to skin and
other tissues due to extreme cold. At or below -15° "Celsius" C (5°
"Fahrenheit" F), blood vessels close to the skin start to narrow (constrict). This
helps to preserve core body temperature. In extreme cold or when the body is
exposed to cold for long periods, this protective strategy can reduce blood flow
in some areas of the body to dangerously low levels. The combination of cold
temperature and poor blood flow can cause severe tissue injury by freezing the
tissue. Frostbite is most likely to happen in body parts farthest from the heart,
and those with a lot of surface area exposed to cold. The initial stages of
frostbite are sometimes called "frostnip", some people can feel these, some not.
Pathophysiology: Cold exposure leads to ice crystal formation, cellular
dehydration, protein denaturation, inhibition of DNA synthesis, abnormal cell
wall permeability with resultant osmotic changes, damage to capillaries, and pH
changes. Rewarming causes cell swelling, erythrocyte and platelet aggregation,
endothelial cell damage, thrombosis, tissue edema, increased compartment space
pressure, bleb formation, localized ischemia, and tissue death.
Underlying responses to these injuries include generation of oxygen free
radicals, production of prostaglandins and thromboxane A2, release of
proteolytic enzymes, and generalized inflammation. Tissue injury is greatest
when cooling is slow, cold exposure is prolonged, rate of rewarming is slow,
and, especially, when tissue is partially thawed and refreezes.
CLINICAL
Symptoms affecting frostbitten body part include the following:
Coldness and firmness
Stinging, burning, numbness
Clumsiness
Pain, throbbing, burning, or electric current-like sensations on rewarming
Physical:
Location
While hands and feet are affected most frequently, shins, cheeks, nose,
ears, and corneas may be involved.
As in thermal burns, frostbite injuries may be classified by degree.
First-degree injuries involve the epidermis, while fourth-degree injuries involve
the epidermis, dermis, subcutaneous tissue, and deeper structures.
Degree of injury
235
First-degree injury - Erythema, edema, waxy appearance, hard white
plaques, and sensory deficit
Second-degree injury - Erythema, edema, and formation of blisters filled
with clear or milky fluid and which are high in thromboxane (These blisters
form within 24 hours of injury.)
Third-degree injury - Presence of blood-filled blisters, which progress to a
black eschar over a matter of weeks
Fourth-degree injury - Full-thickness damage affecting muscles, tendons,
and bone, with resultant tissue loss
Other signs
Excessive sweating
Joint pain
Pallor or blue discoloration
Hyperemia
Skin necrosis
Gangrene
Causes:
Predisposing factors and populations at greatest risk include the
following:
Individuals stranded in cold weather
Soldiers, cold weather rescuers, and laborers working in a cold
environment
Winter and high-altitude athletes
Extremes of age
Homelessness
Altered mental status (eg, head trauma, ethanol or illicit drug abuse,
psychiatric illness)
Exposure to water or dampness
Immobilization
Use of nicotine or other vasoconstrictive drugs
Previous cold injury
Use of inadequate or constrictive clothing
Persons exposed to chronic hand or arm vibration
Underlying illness
Malnutrition
Infection
Peripheral vascular disease
Atherosclerosis
Arthritis
Diabetes
Thyroid disease
Treatment
Prehospital Care:
236
Address life-threatening conditions first.
Replace wet clothing with dry soft clothing to minimize further heat loss.
Initiate rewarming of affected area as soon as possible. Do not attempt
rewarming if a danger of refreezing is present. Avoid rubbing affected area with
warm hands or snow, as this can cause further injury. If affected body part is an
extremity, wrap it in a blanket for mechanical protection during transport. Avoid
alcohol or sedatives, which can enhance heat loss and impair shivering.
It is better to walk with frozen feet to shelter than to attempt rewarming at
the scene; however, walking on frostbitten feet may cause tissue chipping or
fracture.
Emergency Department Care:
Address life-threatening conditions first. Fluid resuscitation, especially in
persons with mountain frostbite, enhances blood flow and tissue perfusion.
Rapidly rewarm affected body part, avoiding further trauma.
An appropriate warming technique is the use of a whirlpool bath or tub of
water at 40-42°C. Mild antibacterial soap may be added. Avoid warmer
temperatures or dry heat because of the risk of thermal injury.
If a tub is not available, use warm wet packs at the same temperature.
Avoid massaging the affected area, as this can cause further injury.
Administer analgesics, such as morphine sulfate, as needed for pain.
Thawing usually takes 20-40 minutes and is complete when distal tip of
affected area flushes. Once thawed, keep body part on sterile sheets, elevated,
and splinted when possible. A cradle may be used over an injured lower
extremity to avoid pressure or trauma.
Debride clear blisters to prevent thromboxane-mediated tissue injury.
Leave hemorrhagic blisters intact to reduce risk of infection.
In patients with an associated dislocation, perform reduction as soon as
thawing is complete. Manage fractures conservatively until postthaw edema has
resolved.
First Aid
One should be aware of factors that can contribute to frostbite, such as
extreme cold, wet clothes, wind chill and poor circulation. This can be caused
by tight clothing or boots, cramped positions, fatigue, certain medications,
smoking, alcohol use or diseases that affect the blood vessels, such as diabetes.
One should wear suitable clothing in cold temperatures and protect
susceptible areas. In cold weather, wear mittens (not gloves); wind-proof, waterresistant, multi-layered clothing; two pairs of socks (synthetic liners next to skin,
then insulator sock); and a scarf and a hat that covers the ears (to avoid
substantial heat loss through the scalp).
One should not wear types of fabric, such as cotton, that retain moisture
when exposed to extreme cold.
Before anticipated prolonged exposure to cold, one should not drink
alcohol or smoke, and get adequate food and rest.
If caught in a severe snowstorm, one should find shelter early or increase
physical activity to maintain body warmth.
237
MEDICATION
The goals of medical management of frostbite are pain control and
prevention of complications, such as further tissue damage or infection.
In addition to therapies described below, there are useful therapies that have not been prospectively validated and doses not standardized. Some of these
include low molecular weight dextran of which daily infusions may be beneficial. This agent may prevent erythrocyte clumping in cold-injured blood vessels.
In addition, low-dose infusions of heparin may prevent microthrombosis.
Marcaine also has been used either for cervical or lumbar sympathetic blockade
to decrease sympathetic tone and relieve pain, but its efficacy is unclear.
Drug Category: Nonsteroidal anti-inflammatory drugs (NSAIDs) -- These
drugs have analgesic and antipyretic activities. Their mechanism of action is not
known, but these agents may inhibit cyclooxygenase activity and prostaglandin
synthesis.
Other mechanisms may exist as well, such as inhibition of leukotriene
synthesis, lysosomal enzyme release, lipoxygenase activity, neutrophil and
platelet aggregation, and various cell-membrane functions.
PROGNOSIS
A new approach to frostbite treatment was developed in the 1980s. The
major emphasis in this method is to re-warm the body as quickly as possible.
This method has proved to be very successful. In one study, about twothirds of
patients with superficial frostbite recovered completely without tissue loss. The
success rate using older methods was only about 35 percent (or about one-third
of patients).
The most serious consequence of frostbite may be amputation. People
who do not require amputation may still experience long-term symptoms. These
symptoms include extreme throbbing pain, a burning sensation or tingling
feelings, color changes of the skin, changes in the shape of nails or loss of nails,
joint stiffness, excessive sweating, and a heightened sensitivity to cold.
FACIAL PARALYSIS
I. Anatomy of the 7th Cranial Nerve
Anatomy of the facial nerve and fallopian canal
Intracranial nerve arises near pons and courses 12mm to porus acousticus.
Meatal portion (10 mm) is anterior to the superior vestibular nerve and superior
to the cochlear nerve.
Intratemporal portion
Labyrinthine segment (3-4 mm) passes through narrowest part of the fallopian
canal. Common site of pathology: temporal bone fractures and Bell's palsy.
Tympanic segment runs from geniculate ganglion to pyramidal turn (11 mm).
Mastoid segment descends 13 mm to exit the stylomastoid foramen.
Extracranial portion
Nerve extends 15-20 mm from stylomastoid foramen to pes anserinus.
Variable branching patterns.
238
Clinical comment: The course of the facial nerve through the posterior
fossa, temporal bone, and parotid gland renders this cranial nerve vulnerable to
many neoplastic, traumatic, and infectious events. Disorders of the nerve
provoke some interest in other medical specialties, but because of his
background in head and neck anatomy and pathology and skill in temporal bone
surgery, the otolaryngologist is most qualified to diagnose and manage paralysis
of the facial nerve. Nevertheless, all clinicians should be able to recognize a
peripheral paralysis and initiate proper evaluation.
Anomalous Courses
Most common anomaly: dehiscence of facial canal.
Common sites: oval window and geniculate ganglion.
Exposed nerve is more susceptible to injury during otologic surgery.
Most course anomalies are within temporal bone:
Prolapse of nerve against stapes
Bifurcation around stapes
Deviation across promontory
Knuckle at the pyramidal (second) turn
Duplication variants
Anomalies are more common in malformations of the ear.
II. Pathophysiology of the Facial Nerve
Mixed Motor-Sensory Nerve
Efferent fibers from the motor nucleus innervate muscles of facial
expression, post-auricular, stylohyoid, posterior digastric, and stapedius
muscles.
Superior salivary nucleus projects efferent (parasympathetic
preganglionic) fibers to sphenopalatine ganglion where postganglionic
fibers then innervate lacrimal glands and mucinous glands of the nose.
Another set of parasympathetic fibers synapse at the submandibular
ganglion. Postganglionic fibers connect the submandibular and sublingual
glands.
Afferent fibers convey taste from anterior two-thirds of tongue to nucleus
tractus solitarius via lingual nerve, chorda tympani, and nervus
intermedius.
Afferent fibers mediate sensation from posterior external auditory canal,
concha, ear lobe, and deep parts of face. Projections unknown.
Nerve Injury and Regeneration
Sunderland classification of nerve injury:
Neuropraxia: reversible conduction block (1° damage).
Axonotmesis: loss of structural continuity of axon with intact endoneurial
sheath (2° damage).
Neurotmesis: 3°: loss of continuity of axons and endoneurial sheaths; 4°:
loss of continuity of axons, sheaths, funiculus; 5°: complete loss of nerve
continuity.
239
Degeneration
Interruption of the continuity of the axon separates the distal axon from its
metabolic source, the neuron or cell body. Wallerian degeneration of the
distal axon and myelin sheath begins within 24 hours.
Macrophages phagocytose degraded myelin and axons.
Regeneration
Neuron metabolism leads to increases in mRNA, enzymes, and structural
proteins.
Axonal stumps swell with axoplasm and proliferation of neurofilaments.
Conditions at the site of injury govern the fate and organization of the
sprouts.
Simple misdirection: the entry of one axon into a tubule destined for a
muscle other than the one previously innervated. Clinical expression:
synkinesis or associated movement.
Complex misdirection: a single axon through branching innervates
tubules to different muscles. Clinical expression: mass movement.
Other sequelae of faulty regeneration: tics, spasms, contractures,
weakness, and gustatory lacrimation.
III. Differential Diagnosis of Peripheral Facial Paralysis
Extracranial
Traumatic
Facial lacerations
Blunt forces
Penetrating wounds
Mandible fractures
Iatrogenic injuries
Newborn paralysis
Neoplastic
Parotid tumors
Tumors of the external canal and middle ear
Facial nerve neurinomas
Metastatic lesions
Congenital absence of facial musculature
Intratemporal
Traumatic
Fractures of petrous pyramid
Penetrating injuries
Iatrogenic injuries
Neoplastic
Glomus tumors
Cholesteatoma
Facial neurinomas
240
Hemangiomas
Meningiomas
Acoustic neurinomas
Squamous cell carcinomas
Rhabdomyosarcoma
Arachnoidal cysts
Metastatic
Infectious
Herpes zoster oticus
Acute otitis media
Chronic otitis media
Malignant otitis externa
Idiopathic
Bell's palsy
Melkersson-Rosenthal syndrome
Congenital: osteopetroses
Intracranial
Iatrogenic injury
Neoplastic
Congenital
Mobius syndrome
Absence of motor units
IV. Evaluation of Facial Paralysis
Examination
The presence of a peripheral facial paralysis demands a complete head
and neck examination with otoscopy and cranial nerve evaluation.
Characteristics of a peripheral paralysis:
At rest: less prominent wrinkles on forehead of affected side, eyebrow
droop, flattened nasolabial fold, corner of mouth turned down.
Unable to wrinkle forehead, raise eyebrow, wrinkle nasolabial fold, purse
lips, show teeth, or completely close eye.
Bell phenomenon: visible vertical rotation of globe on closing affected
eye.
Characteristics of a central facial paralysis:
Because of uncrossed contributions from ipsilateral supranuclear areas,
movements of the frontal and upper orbicularis oculi mm. tend to be spared.
Facial movement may be present on affected side during emotional
expression.
Involvement of tongue.
Presence of lacrimation and salivation.
Electrodiagnostic Testing Expediency of management of acute paralysis
may prevent conversion of minor injury into a more severe form with resulting
sequelae.
241
Nerve Excitability Test
Technique: Using a stimulating electrode (square pulse of 0.3 msec
duration) over the terminal ramifications of the facial nerve, one increases the
current (milliamperes) until movement in the appropriate muscle group is just
visible. Normal values (unaffected side of face) are compared to the side of
paralysis.
Interpretation: A difference of 3.5 mamp or more indicates an unfavorable
prognosis.
Electroneurography (Evoked Electromyography)
Technique: Square wave impulses of 0.2 msec duration and 50-100 volt
amplitude with a frequency of 1/sec are delivered with a bipolar electrode in
front of the tragus while a second bipolar electrode captures the compound
action potentials of underlying facial muscles in the nasolabial fold.
Interpretation: The difference in amplitude of the potentials of the intact
and involved side of the face correlate with the percentage of degenerated motor
fibers (denervation).
Advantage: Quantitative analysis of amount of degeneration.
Disadvantage: Amplitudes are a 24-48 hour delayed representation of
actual events occurring at site of lesion.
Clinical applications: Facial nerves subjected to traumatic injuries of a
magnitude requiring surgical repair undergo 90% degeneration within six days
of injury. In cases of Bell's Palsy, a poor prognosis can be anticipated in patients
reaching 95% or more degeneration within 14 days of onset of the palsy.
Topographic Diagnosis - Topographic testing to determine the anatomical
level of a peripheral lesion is possible because of the mixed function of the
nerve and the branching pattern within the temporal bone.
Tests of clinical value include:
Petrosal nerve
Schirmer test: quantitative evaluation of tear production
Interpretation: When unilateral wetness is reduced by more than 30% of
the total amount of both eyes after 5 minutes or when bilateral tearing is reduced
to less than 25 mm after a 5-minute period, the Schirmer test is considered clinically significant and implies a lesion at or proximal to the geniculate ganglion.
Stapedius nerve
Impedance audiometry can record the presence or absence of stapedius
muscle contraction to sound stimuli 70 to 100 dB above hearing threshold.
Interpretation: Absence of the reflex is due to a lesion proximal to stapedius
nerve (vertical segment of facial nerve). (Caution: The Schirmer's test can give
false negative results.)
Diagnostic Studies
Audiometry
Pure tone audiometry records cochlear nerve function.
Stapedial reflex is part of topographic testing.
242
Speech discrimination, tone decay, auditory evoked potentials are used to
screen for retrocochlear lesions, e.g., tumors of the cerebellopontine
angle.
X-ray
Computed tomography with and without contrast (radiopaque and air) is
preferred for lesions of IAC, posterior fossa, and brain stem. High
resolution scans needed for base of skull lesions.
MRI is best for soft tissue assessment and tumors of the facial nerve.
V. Management of Facial Paralysis
Extracranial Etiologies
Traumatic injuries: lacerations, gunshot wounds, iatrogenic.
Most important areas to repair: main trunk, temporofacial and
cervicofacial divisions.
When immediate repair in contaminated or extensive wounds is not
feasible, proximal and distal stumps should be tagged. The transected ends lose
response to electrical stimulation within 72 hours. If not properly identified,
these endings may become involved in scar tissue. Anastomosis or grafting in
such cases may be impossible.
Methods of repair: direct end-to-end anastomosis and interpositional
grafting. Do not approximate ends under tension!
Iatrogenic injury
Complication of parotid surgery. Tumors are best managed by the
experienced otolaryngologist-head and neck surgeon.
Integrity of nerve should be ascertained prior to closure. Immediate repair
indicated.
Neoplasia
A mass in the parotid associated with facial paralysis is a sign of
malignancy. Two most common cell types: adenoid cystic and undifferentiated.
Sacrifice of involved nerve and nerve adjacent to tumor indicated in high-grade
malignancies: adenoid cystic, high-grade mucoepidermoid carcinoma, expleomorphic adenoma, etc.
Reconstruction: interpositional grafting and 7-12 cranial nerve crossover.
Intratemporal Etiologies
Temporal bone fractures
Signs: bleeding from the external canal, hemotympanum, step-deformity
of the osseous canal, conductive hearing loss (longitudinal fracture),
sensorineural hearing loss (transverse fracture), CSF otorrhea, and facial nerve
involvement (20% of longitudinal fractures and 50% of transverse fractures).
In general, paralysis of immediate onset carries a poor prognosis and paralysis
of delayed onset has a more favorable recovery. All paralysis should be
followed with electrical testing, as exceptions to the maxim exist. Timely
exploration and repair ensure better quality of return of function.
243
Types of pathology: intraneural hematoma, impingement of bone and
transection of nerve. Most common site of injury: adjacent to geniculate
ganglion.
Surgical approaches: Longitudinal fractures are explored through the
middle fossa, and mastoid, if necessary. Facial nerve is examined via
transmastoid, translabyrinthine approach in transverse fractures.
Iatrogenic injury
Incidence 0.6-3.7%
Most common areas: pyramidal turn and the tympanic segment over the
oval window.
Neoplasia
The primary tumor of the facial nerve per se is the facial neurinoma.
Weakness of the face is the most common symptom. Treatment is surgical
removal with grafting of the involved segment of nerve.
Many benign and malignant masses may involve the facial nerve in its
course through the temporal bone: glomus tumors, meningiomas,
cholesteatomas, squamous cell carcinoma, rhabdomyosarcoma, etc. Surgical
removal is necessary in most cases. Radiation therapy may be palliative
depending on cell type, size, and location. If the nerve cannot be spared at the
time of resection, interpositional grafting is warranted.
Idiopathic facial palsy (Bell's Palsy)
Bell's Palsy is the most common cause of facial paralysis (greater than
50% of cases of acute palsy). Unfortunately, this leads to over-diagnosis of the
condition and a false sense of security. Every patient with a facial paralysis
needs a complete evaluation. When the diagnosis of Bell's palsy is made (by
exclusion), the patient must be followed 6 - 9 months or until recovery of facial
movement. Failure of any return of function implies an etiology other than Bell's
palsy. Re-evaluation is mandatory in such cases, as the most commonly
overlooked diagnosis is one of neoplasia.
Etiology is still unknown.
Entrapment theory: an inflammatory response leads to compression and
ischemia of the nerve in the narrowest part of the fallopian canal, the meatal
foramen and labyrinthine segment.
Electrical testing follows the degeneration of the motor fibers.
Decompression of the nerve is indicated when 90-94% degeneration occurs
within 2 weeks of onset.
Steroids are indicated early in the course of the disease. The use of
acyclovir is under investigation. Surgical decompression is accomplished via the
middle fossa by an otologist-neurotologist. Transmastoid decompression is no
more efficacious than steroid therapy.
Infection
Acute suppurative otitis media is caused by gram-positive cocci and
Hemophilus influenza. Invasion into the facial canal through a dehiscence may
evoke an inflammatory response with edema, compression, and ischemia
244
resulting in facial weakness. Treatment includes myringotomy, appropriate
antibiotics, and transmastoid decompression if degeneration progresses.
Facial
paralysis
due
to
chronic
otitis
media
requires
tympanomastoidectomy for eradication of infection or cholesteatoma.
Otalgia, facial weakness and a vesicular eruption on the concha or
external canal (sensory distribution of 7th cranial nerve) characterize herpes
zoster oticus (Ramsay-Hunt Syndrome). Site of pathology: labyrinthine segment
of nerve. Acyclovir is treatment of choice.
Malignant otitis externa is due to Pseudomonas invasion of soft tissue,
cartilage, and bone. Treatment includes debridement of infected tissue,
decompression of facial nerve when involved, and six weeks of semi-synthetic
penicillin in combination with an aminoglycoside. Cipro may have a role in
long-term therapy.
Other Etiologies
Congenital
Mobius syndrome: hypoplasia of 6th and 7th cranial nerve nuclei.
Birth trauma: due to forceps compression or compression of side of face against
sacrum during labor.
Osteopetroses: hereditary bone diseases. May result in bony obliteration
of foramina with compression of cranial nerves. Decompression is indicated on
rare occasion.
Intracranial: Most common causes are neoplastic and iatrogenic.
THE TRIGEMINAL NEURALGIA
The Trigeminal Nerve
(N. Trigeminus; Fifth Or Trifacial Nerve)
The trigeminal nerve is the largest cranial nerve and is the great sensory nerve of
the head and face, and the motor nerve of the muscles of mastication.
It emerges from the side of the pons, near its upper border, by a small
motor and a large sensory root—the former being situated in front of and medial
to the latter.
Motor Root. The fibers of the motor root arise from two nuclei, a
superior and an inferior. The superior nucleus consists of a strand of cells
occupying the whole length of the lateral portion of the gray substance of the
cerebral aqueduct. The inferior or chief nucleus is situated in the upper part of
the pons, close to its dorsal surface, and along the line of the lateral margin of
the rhomboid fossa. The fibers from the superior nucleus constitute the
mesencephalic root: they descend through the mid-brain, and, entering the pons,
join with the fibers from the lower nucleus, and the motor root, thus formed,
passes forward through the pons to its point of emergence. It is uncertain
whether the mesencephalic root is motor or sensory.
245
Sensory Root. The fibers of the sensory root arise from the cells of the
semilunar ganglion which lies in a cavity of the dura mater near the apex of the
petrous part of the temporal bone. They pass backward below the superior
petrosal sinus and tentorium cerebelli, and, entering the pons, divide into upper
and lower roots. The upper root ends partly in a nucleus which is situated in the
pons lateral to the lower motor nucleus, and partly in the locus cжruleus; the
lower root descends through the pons and medulla oblongata, and ends in the
upper part of the substantia gelatinosa of Rolando. This lower root is sometimes
named the spinal root of the nerve. Medullation of the fibers of the sensory root
begins about the fifth month of fetal life, but the whole of its fibers are not
medullated until the third month after birth.
The Semilunar Ganglion (ganglion semilunare [Gasseri]; Gasserian
ganglion) occupies a cavity (cavum Meckelii) in the dura mater covering the
trigeminal impression near the apex of the petrous part of the temporal bone. It
is somewhat crescentic in shape, with its convexity directed forward: medially,
it is in relation with the internal carotid artery and the posterior part of the
cavernous sinus. The motor root runs in front of and medial to the sensory root,
and passes beneath the ganglion; it leaves the skull through the foramen ovale,
and, immediately below this foramen, joins the mandibular nerve. The greater
superficial petrosal nerve lies also underneath the ganglion.
The ganglion receives, on its medial side, filaments from the carotid
plexus of the sympathetic. It give off minute branches to the tentorium cerebelli,
and to the dura mater in the middle fossa of the cranium. From its convex
border, which is directed forward and lateralward, three large nerves proceed,
viz., the ophthalmic, maxillary, and mandibular. The ophthalmic and maxillary
consist exclusively of sensory fibers; the mandibular is joined outside the
cranium by the motor root.
Associated with the three divisions of the trigeminal nerve are four small
ganglia. The ciliary ganglion is connected with the ophthalmic nerve; the
sphenopalatine ganglion with the maxillary nerve; and the otic and submaxillary
ganglia with the mandibular nerve. All four receive sensory filaments from the
trigeminal, and motor and sympathetic filaments from various sources; these
filaments are called the roots of the ganglia.
The Ophthalmic Nerve (n. ophthalmicus), or first division of the
trigeminal, is a sensory nerve. It supplies branches to the cornea, ciliary body,
and iris; to the lacrimal gland and conjunctiva; to the part of the mucous
membrane of the nasal cavity; and to the skin of the eyelids, eyebrow, forehead,
and nose. It is the smallest of the three divisions of the trigeminal, and arises
from the upper part of the semilunar ganglion as a short, flattened band, about
2.5 cm. long, which passes forward along the lateral wall of the cavernous sinus,
below the oculomotor and trochlear nerves; just before entering the orbit,
through the superior orbital fissure, it divides into three branches, lacrimal,
frontal, and nasociliary.
The ophthalmic nerve is joined by filaments from the cavernous plexus of
the sympathetic, and communicates with the oculomotor, trochlear, and
246
abducent nerves; it gives off a recurrent filament which passes between the
layers of the tentorium.
The Lacrimal Nerve (n. lacrimalis) is the smallest of the three branches
of the ophthalmic. It sometimes receives a filament from the trochlear nerve, but
this is possibly derived from the branch which goes from the ophthalmic to the
trochlear nerve. It passes forward in a separate tube of dura mater, and enters the
orbit through the narrowest part of the superior orbital fissure. In the orbit it runs
along the upper border of the Rectus lateralis, with the lacrimal artery, and
communicates with the zygomatic branch of the maxillary nerve. It enters the
lacrimal gland and gives off several filaments, which supply the gland and the
conjunctiva. Finally it pierces the orbital septum, and ends in the skin of the
upper eyelid, joining with filaments of the facial nerve. The lacrimal nerve is
occasionally absent, and its place is then taken by the zygomaticotemporal
branch of the maxillary. Sometimes the latter branch is absent, and a
continuation of the lacrimal is substituted for it.
The Frontal Nerve (n. frontalis) is the largest branch of the ophthalmic,
and may be regarded, both from its size and direction, as the continuation of the
nerve. It enters the orbit through the superior orbital fissure, and runs forward
between the Levator palpebrж superioris and the periosteum. Midway between
the apex and base of the orbit it divides into two branches, supratrochlear and
supraorbital.
The supratrochlear nerve (n. supratrochlearis), the smaller of the two,
passes above the pulley of the Obliquus superior, and gives off a descending
filament, to join the infratrochlear branch of the nasociliary nerve. It then
escapes from the orbit between the pulley of the Obliquus superior and the
supraorbital foramen, curves up on to the forehead close to the bone, ascends
beneath the Corrugator and Frontalis, and dividing into branches which pierce
these muscles, it supplies the skin of the lower part of the forehead close to the
middle line and sends filaments to the conjunctiva and skin of the upper eyelid.
The supraorbital nerve (n. supraorbitalis) passes through the
supraorbital foramen, and gives off, in this situation, palpebral filaments to the
upper eyelid. It then ascends upon the forehead, and ends in two branches, a
medial and a lateral, which supply the integument of the scalp, reaching nearly
as far back as the lambdoidal suture; they are at first situated beneath the
Frontalis, the medial branch perforating the muscle, the lateral branch the galea
aponeurotica. Both branches supply small twigs to the pericranium.
The Nasociliary Nerve (n. nasociliaris; nasal nerve) is intermediate in
size between the frontal and lacrimal, and is more deeply placed. It enters the
orbit between the two heads of the Rectus lateralis, and between the superior and
inferior rami of the oculomotor nerve. It passes across the optic nerve and runs
obliquely beneath the Rectus superior and Obliquus superior, to the medial wall
of the orbital cavity. Here it passes through the anterior ethmoidal foramen, and,
entering the cavity of the cranium, traverses a shallow groove on the lateral
margin of the front part of the cribriform plate of the ethmoid bone, and runs
down, through a slit at the side of the crista galli, into the nasal cavity. It
247
supplies internal nasal branches to the mucous membrane of the front part of the
septum and lateral wall of the nasal cavity. Finally, it emerges, as the external
nasal branch, between the lower border of the nasal bone and the lateral nasal
cartilage, and, passing down beneath the Nasalis muscle, supplies the skin of the
ala and apex of the nose.
The nasociliary nerve gives off the following branches, viz.: the long root
of the ciliary ganglion, the long ciliary, and the ethmoidal nerves.
The long root of the ciliary ganglion (radix longa ganglii ciliaris) usually
arises from the nasociliary between the two heads of the Rectus lateralis. It
passes forward on the lateral side of the optic nerve, and enters the posterosuperior angle of the ciliary ganglion; it is sometimes joined by a filament from
the cavernous plexus of the sympathetic, or from the superior ramus of the
trochlear nerve.
The long ciliary nerves (nn. ciliares longi), two or three in number, are
given off from the nasociliary, as it crosses the optic nerve. They accompany the
short ciliary nerves from the ciliary ganglion, pierce the posterior part of the
sclera, and running forward between it and the choroid, are distributed to the iris
and cornea. The long ciliary nerves are supposed to contain sympathetic fibers
from the superior cervical ganglion to the Dilator pupillж muscle.
The infratrochlear nerve (n. infratrochlearis) is given off from the
nasociliary just before it enters the anterior ethmoidal foramen. It runs forward
along the upper border of the Rectus medialis, and is joined, near the pulley of
the Obliquus superior, by a filament from the supratrochlear nerve. It then
passes to the medial angle of the eye, and supplies the skin of the eyelids and
side of the nose, the conjunctiva, lacrimal sac, and caruncula lacrimalis.
The ethmoidal branches (nn. ethmoidales) supply the ethmoidal cells;
the posterior branch leaves the orbital cavity through the posterior ethmoidal
foramen and gives some filaments to the sphenoidal sinus.
The Ciliary Ganglion (ophthalmic or lenticular ganglion) The ciliary
ganglion is a small, sympathetic ganglion, of a reddish-gray color, and about the
size of a pin’s head; it is situated at the back part of the orbit, in some loose fat
between the optic nerve and the Rectus lateralis muscle, lying generally on the
lateral side of the ophthalmic artery.
Its roots are three in number, and enter its posterior border. One, the long
or sensory root, is derived from the nasociliary nerve, and joins its posterosuperior angle. The second, the short or motor root, is a thick nerve
(occasionally divided into two parts) derived from the branch of the oculomotor
nerve to the Obliquus inferior, and connected with the postero-inferior angle of
the ganglion. The motor root is supposed to contain sympathetic efferent fibers
(preganglionic fibers) from the nucleus of the third nerve in the mid-brain to the
ciliary ganglion where they form synapses with neurons whose fibers
(postganglionic) pass to the Ciliary muscle and to Sphincter muscle of the pupil.
The third, the sympathetic root, is a slender filament from the cavernous plexus
of the sympathetic; it is frequently blended with the long root. According to
248
Tiedemann, the ciliary ganglion receives a twig of communication from the
sphenopalatine ganglion.
Its branches are the short ciliary nerves. These are delicate filaments,
from six to ten in number, which arise from the forepart of the ganglion in two
bundles connected with its superior and inferior angles; the lower bundle is the
larger. They run forward with the ciliary arteries in a wavy course, one set above
and the other below the optic nerve, and are accompanied by the long ciliary
nerves from the nasociliary. They pierce the sclera at the back part of the bulb of
the eye, pass forward in delicate grooves on the inner surface of the sclera, and
are distributed to the Ciliaris muscle, iris, and cornea. Tiedemann has described
a small branch as penetrating the optic nerve with the arteria centralis retinж.
The Maxillary Nerve (n. maxillaris; superior maxillary nerve), or second
division of the trigeminal, is a sensory nerve. It is intermediate, both in position
and size, between the ophthalmic and mandibular. It begins at the middle of the
semilunar ganglion as a flattened plexiform band, and, passing horizontally
forward, it leaves the skull through the foramen rotundum, where it becomes
more cylindrical in form, and firmer in texture. It then crosses the
pterygopalatine fossa, inclines lateralward on the back of the maxilla, and enters
the orbit through the inferior orbital fissure; it traverses the infraorbital groove
and canal in the floor of the orbit, and appears upon the face at the infraorbital
foramen. At its termination, the nerve lies beneath the Quadratus labii
superioris, and divides into a leash of branches which spread out upon the side
of the nose, the lower eyelid, and the upper lip, joining with filaments of the
facial nerve.
Branches.—Its branches may be divided into four groups, according as
they are given off in the cranium, in the pterygopalatine fossa, in the infraorbital
canal, or on the face.
The Middle Meningeal Nerve (n. meningeus medius; meningeal or dural
branch) is given off from the maxillary nerve directly after its origin from the
semilunar ganglion; it accompanies the middle meningeal artery and supplies the
dura mater.
The Zygomatic Nerve (n. zygomaticus; temporomalar nerve; orbital
nerve) arises in the pterygopalatine fossa, enters the orbit by the inferior orbital
fissure, and divides at the back of that cavity into two branches,
zygomaticotemporal and zygomaticofacial.
The zygomaticotemporal branch (ramus zygomaticotemporalis;
temporal branch) runs along the lateral wall of the orbit in a groove in the
zygomatic bone, receives a branch of communication from the lacrimal, and,
passing through a foramen in the zygomatic bone, enters the temporal fossa. It
ascends between the bone, and substance of the Temporalis muscle, pierces the
temporal fascia about 2.5 cm. above the zygomatic arch, and is distributed to the
skin of the side of the forehead, and communicates with the facial nerve and
with the aurićulotemporal branch of the mandibular nerve. As it pierces
the temporal fascia, it gives off a slender twig, which runs between the two
layers of the fascia to the lateral angle of the orbit.
249
The zygomaticofacial branch (ramus zygomaticofacialis; malar branch)
passes along the infero-lateral angle of the orbit, emerges upon the face through
a foramen in the zygomatic bone, and, perforating the Orbicularis oculi, supplies
the skin on the prominence of the cheek. It joins with the facial nerve and with
the inferior palpebral branches of the maxillary.
The Sphenopalatine Branches (nn. sphenopalatini), two in number,
descend to the sphenopalatine ganglion.
The Posterior Superior Alveolar Branches (rami alveolares superiores
posteriores; posterior superior dental branches) arise from the trunk of the nerve
just before it enters the infraorbital groove; they are generally two in number,
but sometimes arise by a single trunk. They descend on the tuberosity of the
maxilla and give off several twigs to the gums and neighboring parts of the
mucous membrane of the cheek. They then enter the posterior alveolar canals on
the infratemporal surface of the maxilla, and, passing from behind forward in the
substance of the bone, communicate with the middle superior alveolar nerve,
and give off branches to the lining membrane of the maxillary sinus and three
twigs to each molar tooth; these twigs enter the foramina at the apices of the
roots of the teeth.
The Middle Superior Alveolar Branch (ramus alveolaris superior
medius; middle superior dental branch), is given off from the nerve in the
posterior part of the infraorbital canal, and runs downward and forward in a
canal in the lateral wall of the maxillary sinus to supply the two premolar teeth.
It forms a superior dental plexus with the anterior and posterior superior alveolar
branches.
The Anterior Superior Alveolar Branch (ramus alveolaris superior
anteriores; anterior superior dental branch), of considerable size, is given off
from the nerve just before its exit from the infraorbital foramen; it descends in a
canal in the anterior wall of the maxillary sinus, and divides into branches which
supply the incisor and canine teeth. It communicates with the middle superior
alveolar branch, and gives off a nasal branch, which passes through a minute
canal in the lateral wall of the inferior meatus, and supplies the mucous
membrane of the anterior part of the inferior meatus and the floor of the nasal
cavity, communicating with the nasal branches from the sphenopalatine
ganglion.
The Inferior Palpebral Branches (rami palpebrales inferiores; palpebral
branches) ascend behind the Orbicularis oculi. They supply the skin and
conjunctiva of the lower eyelid, joining at the lateral angle of the orbit with the
facial and zygomaticofacial nerves.
The External Nasal Branches (rami nasales externi) supply the skin of
the side of the nose and of the septum mobile nasi, and join with the terminal
twigs of the nasociliary nerve.
The Superior Labial Branches (rami labiales superiores; labial
branches), the largest and most numerous, descend behind the Quadratus labii
superioris, and are distributed to the skin of the upper lip, the mucous membrane
250
of the mouth, and labial glands. They are joined, immediately beneath the orbit,
by filaments from the facial nerve, forming with them the infraorbital plexus.
Sphenopalatine Ganglion (ganglion of Meckel) The sphenopalatine
ganglion, the largest of the sympathetic ganglia associated with the branches of
the trigeminal nerve, is deeply placed in the pterygopalatine fossa, close to the
sphenopalatine foramen. It is triangular or heart-shaped, of a reddish-gray color,
and is situated just below the maxillary nerve as it crosses the fossa. It receives a
sensory, a motor, and a sympathetic root.
Its sensory root is derived from two sphenopalatine branches of the
maxillary nerve; their fibers, for the most part, pass directly into the palatine
nerves; a few, however, enter the ganglion, constituting its sensory root. Its
motor root is probably derived from the nervus intermedius through the greater
superficial petrosal nerve and is supposed to consist in part of sympathetic
efferent (preganglionic) fibers from the medulla. In the sphenopalatine ganglion
they form synapses with neurons whose postganglionic axons, vasodilator and
secretory fibers, are distributed with the deep branches of the trigeminal to the
mucous membrane of the nose, soft palate, tonsils, uvula, roof of the mouth,
upper lip and gums, and to the upper part of the pharynx. Its sympathetic root is
derived from the carotid plexus through the deep petrosal nerve. These two
nerves join to form the nerve of the pterygoid canal before their entrance into the
ganglion.
The greater superficial petrosal nerve (n. petrosus superficialis major;
large superficial petrosal nerve) is given off from the genicular ganglion of the
facial nerve; it passes through the hiatus of the facial canal, enters the cranial
cavity, and runs forward beneath the dura mater in a groove on the anterior
surface of the petrous portion of the temporal bone. It then enters the
cartilaginous substance which fills the foramen lacerum, and joining with the
deep petrosal branch forms the nerve of the pterygoid canal.
The deep petrosal nerve (n. petrosus profundus; large deep petrosal nerve)
is given off from the carotid plexus, and runs through the carotid canal lateral to
the internal carotid artery. It then enters the cartilaginous substance which fills
the foramen lacerum, and joins with the greater superficial petrosal nerve to
form the nerve of the pterygoid canal.
The nerve of the pterygoid canal (n. canalis pterygoidei [Vidii]; Vidian
nerve), formed by the junction of the two preceding nerves in the cartilaginous
substance which fills the foramen lacerum, passes forward, through the
pterygoid canal, with the corresponding artery, and is joined by a small
ascending sphenoidal branch from the otic ganglion. Finally, it enters the
pterygopalatine fossa, and joins the posterior angle of the sphenopalatine
ganglion.
Branches of Distribution.—These are divisible into four groups, viz.,
orbital, palatine, posterior superior nasal, and pharyngeal.
The orbital branches (rami orbitales; ascending branches) are two or
three delicate filaments, which enter the orbit by the inferior orbital fissure, and
supply the periosteum. According to Luschka, some filaments pass through
251
foramina in the frontoethmoidal suture to supply the mucous membrane of the
posterior ethmoidal and sphenoidal sinuses.
The palatine nerves (nn. palatini; descending branches) are distributed to
the roof of the mouth, soft palate, tonsil, and lining membrane of the nasal
cavity. Most of their fibers are derived from the sphenopalatine branches of the
maxillary nerve. They are three in number: anterior, middle, and posterior.
The anterior palatine nerve (n. palatinus anterior) descends through the
pterygopalatine canal, emerges upon the hard palate through the greater palatine
foramen, and passes forward in a groove in the hard palate, nearly as far as the
incisor teeth. It supplies the gums, the mucous membrane and glands of the hard
palate, and communicates in front with the terminal filaments of the
nasopalatine nerve. While in the pterygopalatine canal, it gives off posterior
inferior nasal branches, which enter the nasal cavity through openings in the
palatine bone, and ramify over the inferior nasal concha and middle and inferior
meatuses; at its exit from the canal, a palatine branch is distributed to both
surfaces of the soft palate.
The middle palatine nerve (n. palatinus medius) emerges through one of
the minor palatine canals and distributes branches to the uvula, tonsil, and soft
palate. It is occasionally wanting.
The posterior palatine nerve (n. palatinus posterior) descends through
the pterygopalatine canal, and emerges by a separate opening behind the greater
palatine foramen; it supplies the soft palate, tonsil, and uvula. The middle and
posterior palatine join with the tonsillar branches of the glossopharyngeal to
form a plexus (circulus tonsillaris) around the tonsil.
The posterior superior nasal branches (rami nasales posteriores
superiores) are distributed to the septum and lateral wall of the nasal fossa. They
enter the posterior part of the nasal cavity by the sphenopalatine foramen and
supply the mucous membrane covering the superior and middle nasal conchж,
the lining of the posterior ethmoidal cells, and the posterior part of the septum.
One branch, longer and larger than the others, is named the nasopalatine nerve.
It enters the nasal cavity through the sphenopalatine foramen, passes across the
roof of the nasal cavity below the orifice of the sphenoidal sinus to reach the
septum, and then runs obliquely downward and forward between the periosteum
and mucous membrane of the lower part of the septum. It descends to the roof of
the mouth through the incisive canal and communicates with the corresponding
nerve of the opposite side and with the anterior palatine nerve. It furnishes a few
filaments to the mucous membrane of the nasal septum.
The pharyngeal nerve (pterygopalatine nerve) is a small branch arising
from the posterior part of the ganglion. It passes through the pharyngeal canal
with the pharyngeal branch of the internal maxillary artery, and is distributed to
the mucous membrane of the nasal part of the pharynx, behind the auditory tube.
The mandibular nerve (n. mandibularis; inferior maxillary nerve)
supplies the teeth and gums of the mandible, the skin of the temporal region, the
auricula, the lower lip, the lower part of the face, and the muscles of
mastication; it also supplies the mucous membrane of the anterior two-thirds of
252
the tongue. It is the largest of the three divisions of the fifth, and is made up of
two roots: a large, sensory root proceeding from the inferior angle of the
semilunar ganglion, and a small motor root (the motor part of the trigeminal),
which passes beneath the ganglion, and unites with the sensory root, just after its
exit through the foramen ovale. Immediately beneath the base of the skull, the
nerve gives off from its medial side a recurrent branch (nervus spinosus) and the
nerve to the Pterygoideus internus, and then divides into two trunks, an anterior
and a posterior.
The Nervus Spinosus (recurrent or meningeal branch) enters the skull
through the foramen spinosum with the middle meningeal artery. It divides into
two branches, anterior and posterior, which accompany the main divisions of the
artery and supply the dura mater; the posterior branch also supplies the mucous
lining of the mastoid cells; the anterior communicates with the meningeal
branch of the maxillary nerve.
The Internal Pterygoid Nerve (n. pterygoideus internus).—The nerve to
the Pterygoideus internus is a slender branch, which enters the deep surface of
the muscle; it gives off one or two filaments to the otic ganglion.
The anterior and smaller division of the mandibular nerve receives nearly
the whole of the fibers of the motor root of the nerve, and supplies the muscles
of mastication and the skin and mucous membrane of the cheek. Its branches are
the masseteric, deep temporal, buccinator, and external pterygoid.
The Masseteric Nerve (n. massetericus) passes lateralward, above the
Pterygoideus externus, in front of the temporomandibular articulation, and
behind the tendon of the Temporalis; it crosses the mandibular notch with the
masseteric artery, to the deep surface of the Masseter, in which it ramifies nearly
as far as its anterior border. It gives a filament to the temporomandibular joint.
The Deep Temporal Nerves (nn. temporales profundi) are two in
number, anterior and posterior. They pass above the upper border of the
Pterygoideus externus and enter the deep surface of the Temporalis. The
posterior branch, of small size, is placed at the back of the temporal fossa, and
sometimes arises in common with the masseteric nerve. The anterior branch is
frequently given off from the buccinator nerve, and then turns upward over the
upper head of the Pterygoideus externus. Frequently a third or intermediate
branch is present.
The Buccinator Nerve (n. buccinatorus; long buccal nerve) passes
forward between the two heads of the Pterygoideus externus, and downward
beneath or through the lower part of the Temporalis; it emerges from under the
anterior border of the Masseter, ramifies on the surface of the Buccinator, and
unites with the buccal branches of the facial nerve. It supplies a branch to the
Pterygoideus externus during its passage through that muscle, and may give off
the anterior deep temporal nerve. The buccinator nerve supplies the skin over
the Buccinator, and the mucous membrane lining its inner surface.
External Pterygoid Nerve (n. pterygoideus externus).—The nerve to the
Pterygoideus externus frequently arises in conjunction with the buccinator
253
nerve, but it may be given off separately from the anterior division of the
mandibular nerve. It enters the deep surface of the muscle.
The posterior and larger division of the mandibular nerve is for the most
part sensory, but receives a few filaments from the motor root. It divides into
auriculotemporal, lingual, and inferior alveolar nerves.
The Auriculotemporal Nerve (n. auriculotemporalis) generally arises by
two roots, between which the middle meningeal artery ascends. It runs backward
beneath the Pterygoideus externus to the medial side of the neck of the
mandible. It then turns upward with the superficial temporal artery, between the
auricula and condyle of the mandible, under cover of the parotid gland; escaping
from beneath the gland, it ascends over the zygomatic arch, and divides into
superficial temporal branches.
The branches of communication of the auriculotemporal nerve are with
the facial nerve and with the otic ganglion. The branches to the facial, usually
two in number, pass forward from behind the neck of the mandible and join the
facial nerve at the posterior border of the Masseter. The filaments to the otic
ganglion are derived from the roots of the auriculotemporal nerve close to their
origin.
Its branches of distribution are:
Anterior auricular.
Articular.
Branches to the external acoustic meatus.
Parotid.
Superficial temporal.
The anterior auricular branches (nn. auriculares anteriores) are usually
two in number; they supply the front of the upper part of the auricula, being
distributed principally to the skin covering the front of the helix and tragus.
The branches to the external acoustic meatus (n. meatus auditorii externi),
two in number, enter the meatus between its bony and cartilaginous portions and
supply the skin lining it; the upper one sends a filament to the tympanic
membrane.
The articular branches consist of one or two twigs which enter the
posterior part of the temporomandibular joint.
The parotid branches (rami parotidei) supply the parotid gland.
The superficial temporal branches (rami temporales superficiales)
accompany the superficial temporal artery to the vertex of the skull; they supply
the skin of the temporal region and communicate with the facial and
zygomaticotemporal nerves.
The Lingual Nerve (n. lingualis) supplies the mucous membrane of the
anterior two-thirds of the tongue. It lies at first beneath the Pterygoideus
externus, medial to and in front of the inferior alveolar nerve, and is
occasionally joined to this nerve by a branch which may cross the internal
maxillary artery. The chorda tympani also joins it at an acute angle in this
situation. The nerve then passes between the Pterygoideus internus and the
254
ramus of the mandible, and crosses obliquely to the side of the tongue over the
Constrictor pharyngis superior and Styloglossus, and then between the
Hyoglossus and deep part of the submaxillary gland; it finally runs across the
duct of the submaxillary gland, and along the tongue to its tip, lying
immediately beneath the mucous membrane.
Its branches of communication are with the facial (through the chorda
tympani), the inferior alveolar and hypoglossal nerves, and the submaxillary
ganglion. The branches to the submaxillary ganglion are two or three in number;
those connected with the hypoglossal nerve form a plexus at the anterior margin
of the Hyoglossus.
Its branches of distribution supply the sublingual gland, the mucous
membrane of the mouth, the gums, and the mucous membrane of the anterior
two-thirds of the tongue; the terminal filaments communicate, at the tip of the
tongue, with the hypoglossal nerve.
The Inferior Alveolar Nerve (n. alveolaris inferior; inferior dental nerve)
is the largest branch of the mandibular nerve. It descends with the inferior
alveolar artery, at first beneath the Pterygoideus externus, and then between the
sphenomandibular ligament and the ramus of the mandible to the mandibular
foramen. It then passes forward in the mandibular canal, beneath the teeth, as far
as the mental foramen, where it divides into two terminal branches, incisive and
mental.
The branches of the inferior alveolar nerve are the mylohyoid, dental,
incisive, and mental.
The mylohyoid nerve (n. mylohyoideus) is derived from the inferior
alveolar just before it enters the mandibular foramen. It descends in a groove on
the deep surface of the ramus of the mandible, and reaching the under surface of
the Mylohyoideus supplies this muscle and the anterior belly of the Digastricus.
The dental branches supply the molar and premolar teeth. They
correspond in number to the roots of those teeth; each nerve entering the orifice
at the point of the root, and supplying the pulp of the tooth; above the alveolar
nerve they form an inferior dental plexus.
The incisive branch is continued onward within the bone, and supplies the
canine and incisor teeth.
The mental nerve (n. mentalis) emerges at the mental foramen, and
divides beneath the Triangularis muscle into three branches; one descends to the
skin of the chin, and two ascend to the skin and mucous membrane of the lower
lip; these branches communicate freely with the facial nerve.
Two small ganglia, the otic and the submaxillary, are connected with the
mandibular nerve.
Otic Ganglion (ganglion oticum) The otic ganglion is a small,
ovalshaped, flattened ganglion of a reddish-gray color, situated immediately
below the foramen ovale; it lies on the medial surface of the mandibular nerve,
and surrounds the origin of the nerve to the Pterygoideus internus. It is in
relation, laterally, with the trunk of the mandibular nerve at the point where the
motor and sensory roots join; medially, with the cartilaginous part of the
255
auditory tube, and the origin of the Tensor veli palatini; posteriorly, with the
middle meningeal artery.
Branches of Communication.—It is connected by two or three short
filaments with the nerve to the Pterygoideus internus, from which it may obtain
a motor, and possibly a sensory root. It communicates with the glossopharyngeal
and facial nerves, through the lesser superficial petrosal nerve continued from
the tympanic plexus, and through this nerve it probably receives a root from the
glossopharyngeal and a motor root from the facial; its sympathetic root consists
of a filament from the plexus surrounding the middle meningeal artery. The
fibers from the glossopharyngeal which pass to the otic ganglion in the small
superficial petrosal are supposed to be sympathetic efferent (preganglionic)
fibers from the dorsal nucleus or inferior salivatory nucleus of the medulla.
Fibers (postganglionic) from the otic ganglion with which these form synapses
are supposed to pass with the auriculotemporal nerve to the parotid gland. A
slender filament (sphenoidal) ascends from it to the nerve of the Pterygoid canal,
and a small branch connects it with the chorda tympani.
Its branches of distribution are: a filament to the Tensor tympani, and one
to the Tensor veli palatini. The former passes backward, lateral to the auditory
tube; the latter arises from the ganglion, near the origin of the nerve to the
Pterygoideus internus, and is directed forward. The fibers of these nerves are,
however, mainly derived from the nerve to the Pterygoideus internus.
Submandibular
Ganglion
(ganglion
submandibularis).
The
submandibular ganglion is of small size and is fusiform in shape. It is situated
above the deep portion of the submandibular gland, on the hyoglossus, near the
posterior border of the Mylohyoideus, and is connected by filaments with the
lower border of the lingual nerve. It is suspended from the lingual nerve by two
filaments which join the anterior and posterior parts of the ganglion. Through
the posterior of these it receives a branch from the chorda tympani nerve which
runs in the sheath of the lingual; these are sympathetic efferent (preganglionic)
fibers from the facial nucleus or the superior salivatory nucleus of the medulla
oblongata that terminate in the submandibular ganglion. The postganglionic
fibers pass to the submandibular gland, it communicates with the sympathetic by
filaments from the sympathetic plexus around the external maxillary artery.
Its branches of distribution are five or six in number; they arise from the
lower part of the ganglion, and supply the mucous membrane of the mouth and
the duct of the submandibular gland, some being lost in the submandibular
gland. The branch of communication from the lingual to the forepart of the
ganglion is by some regarded as a branch of distribution, through which
filaments pass from the ganglion to the lingual nerve, and by it are conveyed to
the sublingual gland and the tongue.
Trigeminal Nerve Reflexes.—Pains referred to various branches of the
trigeminal nerve are of very frequent occurrence, and should always lead to a
careful examination in order to discover a local cause. As a general rule the
diffusion of pain over the various branches of the nerve is at first confined to
one only of the main divisions, and the search for the causative lesion should
256
always commence with a thorough examination of all those parts which are
supplied by that division; although in severe cases pain may radiate over the
branches of the other main divisions. The commonest example of this condition
is the neuralgia which is so often associated with dental caries—here, although
the tooth itself may not appear to be painful, the most distressing referred pains
may be experienced, and these are at once relieved by treatment directed to the
affected tooth.
Many other examples of trigeminal reflexes could be quoted, but it will be
sufficient to mention the more common ones. Dealing with the ophthalmic
nerve, severe supraorbital pain is commonly associated with acute glaucoma or
with disease of the frontal or ethmoidal air cells. Malignant growths or
empyema of the maxillary antrum, or unhealthy conditions about the inferior
conchж or the septum of the nose, are often found giving rise to “second
division” neuralgia, and should be always looked for in the absence of dental
disease in the maxilla.
It is on the mandibular nerve, however, that some of the most striking
reflexes are seen. It is quite common to meet with patients who complain of pain
in the ear, in whom there is no sign of aural disease, and the cause is usually to
be found in a carious tooth in the mandible. Moreover, with an ulcer or cancer of
the tongue, often the first pain to be experienced is one which radiates to the ear
and temporal fossa, over the distribution of the auriculotemporal nerve.
Trigeminal Neuralgia
The clinical description of severe facial pain, which is now known as
trigeminal neuralgia (TN), can be traced back more than 300 years. Aretaeus of
Cappadocia, known for one of the earliest descriptions of migraine, is credited
with the first indication of TN. He described a headache in which "spasms and
distortions of the countenance took place." John Fothergill was the first to give a
full and accurate description of TN in a paper titled "On a Painful Affliction of
the Face," which he presented to the medical society of London in 1773.
Nicholaus Andre coined the term tic douloureux in 1756.
Idiopathic TN is the most common type of facial pain neuralgia. The pain
typically occurs in the distribution of one of the branches of the trigeminal
nerve, usually on one side. Rarely, it can affect both sides, although
simultaneous bilateral trigeminal neuralgia is uncommon. It involves both the
mandibular and maxillary divisions of the trigeminal nerve in 35% of affected
patients. Isolated involvement of the ophthalmic division is much less common
(2.8% of TN cases).
TN reportedly is one of the most excruciating pain syndromes. It has been
known to drive patients with TN to the brink of suicide. The name tic
douloureux was first used to describe TN and remains synonymous with the
classic form of TN. The tic refers mainly to the visible effects of the brief and
paroxysmal pain that, in classic TN, lasts only a few seconds. The pain is so
severe that it often causes the patient to wince or make an aversive head
movement, as if trying to escape the pain, thus producing an obvious movement,
or tic.
257
Problem: A lack of clear definitions for facial pain has hampered the
understanding of trigeminal neuralgia. The condition has no clear natural
history, and no long-term follow-up study of the progression of the disorder has
ever been published. In an attempt to rationalize the language of facial pain,
recently, a new classification scheme that divides facial pain into several distinct
categories was introduced (Burchiel, 2003):
Trigeminal neuralgia type 1 (TN1): This is the classic form of trigeminal
neuralgia in which episodic lancinating pain predominates.
Trigeminal neuralgia type 2 (TN2): This is the atypical form of trigeminal
neuralgia in which more constant pains (aching, throbbing, burning)
predominate.
Trigeminal neuropathic pain (TNP): This is pain that results from
incidental or accidental injury to the trigeminal nerve or the brain pathways of
the trigeminal system.
T
rigeminal deafferentation pain (TDP): This is pain that results from
intentional injury to the system in an attempt to treat trigeminal neuralgia.
Numbness of the face is a constant part of this syndrome, which has also been
referred to as anesthesia dolorosa or one of its variants.
Symptomatic trigeminal neuralgia (STN): This is trigeminal neuralgia
associated with multiple sclerosis (MS).
Postherpetic neuralgia (PHN): This is chronic facial pain that results from
an outbreak of herpes zoster (shingles), usually in the ophthalmic division (V1)
of the trigeminal nerve on the face and usually in elderly patients.
Geniculate neuralgia (GeN): This is typified by episodic lancinating pain
felt deep in the ear. .
Etiology: The etiology of most cases of TN is chronic vascular
compression and injury to the trigeminal nerve at its entrance into the brainstem
(pons). In one study, 64% of the compressing vessels were identified as an
artery, most commonly the superior cerebellar (81%). Venous compression was
identified in 36% of cases (Anderson, 2006).
Pathophysiology: Vascular compression of the trigeminal nerve appears
to cause demyelination and remyelination of the nerve with persisting
abnormalities of myelination (dysmyelination).
The most common theoretical explanation for TN proposes that high-frequency ectopic impulses are either generated from or augmented by areas of dysmyelination (Burchiel, 1980). These abnormal discharges may ignite a chain reaction of neuronal depolarization in the trigeminal ganglion (Devor, 2002). The
subsequent cascade of neuronal activity is progated centrally into the trige-minal
nucleus and is then perceived by the patient as an overwhelming burst of pain.
Clinical: TN presents with multiple episodes of severe and spontaneous
pain that usually lasts seconds to minutes. The pain is often described as
shooting, lancinating, shocklike, or stabbing. The episodes frequently are
triggered by painless sensory stimulation to perioral trigger zones, eg, a patch of
facial skin, mucosa, or teeth innervated by the ipsilateral trigeminal nerve.
Triggers include touch, certain head movements, talking, chewing, swallowing,
258
shaving, brushing teeth, or even a cold draft. The most commonly affected
dermatomal zones are innervated by the second and third branches of the
trigeminal nerve.
The episodes may be repetitive, recurring, and remitting randomly. Painfree intervals, which might last for years early in the course of TN, typically
grow shorter as the disease progresses. During episodes of pain, some patients
have difficulty talking, eating, and maintaining facial hygiene out of fear of
triggering the pain.
Lab Studies:
The diagnosis of facial pain is almost entirely based on the patient's
history. In most cases of facial pain, no specific laboratory tests are needed. A
blood count and liver function tests are required if therapy with carbamazepine
is contemplated. Oxycarbazine can cause hyponatremia, so the serum sodium
should be tested after institution of therapy.
Although rarely indicated, appropriate blood work for rheumatic diseases,
such as scleroderma (trigeminal neuropathy is reported in up to 5% of patients
with this collagen vascular disease) and systemic lupus erythematosus, should
be undertaken in patients with atypical features of facial pain and a systemic
presentation of collagen vascular disease. Appropriate blood work includes a
sedimentation rate, antinuclear antibody titer, double-stranded DNA, anti-Sm
antibody, lupus erythematosus cell preparation, and complete blood count to
look for hematological abnormalities (eg, hemolytic anemia, leukopenia,
thrombocytopenia). Particularly in the case of scleroderma, creatinine kinase
and aldolase levels may be elevated with muscle involvement. Antibody titers to
SCL-86 and SCL-70 may also be present.
When surgical procedures are contemplated, appropriate and routine
preoperative laboratory tests are in order.
Imaging Studies:
In cases of typical (TN1) and atypical TN (TN2), a brain MRI with
contrast is required. An MRI is sensitive for the exclusion of intracranial lesions
that can rarely cause trigeminal neuralgia.
Obtain contrast-enhanced brain MRIs prior to surgery to evaluate for
vascular malformations or other lesions. Devote particular attention to the
posterior fossa. High-resolution imaging of the nerve at the brainstem entry zone
may reveal vascular compression of the nerve (Anderson,
Medical therapy or treatment:
The most effective medication for the treatment of trigeminal neuralgia
(TN) is carbamazepine. It acts by inhibiting the neuronal sodium channel activity, thereby reducing the excitability of neurons. The effective dose ranges
from 600-1200 mg/d,with serum concentrations between 40-100 mcg/mL.However, many adverse CNS effects (eg, vertigo, sedation, ataxia, diplopia) are associated with carbamazepine, which may make it difficult to use in elderly patients. The dose may be tapered once pain is controlled, since remission may
occur.
259
Obtain a blood count during the first few weeks of therapy and yearly
thereafter. Agranulocytosis and aplastic anemia are extremely rare adverse
effects, but suppression of the WBC count in the range of 200-3000 103/ L is
not uncommon. This mild suppression of the WBC count does not warrant
discontinuation of carbamazepine therapy. Hepatic function should also be
monitored. Up to 70% of patients receive complete or acceptable partial relief, at
least temporarily.
Oxycarbazine is a newer agent that may have fewer side effects, but it can
cause hyponatremia, which should be monitored with serial serum sodium
measurements in the first few weeks of therapy.
Gabapentin, lamotrigine topiramate, and several other newer
anticonvulsants are being used to treat trigeminal neuralgia. Further outcome
studies on their use in the treatment of trigeminal neuralgia are neede
Surgical therapy: In some studies, more than 50% of patients with TN
eventually had some kind of surgical procedure. Experience would indicate that
medical management eventually fails in most patients with TN, and those
patients undergo surgery.
Microvascular decompression (MVD) is the classic and most effective
surgical procedure. It involves a posterior fossa craniotomy and dissection of
vascular elements that compress the trigeminal nerve in the subarachnoid space.
Teflon felt is used to pad the nerve away from the offending artery or vein.
The effectiveness of MVD is based on the hypothesis that compression
from vessels in the vicinity of the trigeminal nerve leads to abnormal nerve
activity.
Complications
Morbidity associated with trigeminal nerve decompression stems from
hemorrhage, infection, and possible damage to the brainstem around the area of
decompression.
In centers where MVD is frequently performed, complications include
facial dysesthesia (0.3%), facial numbness (0.15%), cerebellar injuries and
hearing loss (<1%), and CSF leakage (<2%).
With thermocoagulation, dysesthesia can occur in 5-25% of patients,
although this complication is uncommon when the degree of facial numbness is
controlled. Corneal numbness can occur in up to 15% of patients, and masseter
weakness can occur in about 4%. Many of these complications are reversible
over time
Causes and symptoms
The presumed cause of TN is a blood vessel pressing on the trigeminal
nerve as it exits the brainstem. This compression causes the wearing away of
the protective coating around the nerve (the myelin sheath). TN may be part of
the normal aging process—as blood vessels lengthen they can come to rest and
pulsate against a nerve. TN symptoms can also occur in people with multiple
sclerosis, a disease caused by the deterioration of myelin throughout the body, or
may be caused by damage to the myelin sheath by compression from a tumor.
260
This deterioration causes the nerve to send abnormal signals to the brain. In
some cases the cause is unknown.
A number of theories have been advanced to explain trigeminal
neuralgia, but none explains all the features of the disorder. The trigeminal nerve
is made up of a set of branches radiating from a bulblike ganglion (nerve center)
just above the joint of the jaw. These branches divide and subdivide to innervate
the jaw, nose, cheek, eye, and forehead. Sensation is conveyed from the surfaces
of these parts to the upper spinal cord and then to the brain; motor commands
are conveyed along parallel fibers from the brain to the muscles of the jaw. The
sensory fibers of the trigeminal nerve are specialized for the conveyance of
cutaneous (skin) sensation, including pain.
In trigeminal neuralgia, the pain-conducting fibers of the trigeminal nerve
are somehow stimulated, perhaps self-stimulated, to send a flood of impulses to
the brain. Many physicians assume that compression of the trigeminal nerve
near the spinal cord by an enlarged loop of the carotid artery or a nearby vein
triggers this flood of impulses. Compression is thought to cause trigeminal
neuralgia when it occurs at the root entry zone, a. 19–.39 in (0.5–1.0 cm) length
of nerve where the type of myelination changes over from peripheral to central.
Pressure on this area may cause demyelination, which in turn may cause
abnormal, spontaneous electrical impulses (pain).
Compression is apparently the cause in some cases of trigeminal neuralgia,
but not in others. Other theories focus on complex feedback mechanisms
involving the subnucleus caudalis in the brain. Multiple sclerosis, which
demyelinates nerve fibers, is associated with a higher rate of trigeminal
neuralgia. Brain tumors can also be correlated with the occurrence of trigeminal
neuralgia. Ultimately, however, the exact mechanisms of trigeminal neuralgia
remain a mystery.
Trigeminal neuralgia was first described by the Arab physician Jurjani in
the eleventh century. Jurjani was also the first physician to advance the vascular
compression theory of trigeminal neuralgia. French physician Nicolaus Andrй
gave a thorough description of trigeminal neuralgia in 1756 and coined the term
tic douloureux. English physician John Fothergill also described the syndrome
in the middle 1700s, and the disorder has sometimes been called after him.
Knowledge of trigeminal neuralgia slowly grew during the twentieth century. In
the 1960s, effective treatment with drugs and surgery began to be available.
The pains of trigeminal neuralgia have several distinct characteristics,
including:
They are paroxysmal, pains that start and end suddenly, with painless
intervals between.
They are usually extremely intense.
They are restricted to areas innervated by the trigeminal nerve.
As seen on autopsy, nothing is visibly wrong with the trigeminal nerve.
261
About 50% of patients have trigger zones, areas where slight stimulation
or irritation can bring on an episode of pain. Painful stimulation of the trigger
zones is actually less effective than light stimulation in triggering an attack.
The disorder comes and goes in an unpredictable way; some patients show
a correlation of attack frequency or severity with stress or menstrual cycle.
Stimulation of the face, lips, or gums, such as talking, eating, shaving,
tooth-brushing, touch, or even a current of air, may trigger the severe knifelike
or shocklike pain of trigeminal neuralgia, often described as excruciating.
Trigger zones may be a few square millimeters in size, or large and diffuse. The
pain usually starts in the trigger zone, but may start elsewhere. Approximately
17% of patients experience dull, aching pain for days to years before the onset
of paroxysmal pain; this has been termed pretrigeminal neuralgia.
The pain of trigeminal neuralgia is severe enough that patients often
modify their behaviors to avoid it. They may suffer severe weight loss from
inability to eat, become unwilling to talk or smile, and cease to practice oral
hygiene. Trigeminal neuralgia tends to worsen with time, so that a patient whose
pain is initially well-controlled with medication may eventually require surgery.
Who is affected? TN occurs most often in people over age 50, but it can
occur at any age. The disorder is more common in women than in men. There
is some evidence that the disorder runs in families, perhaps because of an
inherited pattern of blood vessel formation.
Diagnosis
Trigeminal neuralgia is a possible diagnosis for any patient presenting
with severe, stabbing, paroxysmal pain in the jaw or face. However, the most
common causes of facial pain are dental problems and diseases of the mouth.
Trigeminal neuralgia must also be differentiated from migraine headaches and
from other cranial neuralgias (i.e., neuralgias affecting cranial nerves other than
the trigeminal). Many persons with trigeminal neuralgia see multiple physicians
before getting a correct diagnosis, and may have multiple dental procedures
performed in an effort to relieve the pain.
There is no definitive, single test for trigeminal neuralgia. Imaging studies
such as computed tomography (CT) scans or magnetic resonance imaging
(MRI) may help to rule out other possible causes of pain and to indicate
trigeminal neuralgia. High-definition MRI angiography of the trigeminal nerve
and brain stem is often able to spot compression of the trigeminal nerve by an
artery or vein. Trial and error also has its place in the diagnostic process; the
physician may initially give the patient carbamazepine (an anticonvulsant) to
see if this diminishes the pain. If so, this is positive evidence for the diagnosis of
trigeminal neuralgia.
Treatment team
Many different sorts of health care professionals may be consulted by
patients with trigeminal neuralgia, including dentists, neurologists,
neurosurgeons, oral surgeons, and ear, nose, and throat surgeons. A referral to a
neurologist should always be sought, as trigeminal neuralgia is essentially a
neurological problem.
262
Treatment
Treatment is primarily with drugs or surgery. Drugs are often preferred
because of their lower risk, but may have intolerable side effects such as nausea
or ataxia (loss of muscle coordination). The two most effective drugs are
carbamazepine (an anticonvulsant often used in treating epilepsy), used for
trigeminal neuralgia since 1962, and gabapentin. Drugs are prescribed initially
in low doses and increased until an effective level is found. Other drugs in use
for trigeminal neuralgia are phenytoin, baclofen, clonazepam, lamotrigine
topiramate, and trileptal.
Carbamazepine, which inhibits the activity of sodium channels in the cell
membranes of neurons (thereby reducing their excitability), is deemed the most
effective medication for trigeminal neuralgia. Unfortunately, it has many side
effects, including vertigo (dizziness), ataxia, and sedation (mental dullness).
This may make it harder to treat elderly patients, who are more likely to have
trigeminal neuralgia. Carbamazepine provides complete or partial relief for as
many as 70% of patients. Phenytoin is also a sodium channel blocker, and also
has adverse side effects, including hirsutism (increased facial hair), coarsening
of facial features, and ataxia.
For patients whose pain does not respond adequately to medication, or
who cannot tolerate the medication itself due to side effects, surgery is
considered. Approximately 50% of trigeminal neuralgia patients eventually
undergo surgery of some kind for their condition. The most common procedure
is microvascular decompression, also known as the Jannetta procedure after its
inventor. This involves surgery to separate the vein or artery compressing the
trigeminal nerve. Teflon or polivinyl alcohol foam is inserted to cushion the
trigeminal nerve against the vein or artery. This procedure is often effective, but
some physicians argue that since other procedures that disturb or injure the
trigeminal nerve are also effective, the benefit of microvascular decompression
surgery is not relief of compression but disturbance of the trigeminal nerve,
causing nonspecific nerve injury that leads to a change in neural activity.
Other surgical procedures are performed, some of which focus on
destroying the pain-carrying fibers of the trigeminal nerve. The most high-tech
and least invasive procedure is gamma-ray knife surgery, which uses
approximately 200 convergent beams of gamma rays to deliver a high (and
highly localized) radiation dose to the trigeminal nerve root. Almost 80% of
patients undergoing this procedure experience significant relief with this
procedure, although about 10% develop facial paresthesias (odd, non-painful
sensations not triggered by any external stimulus).
Clinical trials
As of mid-2004, one clinical trial related to trigeminal neuralgia was
recruiting patients. This study, titled "Randomized Study of L-Baclofen in
Patients with Refractory Trigeminal Neuralgia," was being carried out at the
University of Pennsylvania, Pittsburgh, and was sponsored by the FDA Office
of Orphan Products Development (dedicated to promoting the development of
treatments for diseases too rare to be considered profitable by pharmaceutical
263
companies). Its goal is to test the effectiveness and safety of the drug L-baclofen
in patients with refractory (treatment-resistant) trigeminal neuralgia. The contact
is Michael J. Soso at the University of Pittsburgh School of Medicine,
Pittsburgh, Pennsylvania, 15261, telephone (412) 648-1239. Forms of baclofen
have been used for the treatment of trigeminal neuralgia since 1980.
Introduction
Imagine having a jab of lightning-like pain shoot through your face when
you brush your teeth or put on makeup. Sound excruciating? If you have
trigeminal neuralgia, attacks of such pain are frequent and can often seem
unbearable.
You may initially experience short, mild attacks, but trigeminal neuralgia
can progress, causing longer, more frequent bouts of searing pain. These painful
attacks can be spontaneous, but they may also be provoked by even mild
stimulation of your face, including brushing your teeth, shaving or putting on
makeup. The pain of trigeminal neuralgia may occur in a fairly small area of
your face, or it may spread rapidly over a wider area.
Because of the variety of treatment options available, having trigeminal
neuralgia doesn't necessarily mean you're doomed to a life of pain. Doctors
usually can effectively manage trigeminal neuralgia, either with medications or
surgery.
Signs and symptoms
An attack of trigeminal neuralgia can last from a few seconds to about a
minute. Some people have mild, occasional twinges of pain, while other people
have frequent, severe, electric-shock-like pain. The condition tends to come and
go. You may experience attacks of pain off and on all day, or even for days or
weeks at a time. Then, you may experience no pain for a prolonged period of
time. Remission is less common the longer you have trigeminal neuralgia.
People who have experienced severe trigeminal neuralgia have described the
pain as:
Lightning-like or electric-shock-like
Shooting
Jabbing
Like having live wires in your face
Trigeminal neuralgia usually affects just one side of your face. The pain
may affect just a portion of one side of your face or spread in a wider pattern.
Rarely, trigeminal neuralgia can affect both sides of your face, but not at the
same time.
Causes
The condition is called trigeminal neuralgia because the painful facial
areas are those served by one or more of the three branches of your trigeminal
nerve. This large nerve originates deep inside your brain and carries sensation
from your face to your brain. The pain of trigeminal neuralgia is due to a
disturbance in the function of the trigeminal nerve. Trigeminal neuralgia is also
known as tic douloureux.
264
The cause of the pain usually is due to contact between a normal artery or
vein and the trigeminal nerve at the base of your brain. This places pressure on
the nerve as it enters your brain and causes the nerve to misfire. Physical nerve
damage or stress may be the initial trigger for trigeminal neuralgia.
After the trigeminal nerve leaves your brain and travels through your
skull, it divides into three smaller branches, controlling sensation throughout
your face:
The first branch controls sensation in your eye, upper eyelid and forehead.
The second branch controls sensation in your lower eyelid, cheek, nostril,
upper lip and upper gum.
The third branch controls sensations in your jaw, lower lip, lower gum
and some of the muscles you use for chewing.
You may feel pain in the area served by just one branch of the trigeminal
nerve, or the pain may affect all branches on one side of your face.
Besides compression from blood vessel contact, other less frequent
sources of pain to the trigeminal nerve may include:
Compression by a tumor
Multiple sclerosis
A stroke affecting the lower part of your brain, where the trigeminal nerve
enters your central nervous system
A variety of triggers, many subtle, may set off the pain. These triggers
may include:
Shaving
Stroking your face
Eating
Drinking
Brushing your teeth
Talking
Putting on makeup
Encountering a breeze
Smiling
Trigeminal neuralgia affects women more often than men. The disorder is
more likely to occur in people who are older than 50. About 5 percent of people
with trigeminal neuralgia have other family members with the disorder, which
suggests a possible genetic cause in some cases.
When to seek medical advice
Some people mistake the pain of trigeminal neuralgia for a toothache or a
headache. It's not uncommon for people to believe that their facial pain is dentalrelated, particularly when the pain seems to stem from the gumline or is located
near a tooth.
If you experience facial pain, particularly prolonged pain or pain that
hasn't gone away with use of over-the-counter pain relievers, see your dentist or
doctor.
Screening and diagnosis
265
If you go to your dentist, an examination of your mouth can reveal
whether a problem with your teeth or gums is causing your pain.
If you go to your doctor, he or she will want to ask about your medical
history and have you describe your pain — how severe it is, what part of your
face it affects, how long pain lasts and what seems to trigger episodes of pain.
You'll also undergo a neurologic examination. During this examination, your
doctor examines and touches parts of your face to try to determine exactly where
the pain is occurring and — if it appears that you have trigeminal neuralgia —
which branches of the trigeminal nerve may be affected.
Your doctor may exclude other possible conditions based on your medical
history, the examination, and a magnetic resonance imaging (MRI) scan of your
head.
Treatment
Medications are the usual initial treatment for trigeminal neuralgia.
Medications are often effective in lessening or blocking the pain signals sent to
your brain. A number of drugs are available. If you stop responding to a
particular medication or experience too many side effects, switching to another
medication may work for you.
Medications
Carbamazepine (Tegretol, Carbatrol). Carbamazepine, an anticonvulsant drug, is
the most common medication that doctors use to treat trigeminal neuralgia. In
the early stages of the disease, carbamazepine controls pain for most people.
However, the effectiveness of carbamazepine decreases over time. Side effects
include dizziness, confusion, sleepiness and nausea.
Baclofen. Baclofen is a muscle relaxant. Its effectiveness may increase when it's
used in combination with carbamazepine or phenytoin. Side effects include
confusion, nausea and drowsiness.
Phenytoin (Dilantin, Phenytek). Phenytoin, another anticonvulsant medication,
was the first medication used to treat trigeminal neuralgia. Side effects include
gum enlargement, dizziness and drowsiness.
Oxcarbazepine (Trileptal).Oxcarbazepine is another anticonvulsant medication
and is similar to carbamazepine.Side effects include dizziness and double vision.
Doctors may sometimes prescribe other medications, such as lamotrignine
(Lamictal) or gabapentin (Neurontin).
Some people with trigeminal neuralgia eventually stop responding to
medications, or they experience unpleasant side effects. For those people,
surgery, or a combination of surgery and medications, may be an option.
Surgery
The goal of a number of surgical procedures is to either
damage or destroy the part of the trigeminal nerve that's the source of your pain.
Because the success of these procedures depends on damaging the nerve, facial
numbness of varying degree is a common side effect. These procedures involve:
Alcohol injection. Alcohol injections under the skin of your face, where
the branches of the trigeminal nerve leave the bones of your face, may offer
temporary pain relief by numbing the areas for weeks or months. Because the
266
pain relief isn't permanent, you may need repeated injections or a different
procedure.
Glycerol injection. This procedure is called percutaneous glycerol
rhizotomy (PGR). "Percutaneous" means through the skin. Your doctor inserts a
needle through your face and into an opening in the base of your skull. The
needle is guided into the trigeminal cistern, a small sac of spinal fluid that
surrounds the trigeminal nerve ganglion (the area where the trigeminal nerve
divides into three branches) and part of its root. Images are made to confirm that
the needle is in the proper location. After confirming the location, your doctor
injects a small amount of sterile glycerol. After three or four hours, the glycerol
damages the trigeminal nerve and blocks pain signals. Initially, PGR relieves
pain in most people. However, some people have a recurrence of pain, and many
experience facial numbness or tingling.
Balloon compression. In a procedure called percutaneous balloon
compression of the trigeminal nerve (PBCTN), your doctor inserts a hollow
needle through your face and into an opening in the base of your skull. Then, a
thin, flexible tube (catheter) with a balloon on the end is threaded through the
needle. The balloon is inflated with enough pressure to damage the nerve and
block pain signals. PBCTN successfully controls pain in most people, at least for
a while. Most people undergoing PBCTN experience facial numbness of varying
degrees, and more than half experience nerve damage resulting in a temporary
or permanent weakness of the muscles used to chew.
Electric current. A procedure called percutaneous stereotactic
radiofrequency thermal rhizotomy (PSRTR) selectively destroys nerve fibers
associated with pain. Your doctor threads a needle through your face and into an
opening in your skull. Once in place, an electrode is threaded through the needle
until it rests against the nerve root.
An electric current is passed through the tip of the electrode until it's
heated to the desired temperature. The heated tip damages the nerve fibers and
creates an area of injury (lesion). If your pain isn't eliminated, your doctor may
create additional lesions.
PSRTR successfully controls pain in most people. Facial numbness is a
common side effect of this type of treatment. The pain may return after a few
years.
Microvascular decompression (MVD). A procedure called microvascular
decompression (MVD) doesn't damage or destroy part of the trigeminal nerve.
Instead, MVD involves relocating or removing blood vessels that are in contact
with the trigeminal root and separating the nerve root and blood vessels with a
small pad. During MVD, your doctor makes an incision behind one ear. Then,
through a small hole in your skull, part of your brain is lifted to expose the
trigeminal nerve. If your doctor finds an artery in contact with the nerve root, he
or she directs it away from the nerve and places a pad between the nerve and the
artery. Doctors usually remove a vein that is found to be compressing the
trigeminal nerve.
267
MVD can successfully eliminate or reduce pain most of the time, but as
with all other surgical procedures for trigeminal neuralgia, pain can recur in
some people. While MVD has a high success rate, it also carries risks. There are
small chances of decreased hearing, facial weakness, facial numbness, double
vision, and even a stroke or death. The risk of facial numbness is less with MVD
than with procedures that involve damaging the trigeminal nerve.
Severing the nerve. A procedure called partial sensory rhizotomy (PSR)
involves cutting part of the trigeminal nerve at the base of your brain. Through
an incision behind your ear, your doctor makes a quarter-sized hole in your skull
to access the nerve. This procedure usually is helpful, but almost always causes
facial numbness. And it's possible for pain to recur. If your doctor doesn't find
an artery or vein in contact with the trigeminal nerve, he or she won't be able to
perform an MVD, and a PSR may be done instead.
Radiation. Gamma-knife radiosurgery (GKR) involves delivering a
focused, high dose of radiation to the root of the trigeminal nerve. The radiation
damages the trigeminal nerve and reduces or eliminates the pain. Relief isn't
immediate and can take several weeks to begin. GKR is successful in
eliminating pain more than half of the time. Sometimes the pain may recur. The
procedure is painless and typically is done without anesthesia. Because this
procedure is relatively new, the long-term risks of this type of radiation are not
yet known.
TUMORS OF MAXILLOFACIAL REGION
Tumor like formations.
ATEROMA (Epidermoid cyst). Cyst of sebaceous gland, formed as a
result of difficulty of secretion of contents through canal of gland. It is most
often localized on skin of hair-bearing parts of head, less often on the skin of the
face. At the trauma or infection sometimes there is a suppuration of the ateromа.
DERMOID CYST. Arises at embryogenesis disorder and contains
elements ectoderm. It is rounded in shape formation of the various sizes, with
precise boundaries, soft elastic consistences. Contents – secretion of sebaceous
gland with the unpleasant smell, often containing hair. It is localized in various
departments of the face and neck. At location on the neck it grows to greater
sizes. The internal surface of cyst looks like a skin and is lined with stratified
pavement epithelium.
Treatment is surgical.
Retentional CYST. It is found mainly on the mucous membrane of the
oral cavity (lower lip). It arises due to difficulty of drainage of secretion from
glandulo-secretory organs
At the trauma of mucous membrane of lips the secretion of mucus from
small alveolar glands is broken, that leads to formation of retentional cyst. At
occlusion of ducts of sublingual salivary gland the cysts of floor of the mouth of
greater sizes are formed called ranula.
268
Clinical picture. Rounded formation within the capsule, with thin coat,
projecting over the mucous membrane. Color is transparent, sometimes with the
bluish shade, contains slime.
Treatment is surgical shelling out of cyst.
EPULIS. The collective term designating formations with various origin,
localized in the area of gingival papillae and around the neck of tooth. In some
cases it can be true tumours, such as angioma, fibroma, the peripheral form of
osteoblastoclastoma. Often these formations are results of inflammatory
process, consequence of growth of granulating tissue with an outcome of
fibrosis, sometimes with the angiomatosis.
Clinical picture. Formation on the stalk of the dark red color, located in
the area of neck of tooth, with a greater or smaller degree of bleeding. The most
common location is area of canines and premolars of the lower jaw.
Treatment is surgical. Excision of formations with coagulation of deep tissues
after preliminary curettage
At chronic inflammatory process is an elimination of the causal factor. At
the peripheral form of osteoblastoclastoma is an economical resection of an
alveolar process.
The forecast is favorable.
OSTEOBLASTOCLASTOMA (huge cell tumour). Primary osteogenic
tumour, having of huge multinuclear cells of type of osteoclasts. Moreoften the
tumours are found in gnathic bones. There are central OBC, developing in
thickness of a bone, and peripheral OBC or so-called huge cells epulis.
Radiologically there are 3 forms of a tumours: cellular,cystic, and lytic. At
cellular (trabecular) form the basic radiological symptom in a zone of bone is
the center of destruction, resembling of aggregation of various cells
OSTEOCLASTOMA
Multiple radiolucent or mixed radiolucent/radiopaque lesions of the
mandible may present as incidental findings on radiographs or as the chief
complaint of a patient. This article is not intended to be an all-inclusive
discussion of such lesions, but rather it confines itself to an overview of the
major odontogenic cysts and tumors with a brief discussion of other mandibular
lesions that often are given the appellation of cyst but are not true cystic lesions.
Although often similar in radiographic presentation, malignant tumors
(both primary and metastatic), benign salivary tumors, and vascular lesions are
not addressed herein. However, such lesions should be included in the
differential diagnoses of a patient presenting with mandibular radiolucency or
swelling. As a corollary, before the biopsy of any such lesions, the area should
be aspirated to exclude a vascular lesion
Odontogenic cysts are defined as epithelial-lined structures derived from
odontogenic epithelium. Most odontogenic cysts are defined more by their
location than by any histologic characteristics. Accordingly, the surgeon must
provide the pathologist with appropriate history and radiographs when
submitting such specimens for examination.
Periapical cyst
269
A periapical (radicular) cyst is the most common odontogenic cyst. The
usual etiology is a tooth that becomes infected, leading to necrosis of the pulp.
Toxins exit the apex of the tooth, leading to periapical inflammation. This
inflammation stimulates the Malassez epithelial rests, which are found in the
periodontal ligament, resulting in the formation of a periapical granuloma that
may be infected or sterile. Eventually, this epithelium undergoes necrosis caused
by a lack of blood supply, and the granuloma becomes a cyst. The lesions are
not usually clinically detectable when small but most often are discovered as
incidental findings on radiographic survey.
Radiographically, distinguishing between a granuloma and a cyst is
impossible, although some say that if the lesion is quite large it is more likely to
be a cyst. They both present as radiolucent lesions in association with the apex
of a nonvital tooth. Occasionally, these lesions can become quite large because
they grow in response to pressure. However, granulomas and cysts are not
neoplastic.
Microscopically, the epithelium is a nondescript stratified squamous
epithelium without keratin formation. Inflammatory changes may be observed in
the cyst wall, and these changes, in turn, may lead to epithelial changes (eg,
ulceration, atrophy, hyperplasia). In particularly inflamed lesions, cholesterol
slits and/or foamy macrophages may be apparent.
Several treatment options exist for such cysts. Many cysts resolve with
endodontic therapy of the involved tooth. Those lesions should be monitored
radiographically to ensure such resolution. Lesions that fail to resolve with such
therapy should be surgically removed and histopathologically examined.
Although these cysts arise from a mature resting epithelium and thus have a
relatively low growth potential, a squamous cell carcinoma occasionally may
arise de novo in a radicular cyst, thus the recommendation for histopathologic
examination of all tissues removed.
Dentigerous cyst
The second most common odontogenic cyst is the dentigerous cyst, which
develops within the normal dental follicle that surrounds an unerupted tooth.
The dentigerous cyst is not thought to be neoplastic. It most frequently is found
in areas where unerupted teeth are found: mandibular third molars, maxillary
third molars, and maxillary canines, in decreasing order of frequency. These
cysts can grow very large and can move teeth, but, more commonly, they are
relatively small. Most dentigerous cysts are asymptomatic, and their discovery is
usually an incidental finding on radiography.
The usual radiographic appearance is that of a well-demarcated
radiolucent lesion attached at an acute angle to the cervical area of an unerupted
tooth. The border of the lesion may be radiopaque. The radiographic
differentiation between a dentigerous cyst and a normal dental follicle is based
merely on size.
However, histologically, a distinction other than size is found. The dental
follicle normally is lined by the reduced enamel epithelium, while the dentige270
rous cyst is lined with a stratified squamous nonkeratinizing epithelium. Dystrophic calcification and clusters of mucous cells may be found within the cysts.
Dentigerous cysts develop from follicular epithelium, and follicular
epithelium has greater potential for growth, differentiation, and degeneration
than the epithelium from which radicular cysts arise. Occasionally, other more
ominous lesions arise within the walls of the dentigerous cyst, including
mucoepidermoid carcinoma arising from mucous cells within the cyst walls,
ameloblastoma (see Odontogenic tumors; 17% of ameloblastomas arise within a
dentigerous cyst), and squamous cell carcinoma. As previously mentioned,
dentigerous cysts also can become quite large and can place the patient at risk
for pathologic jaw fracture.
These findings comprise most of the medical rationale for removal of
impacted third molars with pericoronal radiolucencies; however, impacted teeth
with small pericoronal radiolucencies (suggesting the presence of normal dental
follicle rather than dentigerous cyst) also may be monitored with serial
radiographic examination. Any increase in the size of the lesion should prompt
removal and histopathologic examination. Any lesion that appears larger than a
normal dental follicle indicates removal and histopathologic examination.
Primordial cyst
By definition, the primordial cyst develops instead of a tooth. Presumably,
the dental follicle forms and subsequently undergoes cystic degeneration
without ever completing odontogenesis. This is the rarest odontogenic cyst, and
lesions designated as primordial cysts may represent residual cysts. The
histology of these lesions is a nondescript stratified squamous epithelium. A
complete dental history is important to establish a diagnosis of primordial
(versus residual) cyst, although such a diagnosis often has little clinical
significance in terms of treatment planning and decision making.
Residual cyst
Residual cyst is a term of convenience because no teeth are left by which
to identify the lesion. Most commonly, these actually are retained periapical
cysts from teeth that have been removed. The histology is a nondescript
stratified squamous epithelium.
Lateral periodontal cyst
The name lateral periodontal cyst is a misnomer. These cysts are not
inflammatory, they do not arise from periodontitis, and they are not a
phenomenon associated with lateral canals within the tooth structure. These
cysts are always well demarcated, relatively small, and radiolucent (sometimes
with a radiopaque roof). They most commonly are associated with the
mandibular premolar area and occasionally are found in the maxillary anterior.
They usually are not clinically apparent but, rather, are detected through
radiographic examination. These cysts have a distinctive histology consisting of
a thick fibrous noninflamed cyst wall, and the lining epithelium is made of thin
cuboidal cells. This lining is incomplete and easily sloughs away with mural
thickenings of clear cells at periodic intervals. These cysts develop from the
271
postfunctional dental lamina, and no good explanation is known for the
localization that is shown.
Gingival cyst of the newborn
Gingival cysts of newborns generally occur in multiples but occasionally
occur as solitary nodules. They are located on the alveolar ridges of newborns or
young infants. These structures originate from remnants of the dental lamina and
are located in the corium below the surface epithelium. Occasionally, they may
become large enough to be clinically noticeable as discrete white swellings on
the ridges. They are generally asymptomatic and do not produce any discomfort
for the infant.
Bohn nodules and Epstein pearls are 2 similar lesions with which gingival
cysts sometimes may be confused; however, the location and etiology of these
lesions are somewhat different. Epstein pearls are cystic keratin-filled nodules
found along the midpalatine raphe and are thought to be derived from entrapped
epithelial remnants along the line of fusion. Bohn nodules are keratin-filled cysts
scattered all over the palate, but they are most apparent at the junction of the
hard and soft palate. These are thought to be derived from palatal salivary gland
structures.
Histologically, the gingival cyst of the newborn is a true cyst with a thin
epithelial lining. The lumen usually is filled with keratin but may contain some
inflammatory cells, dystrophic calcifications, and hyaline bodies, such as those
often found in dentigerous cysts.
No treatment is required for these lesions, which usually disappear either
by opening onto the surface mucosa or through disruption by erupting teeth.
These cysts are most likely what older literature describes as predeciduous
dentition.
Gingival cyst of the adult
Gingival cysts of the adult are found only in soft tissue in the lower
premolar areas. These cysts present as tense, fluctuant, vesicular, or bullous
lesions. Histologically, they look like lateral periodontal cysts, and they
probably represent the same lesion when found in soft tissue.
Odontogenic keratocyst
The odontogenic keratocyst (OKC) is the most important of the
odontogenic cysts. This cyst may have any clinical appearance; it is a great
mimic, and the diagnosis is a histologic one. These lesions are different from
other cysts; they are aggressive and can be difficult to remove. OKCs can grow
quite rapidly, and recurrences are frequent. This is the third most common
odontogenic cyst and belongs in the differential diagnoses of any radiolucency
of the jaws. Although 40% of OKCs appear in a dentigerous relationship, 9% of
dentigerous cysts are OKCs when the histology is examined. These cysts also
are found as part of the basal cell nevus syndrome, also known as Gorlin
syndrome (see Basal cell nevus syndrome).
Histologically, these cysts are formed with a stratified squamous
epithelium that produces orthokeratin (10%), parakeratin (83%), or both types of
keratin (7%). The epithelial lining appears corrugated when viewed under a
272
microscope. A well-polarized hyperchromatic basal layer is observed, and the
cells remain basaloid almost to the surface. No rete ridges are present; therefore,
the epithelium often sloughs from the connective tissue (94% of the time). The
epithelium is thin, and mitotic activity is frequent; therefore, OKCs grow in a
neoplastic fashion and not in response to internal pressure. The lumen frequently
is filled with a foul-smelling cheeselike material that is not pus but rather
collected degenerating keratin.
The lesions grow in a multilocular bosselated fashion with daughter cysts
that extend into the surrounding bone. Because of this relationship, the tendency
for recurrence is high, particularly if the original surgical treatment does not
result in complete removal of the lesion. Enucleations with peripheral ostectomy
and/or cryosurgery are the most common forms of treatment. Long-term
(lifetime) follow-up radiography is imperative. If these lesions are left untreated,
they can become quite large and locally destructive.
The variant of OKC that produces only orthokeratin acts somewhat
differently than other OKCs. These almost always are found in a dentigerous
association, usually around the mandibular third molar, and they are much less
aggressive. They do not have a hyperchromatic basal layer; in fact, the basal
layer is flattened. They are not
Basal cell nevus syndrome
This symptom complex includes hypertelorism, midface hypoplasia,
relative frontal bossing and prognathism, mental retardation, schizophrenia,
multiple basal cell carcinomas, calcification of the falx cerebri, bifid ribs, palmar
pitting (the pits later develop into basal cell carcinoma), and multiple OKCs.
Multiple OKCs are diagnostic for basal cell nevus syndrome until proven
otherwise. This is a hereditary disease with autosomal dominant inheritance and
high penetration. Of patients with OKC, 5% have basal cell nevus syndrome.
Early identification of these patients and their lesions is key to improving longterm survival and quality of life
Traumatic bone cyst
The traumatic bone cyst also is known as solitary bone cyst, hemorrhagic
cyst, extravasation cyst, unicameral bone cyst, simple bone cyst, and idiopathic
bone cavity.
The traumatic bone cyst is a relatively frequent lesion both in the jaws and
elsewhere in the skeleton. The specific etiology of the lesion is unknown,
although several mechanisms have been proposed. The most widely accepted is
that these lesions originate from intramedullary hemorrhage caused by trauma.
In these cases, failure of organization of the blood clot occurs followed by
subsequent degeneration of the clot, eventually leading to an empty bone cavity.
Restricted venous drainage leads to increasing edema, which in turn causes
continued resorption of trabeculae and expansion of the lesion. Expansion of the
lesion tends to stop when cortical bone is reached, thus these lesions are not
characterized by any cortical expansion. Instead, they usually are incidental
findings on radiographs taken for other purposes. However, it is not unusual for
a patient to be unable to recall any trauma to the involved jaw.
273
The lesion most commonly is found in young persons (median age, 18 y);
the male-to-female incidence ratio is 3:2. The lesions occasionally have been
reported in the maxilla but are far more common in the mandible. When the
cavities are opened surgically, they are generally either empty or filled with a
small amount of straw-colored fluid. Shreds of necrotic clot and fragments of
fibrous connective tissue have been reported less commonly. Histologically,
these cysts may have a thin connective tissue membrane lining or no lining at
all. Radiographically, these lesions tend to appear as smoothly outlined
radiolucencies that scallop around the roots of the teeth. They do not displace
teeth or resorb roots, and the lamina dura is left intact. They may range from
very small (<1 cm) to very large (involving most of the mandible). They tend to
occur above the inferior alveolar canal.
These lesions usually are surgically explored to establish a diagnosis,
which is made upon finding an empty cavity. No further treatment is generally
necessary because surgical manipulation causes the cavity to fill with blood.
Soft tissues are closed, and the lesion tends to heal without further intervention.
The extreme rarity of such lesions in older patients suggests that the lesions may
be self-limiting and/or subject to resolution over time
Treatment
The treatment of ameloblastoma is surgical excision with wide free
margins (see “Surgical considerations”). Appropriate reconstruction may be
performed at the same time. All patients with ameloblastoma, regardless of
surgical treatment method or histologic type, must be monitored
radiographically throughout their lifetime. If excision is inadequate, recurrence
is common.
Surgical considerations
The maxillary ameloblastoma is not confined by the strong cortical plate
found in the mandible. In addition, the posterior maxilla lies in close relationship
to many vital structures. These factors make strong arguments for aggressive
and definitive surgical treatment of the maxillary ameloblastoma.
In the mandible, 1-cm clear margins are considered the standard. This
may be accomplished with block or segmental resection, depending on the
relationship of the lesion to the inferior cortical border.
The single exception to this may be the unicystic ameloblastoma. This
variant most commonly appears in late adolescence and, as the name suggests, is
characterized by a unicystic radiolucency that most commonly is found in the
area of the mandibular third molars. Unlike other types of ameloblastomas, it is
believed that this lesion is encapsulated and can be removed with
enucleation/curettage procedures alone. These lesions may recur, and
recurrences may require more aggressive treatment. Most authors believe that if
left untreated, this lesion becomes an ameloblastoma of one of the classic
varieties, leading to the corollary conclusion that these lesions simply represent
an early stage in the development of ameloblastoma.
For peripheral ameloblastoma, a more conservative excision with close
clinical follow-up is the standard of care.
274
Relationship to other lesions
Basal cell carcinoma: Basal cell carcinoma is another infiltrative,
essentially nonmetastasizing adnexal neoplasm. Basal cell carcinomas and
ameloblastomas are slow growing but persistent, and they may cause death via
local extension into vital structures. If one considers that the tooth is an oral
adnexal structure, then it is easy to understand why ameloblastoma may be seen
as an analogue to basal cell carcinoma.
Craniopharyngioma: This pituitary tumor arises from Rathke pouch, part
of the oral stomadeum that histologically appears somewhat like ameloblastoma.
However, it is actually more like the Gorlin cyst.
Peripheral ameloblastoma: This lesion is histologically identical to the
central ameloblastoma, but it does not involve bone and is confined entirely to
the gingiva. It has a lower potential for growth and invasion than the central
ameloblastoma, and, quite possibly, it is responsible for reported cases of basal
cell carcinoma in the gingiva.
Malignant ameloblastoma: Approximately 2% of ameloblastomas
metastasize, usually to the lungs. It is thought that these lesions actually may be
the result of aspiration of material from fungating lesions in the oral cavity and,
therefore, do not represent true metastases.
Ameloblastic carcinoma: These are cytologically malignant lesions with
hyperchromatism, pleomorphism, and high mitotic activity. Real metastases
occur with ameloblastic carcinoma.
Osteoblastoma
Osteoblastoma is a rare primary neoplasm of bone, categorized as a
benign bone tumor. However, an aggressive type of osteoblastoma has been
described that has characteristics similar to osteosarcoma. Osteoblastoma is
closely related to osteoid osteoma. It differs from osteoid osteoma in its ability
to grow larger than 2 cm in diameter.
The clinical course of osteoblastoma often makes it difficult to diagnose.
The tumor may have a slow indolent course or display characteristics that are
confused with malignancy. Other diagnoses that share similar clinical,
radiographic, and histological features with conventional osteoblastoma include
osteoid osteoma, giant cell tumor, and fibrous dysplasia. Osteoblastomas may
also have features that mimic malignant tumors such as osteosarcoma.
Osteoblastoma is about 20 times less common than osteosarcoma.
History of the Procedure: In his original descriptions of osteoblastoma,
Lichtenstein termed the lesion an "osteogenic fibroma of bone." In 1954, Dahlin
and Johnson reported 11 unusual tumors, all of which appeared to originate within bone. They chose to call the tumor a giant osteoid osteoma for its histologic
similarity to osteoid osteoma. The current and accepted name, osteoblastoma,
was obtained from independent publications by Lichtenstein and Jaffe.
Problem: Osteoblastoma is a bone-forming lesion. It may be found within
the cortex, medullary canal, or periosteal tissues. Multicentric foci within a
single bone have also been described. A slight predominance of metaphyseal
275
over diaphyseal lesions has been reported, with very few lesions being described
as epiphyseal in location.
According to the staging system of benign bone tumors (Enneking, 1986),
most osteoblastomas are stage 2 lesions. Stage 2 lesions are characterized by
benign cytologic characteristics, remain intracapsular, and do not metastasize.
Aggressive stage 3 osteoblastomas have been reported; these lesions display
predominantly benign cytologic features and extend extracapsularly
Etiology: The exact etiology of osteoblastoma is unknown.
Pathophysiology: Neoplastic cells grow within the bone and contain
numerous osteoblasts that produce osteoid and woven bone. Cortical expansion
is often present, but the outer rim of tumor is always covered by periosteum and
by a thin rim of reactive bone. The size of osteoblastomas in the largest series
reported ranged from 1-11 cm with a mean of 3.2 cm (Lucas, 1994).
More aggressive osteoblastomas differ from the conventional types in that
they display resorption of all cortex, destruction of bone, and extension into
surrounding soft tissues.
Clinical: Osteoblastoma most commonly occurs during the first 3 decades
of life. Patients' primary symptom is pain, often characterized as a dull and achy.
Unlike osteoid osteoma, the pain of osteoblastoma is more generalized and less
likely to be relieved by salicylates.
Osteoblastoma may affect any bone, but it is most frequently found within
the vertebral column and long tubular bones. Patients who have these tumors in
the spine may initially present with neurologic symptoms resulting from spinal
cord or nerve root compression. In addition, scoliosis or torticollis may be a
presenting sign. In a recent review of osteoblastomas and osteoid osteomas of
the spine, 9 of 13 patients were noted to have neurologic disorders before
treatment, and 8 of 13 had a preoperative deformity, namely, scoliosis,
torticollis, or both (Ozaki, 2002).
INDICATIONS
The presence of osteoblastoma is an indication for treatment, as
osteoblastoma is not self-resolving and ascertaining the diagnosis is often
difficult. In making the diagnosis, it is important to differentiate aggressive
(stage 3) lesions from osteosarcoma. Once the diagnosis has been established,
surgical treatment is indicate Diagnostic Procedures:
Biopsy of the lesion is necessary to provide tissue for histologic diagnosis.
A core-needle biopsy is often necessary to obtain a specimen for diagnosis.
Open biopsy may also be performed. The use of incisional or excisional biopsy
depends on the anatomic location of the lesion, the experience of the person
performing the biopsy, and any associated defects (eg, aneurysmal bone cyst).
Histologic Findings: Osteoblastoma has been characterized as a cellular
osteoblastic tissue with active intercellular production of osteoid material and
primitive woven bone. General histologic findings of osteoblastoma include the
following:
276
Most importantly, immature bony trabeculae lined with osteoblasts: The
trabeculae may have extensive ossification, whereas others may be without
mineralization.
A highly vascularized connective tissue: The stroma consists of widely
dilated capillaries, and areas of large dilated blood sinusoids may be present.
Mitotic activity: Mitotic activity is nil to minimal in 89% of cases in the
largest reported series.
Histologic findings are very important in making the diagnosis of
osteoblastoma versus osteosarcoma, but the differentiation is often difficult.
Histologic hints in separating the two diagnoses include the following:
Osteoblastoma has a very low rate of mitosis and minimal cytologic
atypia.
Osteoblastoma has a tendency for peripheral maturation and does not
permeate the surrounding bone, unlike osteosarcoma.
Osteoblastoma rarely has a cartilaginous matrix that is often present with
osteosarcoma.
Medical therapy: Radiation therapy or chemotherapy is controversial in
the treatment of osteoblastoma. Many authors believe it is doubtful that either
have a definitive effect on the lesion. Cases of postirradiation sarcoma have
been reported after use of these modalities. However, it is possible that the
original histologic diagnosis was incorrect and the initial lesion was an
osteosarcoma, since histologic differentiation of these two entities can be very
difficult.
Surgical therapy: The treatment goal is complete surgical excision of the
lesion. The type of excision depends on the location of the tumor. For stage 1
and 2 lesions, the recommended treatment is extensive intralesional excision,
using a high-speed burr. Extensive intralesional resections ideally consist of
removal of gross and microscopic tumor and a margin of normal tissue. For
stage 3 lesions, wide resection is recommended because of the need to remove
all tumor-bearing tissue. Wide excision is defined here as the excision of tumor
and a circumferential cuff of normal tissue around the entity. This type of
complete excision is usually curative for osteoblastoma.
Preoperative details: The defect created by any surgical excision must be
planned carefully preoperatively. Larger resections may require bone grafting
and internal fixation. Also, embolization may be indicated for a hypervascular
tumor with a secondary aneurysmal bone cyst in order to reduce intraoperative
bleeding and facilitate complete excision.
Intraoperative details: Aggressive lesions need to be removed with wide
resection. If required, internal fixation must be planned for stabilization. For
example, vertebral lesions that have undergone wide resection may require facet
resections and produce an unstable spine, requiring internal fixation to stabilize
the spine
Postoperative details: The surgical specimen margins should be tumor free
in order to ensure complete excision of the tumor, regardless of the method of
resection.
277
Follow-up care: Monitoring for signs and symptoms of infection and
bleeding is important within the postoperative period. It is also important to
evaluate for local recurrence with plain radiographs on a regular basis.
PROGNOSIS
Osteoblastoma has a reported recurrence rate of approximately 10-20%.
Tumor relapse is most likely the result of inadequate resection of the initial
lesion, such as within the spine, where it might not always be possible to remove
the entire lesion. For stage 2 osteoblastomas, the recurrence rate after extensive
intralesional excision approaches 0%. The recurrence rate after marginal
excision in which the reactive zone of bone is not removed is higher. For stage 3
osteoblastomas, the recurrence rate after wide excision is negligible. The
recurrence rate after intralesional excision is higher, as would be predicted
Fibroma
Fibromas (or fibroid tumors or fibroids) are benign tumors that are
composed of fibrous or connective tissue. They can grow in all organs, arising
from mesenchyme tissue. The term " fibroblastic" or "fibromatous" is used to
describe tumors of the fibrous connective tissue. When the term fibroma is used
without modifier, it is usually considered benign, with the term fibrosarcoma
reserved for malignant tumors.The term fibroid can also refer to tumors of
smooth muscle, as in uterine fibroids.
Pathophysiology: Although Helwig originally suggested that fibrous
papule was derived from preexisting or involuting melanocytic nevi,
immunostaining has refuted this and has confirmed a relationship to factor
XIIIa–positive dermal dendrocytes. They are best considered a variant of
solitary angiofibroma.
Hard Fibroma
The hard fibroma (fibroma durum) consists of many fibres and few cells,
e.g. in skin it is called dermatofibroma (fibroma simplex or nodulus cutaneous),
in skin there also might be histiocytomas, which contain more cells. A special
form is the keloid, which derives from hyperplastic growth of scars.
Soft Fibroma
The soft fibroma (fibroma molle) or fibroma with a shaft (acrochordon,
skin tag, fibroma pendulans) consist of many loosely connected cells and less
fibroid tissue. It mostly appears at the neck, armpits or groins.
Other Types of Fibroma
The fibroma cavernosum or angiofibroma, consists of many often dilated
vessels, it is a vasoactive tumor occurring almost exclusively in adolescent
males.
The cystic fibroma (fibroma cysticum) has central softening or dilated
lymphatic vessels. The myxofibroma (fibroma myxomatodes) is produced by
liquefaction of the underlying soft tissue.
The cemento-ossifying fibroma is hard and fibrous, most frequently seen
in the jaw or mouth, sometimes in connection with a fracture or another type
of injury.
278
Other fibromas: chondromyxoid fibroma, desmoplasmic fibroma,
nonossifying fibroma, ossifying fibroma, perifollicular fibroma, pleomorphic
fibroma etc.
CLINICAL
History: Family history is not considered relevant.
Physical: The lesions are clinically indistinct.
Fibrous papule of the face usually occurs as single lesion, but,
occasionally, several lesions may be present.
Most lesions are located on the nose and, less commonly, on the cheeks,
chin, neck, and, rarely, on the lip or forehead.
Fibrous papules are usually dome-shaped lesions with a shiny, skincolored appearance. Occasionally, lesions are sessile, polypoid, or
papillomatous.
Most of the lesions are firm and indurated.
Size usually ranges from 1-5 mm in diameter.
Similar papules may be present on the fingers or oral mucosa, where they
have been described as reactive nodular hyperplasia or giant cell fibroma.
Histologic Findings: Most fibrous papules are characterized by a
proliferation of fibroblasts, a fibrotic stroma, and dilated blood vessels.
Occasionally, a sparse inflammatory cell infiltrate of lymphocytes is present.
Acanthosis and an increased number of large polygonal melanocytes may
be present in the basal layer. Elastic tissue may be markedly diminished or
entirely absent.
Dermal dendritic cells usually stain for factor XIIIa.
Several histological subtypes have been described, which might cause
diagnostic difficulties. Hypercellular fibrous papules are characterized by a
dense infiltrate of round fibroblasts, often with a nevoid appearance (differential
diagnoses include malignant melanoma and atypical fibroxanthoma).
Clear cell fibrous papules show a proliferation of round clear cells, some
with cytoplasm ranging form finely granular to foamy, resembling histiocytes or
clear epithelial cells; differential diagnoses include clear cell neoplasm and
metastasis (Lee, 2005).
Pleomorphic fibrous papules are characterized by bizarre, stellate
fibroblasts (differential diagnosis includes pleomorphic fibroma).
Pigmented fibrous papules demonstrate prominent melanocytic
hyperplasia and dermal macrophages (differential diagnoses include nevus and
melanoma).
Inflammatory fibrous papules have dense, diffuse dermal infiltrates of
predominantly mixed small and large lymphocytes with plasma cells,
histiocytes, and rare eosinophils and neutrophils (differential diagnoses include
lymphoma, lymphocytic infiltrate, and an infectious process).
TREATMENT
Surgical Care: The lesion is benign, but may be removed to confirm the
diagnosis or for cosmetic reasons. Surgical procedures include curettage, shave
excision, or elliptical excision.
279
Prognosis:
Fibrous papule of the face is a benign lesion.
Peripheral Fibroma A fairly-common overgrowth of fibrous c.t. in the
face of injury manifesting as a pale, firm raised gingival lesion covered, often,
with ulcerated mucosa; removal of the irritant and surgical removal of the lesion
will cure peripheral fibromas.
Etiology: Trauma
Location(s): Gingiva
Clinical Features: Raised pale pink lesions covered with intact gingival
stippled mucosa; sometimes the overlying mucosa is ulcerated.
Radiographic Features: None
Microscopic Features: Fibrous c.t.; may be bone or "cementum" formation
Complications: None; may recur if not completely removed or if irritant
isn't eliminated.
Treatment: Surgical excision and removal of irritant
Prognosis: Excellent
Pathogenesis: Proliferation of fibrous c.t. and bone in the face of repeated
injury.
ANGIOMAS
(Hemangioma; Vascular Nevus; Lymphangioma)
Localized vascular lesions of the skin and subcutaneous tissues, rarely of
the CNS, that result from hyperplasia of blood or lymph vessels.
Angiomas, which occur in about 1/3 of newborn infants, are usually
congenital or appear shortly after birth. Most disappear spontaneously
(immature hemangiomas); some persist and create cosmetic problems.
Complications may follow overtreatment, posttraumatic ulceration, or localized
tissue hypertrophy from a persistent angioma of the CNS the face, or an
extremity.
Classification and Treatment of Congenital Hemangiomas
1. Nevus flammeus (portwine stain): A flat, pink, red, or purplish lesion
present at birth. These lesions represent vascular ectasia and are very commonly
present in the nuchal area. Nevus flammeus of the trigeminal area may be a
component of the Sturge-Weber syndrome (leptomeningeal angiomatosis with
intracranial calcification). A nevus flammeus usually will not fade, though
splotchy small red macular lesions in the area above the nose and on the eyelids
may disappear in a few months. For treatment, the pulsated tunable dye lasers
are being used with excellent results in many cases. A nevus flammeus can
usually be hidden with an opaque cosmetic cream prepared by a cosmetician to
match the patient's skin color.
2. Capillary hemangioma (strawberry mark): A raised, bright red lesion
consisting of proliferations of endothelial cells. It develops shortly after birth
and tends to enlarge slowly during the first several months of life. About 75 to
95% usually involute spontaneously within 5 to 7 yr; regression is usually
complete, but at times a brownish pigmentation and scarring or wrinkling of the
skin remains. Since spontaneous regression is the usual course, treatment is not
280
indicated unless a lesion on or near the eye or a body orifice (eg, urethra, anus)
might interfere with function. When treatment is required, oral prednisone 10
mg bid or tid should be given as soon as possible and for at least 2 wk. If
resolution starts, the prednisone should be decreased slowly; if not, it should be
stopped. Unless complications are life-threatening or vital organs compromised,
surgical excision or other destructive procedures should be avoided because
these result in more scarring than when the lesion is allowed to involute
spontaneously.
3. Cavernous hemangioma: A raised red or purplish lesion composed of
large vascular spaces. The blood vessels and frequently the lymphatics are often
mature, in which case the lesion may contain numerous arteriovenous shunts and
vascular malformations. Cavernous hemangiomas rarely involute spontaneously.
Partial involution may follow ulceration, trauma, or hemorrhage. Treatment
must suit the type of lesion. In children, systemic prednisone (as for a capillary
hemangioma) occasionally may induce spontaneous resolution. Surgical
excision may be considered, especially if the lesion causes increased growth of
an extremity. Small surface nodules may be excised individually or destroyed by
electrocoagulation.
4. Spider angioma (vascular spider): A bright red, faintly pulsatile lesion
consisting of a central arteriole with slender projections like spider legs.
Compression of the central vessel temporarily obliterates the lesion. Vascular
spiders are not congenital. One lesion or small numbers that are unrelated to
internal disease may be seen in children or adults. Most patients with hepatic
cirrhosis develop many vascular spiders that may become quite prominent.
Many women develop lesions during pregnancy or while taking oral
contraceptives. As spiders are asymptomatic and usually resolve spontaneously
about 6 to 9 mo postpartum or after discontinuing oral contraceptives, treatment
is not usually required. If resolution is not spontaneous or treatment is required
for cosmetic purposes, the central arteriole can be destroyed with fine needle
electrodesiccation. The superiority of cosmetic results with lasers is not
established.
5. Lymphangiomas: Elevated lesions composed of dilated, cystic
lymphatic vessels; usually yellowish tan but occasionally reddish if small blood
vessels are intermingled. Puncture of the lesion yields a colorless fluid.
Treatment consists of deep excision.
CANCER OF THE ORAL CAVITY
Cancers involve body tissues whose cells are dividing and growing faster
than cells are dying. The result of excessive cell growth is an enlarged mass
called a tumor. Cancers may fail to remain within the boundaries of normal
tissues and therefore spread or invade surrounding healthy tissues.
Unfortunately, cancer of the head and neck is diagnosed relatively frequently.
The most common oral cancers involve the surface tissue (epithelium) of the
mouth and pharynx.
RISKS FACTORS FOR DEVELOPING ORAL CANCER
281
Genetic Factors. Genetic factors involved in the development of cancer
include:
1. Hereditary predisposition. An individual with a hereditary predisposition for
cancer is a person with an increased likelihood of developing cancer due to
inherited genes. These inherited genes make body cells more sensitive to
environmental factors, such as sunlight and tobacco, and, therefore change
normal body cells into cancer cells.
2. Oncogenes. Cancers can be derived from mutations in genes that change cell
growth patterns. These genes are called oncogenes. Such a mutation in an
oncogene can convert ordinary body cells into cancer cells. Cancers caused by
oncogenes are not inherited. To date, scientists have discovered more than 100
oncogenes, and several oncogenes have been associated with cancers of the head
and neck.
3. Tumor suppressor genes. Normal tumor suppressor genes are anti-cancer
genes that slow down or stop growth of normal body cells. Mutations of tumor
suppressor genes can also cause the development of oral and pharyngeal
cancers.
4. Tobacco and Alcohol. Use of tobacco and alcohol are the major risk factors
for developing oral cancer. Tobacco and alcohol contains substances that are
carcinogenic or promote cancer. Cigarettes smoke and substances in smokeless
tobacco have received considerable attention as carcinogens that promote oral
cancer. Studies also indicate that smoking in combination with consumption of
alcohol produces an even greater risk for oral cancer than use of either substance
alone.
5. Radiation. Radiation of high dosage and prolonged duration can produce
cancer. There is no evidence that routine dental X-rays are carcinogenic,
especially with today's high speed, low dosage machines.
6. Traumatic irritation. Prolonged irritation from broken teeth, rough dental
restorations, and ill-fitting dentures are considered a possible causes for oral
cancer.
7. Viruses. Some viruses can cause cancer in human cells. Studies have
indicated a link to oral cancer with infections from herpes simplex type- I virus,
Epstein-Barr virus, and human papillomavirus.
DIAGNOSIS AND EVALUATION. Early diagnosis is the single factor
in successfully combating oral cancer. Therefore, it is crucial that the patient
seeks immediate and proper treatment for the disease. If tissue of the mouth is
suspected to be cancerous, a biopsy is necessary. A biopsy involves surgically
removing diseased tissue for microscopic evaluation and diagnosis by a
pathologist.
PRE-CANCEROUS LESIONS OF THE MOUTH. Pre-cancerous refers
to early changes of normal cells that are changing into cancer cells. Recognition
of pre-cancerous tissue of the mouth is an important role in the diagnosis and
prevention of oral cancer by the dental health professional. Many times, precancerous tissue goes unnoticed by an individual because it is not painful. As a
general rule, pre-cancerous or early cancers do not heal.
282
Leukoplakia. Leukoplakia is a term that describes a 'white patch' on the
surface of oral tissues. Leukoplakias have been associated with pre-cancerous
tissues. In general, leukoplakias are suspected as pre- or early cancer if the white
area cannot be scraped off the surface tissue and cannot be attributed to another
disease.
Erythroplakia. Erythroplakia occurs in the oral cavity as a distinct and
well-defined patch with a bright red and velvety surface. Erythroplakias are
relatively rare lesions of the oral cavity. Erythroplakias are almost always precancerous.
DESCRIPTION OF SELECTED ORAL CANCERS
Cancers
can
involve growth changes to any tissue of the oral cavity. Cancers of the mouth
may be benign or malignant tumors. A benign tumor is a mass limited within a
connective tissue capsule. This type of tumor does not spread or invade adjacent
tissue and is therefore usually not life-threatening. A malignant tumor, on the
other hand, is a mass capable of spreading and invading other healthy tissues of
the body. The spread of cancer cells occurs by traveling within the bloodstream
or lymph system. A malignant tumor can then form additional sites of cancers
away from the original tumor, a process called metastasis. Malignant tumors are
dangerous and difficult to control. Well-developed malignant cancers can be life
threatening.
Squamous Cell Carcinoma. Squamous cell carcinoma is the most
frequently occurring malignant cancer of the oral region. This type of cancer
involves cell growth changes of the surface tissue of the oral region, the
epithelium. Nearly 90% of all oral cancers are squamous cell carcinomas. The
most frequent location of this cancer is on the lips, the tongue, and the floor of
the mouth. A chronic (long-term) mouth ulcer that does not heal, a lesion
attached to deeper tissues, and a red-velvety lesion are all suspect squamous cell
carcinomas. If left untreated, squamous cell carcinomas undergo metastasis and
involve vital organs of the body. Many times death is the result of complications
to the heart and lungs.
Basal Cell Carcinoma. Basal cell carcinoma is a tumor located on the
surface of skin that has hair. Basal cell carcinomas are associated with
prolonged exposure of skin to the sun. Lesions initially appear as elevated
blisters, followed by a period of ulceration and healing. Continued cycles of
blistering, ulcerating, and healing of the same tissue occurs as deeper tissues are
slowly invaded. Metastasis of basal cell carcinomas is rare, and survival rate of
individuals diagnosed with basal cell carcinoma is excellent.
Fibromas. Fibromas are benign tumors whose cell origin lies in the
connective tissue supporting teeth within tooth sockets, the periodontal
membrane.
Osteomas. Osteomas are benign tumors whose cell origin is the bone of
the upper and lower jaw.
Malignant tumors of connective tissue origin. Malignant tumors of
connective tissue origin (the periodontal membrane, bone and cartilage) include
fibrosarcomas, osteosarcomas and chondrosarcomas.
Tumors of the teeth.
283
Tumors involving cells of the teeth are termed odontogenic tumors. Salivary
gland tumors. Salivary gland tumors are varied in their degree of malignancy
and tumor cell origin. Salivary gland tumors can be benign or malignant. This
type of tumor is very rare.
TREATMENT OF ORAL CANCER. The three major treatment
considerations for oral cancer include:
Surgical removal of diseased tissue. This is the best management for
treatment of cancer if possible. The advantages for surgery are prompt treatment
and the ability of physicians to study tissue removed during surgery with a
microscope. With this study, it can be known that the entire tumor has been
removed. The disadvantage of surgical treatment is normal tissue loss in the
attempt to remove all diseased tissue. Another consequence to surgery is the risk
of loss of function and cosmetic defects.
Radiation therapy. This is an effective form of treatment for isolated areas
of cancer. As the size of the tumors enlarge, larger doses of radiation are
necessary to kill cancer cells. The advantages of radiation therapy are the
preservation of normal tissues and the maintenance of normal tissue function.
The major disadvantage of radiation therapy includes inflammation of the lining
of the mouth, destruction of salivary gland tissue that can result in dry mouth,
changes in taste sensations, dental decay, damage to small blood vessels, and the
risk of necrosis to bone tissue.
Chemotherapy. Chemotherapy is dependent on the cancer diagnosis.
Therapy can be designed for a systemic approach (one that treats the entire
body) or a regional approach (one that treats the only the area of the cancer).
The appropriate medication and duration of treatment is determined by
evaluating the location of the cancer and the severity of the disease process.
There are many side effects to chemotherapy, including complications affecting
cells of the bone marrow, the gut, and lining of the mouth.
Treatment of most cancers of the head and neck involve a combination of
surgery, radiation and chemotherapy.
Oral cancer
Definition: Oral cancer is cancer of the mouth.
Causes, incidence, and risk factors
Oral or mouth cancer most commonly involves the tissue of the lips or the
tongue. It may also occur on the floor of the mouth, cheek lining, gingiva
(gums), or palate (roof of the mouth). Most oral cancers look very similar under
the microscope and are called squamous cell carcinomas. These are malignant
and tend to spread rapidly.
Smoking and other tobacco use are associated with 70-80% of oral cancer
cases. Smoke and heat from cigarettes, cigars, and pipes irritate the mucous
membranes of the mouth. Use of chewing tobacco or snuff causes irritation from
direct contact with the mucous membranes. Heavy alcohol use is another highrisk activity associated with oral cancer.
Other risks include poor dental and oral hygiene and chronic irritation (such as that from rough teeth, dentures, or fillings).Some oral cancers begin as leu284
koplakia or mouth ulcers.Oral cancer accounts for about8%of all malignant growths. Men are affected twice as often as women, particularly men older than 40.
Symptoms
Skin lesion, lump, or ulcer:
On the tongue, lip, or other mouth area
Usually small
Most often pale colored, may be dark or discolored
May be a deep, hard edged crack in the tissue
Usually painless initially
May develop a burning sensation or pain when the tumor is advanced
Additional symptoms that may be associated with this disease:
Tongue problems
Swallowing difficulty
Mouth sores
Abnormal taste
Signs and tests
An examination of the mouth by the health care provider or dentist shows
a visible or palpable (can be felt) lesion of the lip, tongue, or other mouth area.
As the tumor enlarges, it may become an ulcer and bleed. Speech difficulties,
chewing problems, or swallowing difficulties may develop, particularly if the
cancer is on the tongue.
A tongue biopsy, gum biopsy, and microscopic examination of the lesion
confirm the diagnosis of oral cancer.
Treatment
Surgical excision (removal) of the tumor is usually recommended if the
tumor is small enough. Radiation therapy and chemotherapy would likely be
used when the tumor is larger or has spread to lymph nodes in the neck. Surgery
may be necessary for large tumors.
Rehabilitation may include speech therapy or other therapy to improve
movement, chewing, swallowing, and speech.
Support Groups
The stress of illness can often be eased by joining a support group of
people who share common experiences and problems. See cancer - support
group.
Expectations (prognosis). Approximately 50% of people with oral cancer
will live more than 5 years after diagnosis and treatment. If the cancer is
detected early, before it has spread to other tissues, the cure rate is nearly 75%.
Unfortunately, more than 50% of oral cancers are advanced at the time the
cancer is detected. Most have spread to the throat or neck.
Approximately 25% of people with oral cancer die because of delayed
diagnosis and treatment.
Complications
Postoperative disfigurement of the face, head and neck
285
Complications of radiation therapy, including dry mouth and difficulty
swallowing
Other metastasis (spread) of the cancer
Calling your health care provider
This disorder may be discovered when the dentist performs a routine
cleaning and examination.
Call for an appointment with your health care provider if a lesion of the
mouth or lip or a lump in the neck are present and do not clear within 1
month. Early diagnosis and treatment of oral cancer greatly increases the
chances of survival.
Prevention
You should have the soft tissue of the mouth examined once a year. Many
oral cancers are discovered by routine dental examination.
Other tips:
Minimize or avoid smoking or other tobacco use
Minimize or avoid alcohol use
Practice good oral hygiene
Have dental problems corrected
Oral Cavity Cancer
The oral cavity extends from the skin-vermilion junctions of the anterior
lips to the junction of the hard and soft palates above and to the line of
circumvallate papillae below and is divided into the following specific areas:
Lip.
Anterior two thirds of tongue.
Buccal mucosa.
Floor of mouth.
Lower gingiva.
Retromolar trigone.
Upper gingiva.
Hard palate.
The main routes of lymph node drainage are into the first station nodes
(i.e., buccinator, jugulodigastric, submandibular, and submental). Sites close to
the midline often drain bilaterally. Second station nodes include the parotid,
jugular, and the upper and lower posterior cervical nodes.
Early cancers (stage I and stage II) of the lip and oral cavity are highly
curable by surgery or by radiation therapy, and the choice of treatment is
dictated by the anticipated functional and cosmetic results of treatment and by
the availability of the particular expertise required of the surgeon or radiation
oncologist for the individual patient.The presence of a positive margin or a
tumor depth >5 mm significantly increases the risk of local recurrence and
suggests that combined modality treatment may be beneficial.
Advanced cancers (stage III and stage IV) of the lip and oral cavity
represent a wide spectrum of challenges for the surgeon and radiation
oncologist. Except for patients with small T3 lesions and no regional lymph
286
node and no distant metastases or who have no lymph nodes >2 cm, for whom
treatment by radiation therapy alone or surgery alone might be appropriate, most
patients with stage III or stage IV tumors are candidates for treatment by a
combination of surgery and radiation therapy.Furthermore, because local
recurrence and/or distant metastases are common in this group of patients, they
should be considered for clinical trials. Such trials evaluate the potential role of
radiation modifiers or combination chemotherapy combined with surgery and/or
radiation therapy.
Patients with head and neck cancers have an increased chance of developping a second primary tumor of the upper aerodigestive tract. A study has shown
that daily treatment of these patients with moderate doses of isotretinoin (13-cisretinoic acid) for 1 year can significantly reduce the incidence of second tumors.
No survival advantage has yet been demonstrated, however, in part due to
recurrence and death from the primary malignancy.Additional trials are ongoing.
The rate of curability of cancers of the lip and oral cavity varies
depending on the stage and specific site. Most patients present with early
cancers of the lip, which are highly curable by surgery or by radiation therapy
with cure rates of 90% to 100%. Small cancers of the retromolar trigone, hard
palate, and upper gingiva are highly curable by either radiation therapy or
surgery, with survival rates of as much as 100%. Local control rates of as much
as 90% can be achieved with either radiation therapy or surgery in small cancers
of the anterior tongue, the floor of the mouth, and buccal mucosa.
Moderately advanced and advanced cancers of the lip also can be
controlled effectively by surgery or radiation therapy or a combination of these.
The choice of treatment is generally dictated by the anticipated functional and
cosmetic results of the treatment. Moderately advanced lesions of the retromolar
trigone without evidence of spread to cervical lymph nodes are usually curable
and have shown local control rates of as much as 90%; such lesions of the hard
palate, upper gingiva, and buccal mucosa have a local control rate of as much as
80%. In the absence of clinical evidence of spread to cervical lymph nodes,
moderately advanced lesions of the floor of the mouth and anterior tongue are
generally curable, with survival rates of as much as 70% and 65%, respectively.
Incidence, Epidemiology and Pathology
Although cancers of the head and neck region only account for five
percent of all cancers reported yearly in the human body, 30 percent of these
cancers occur in the oral cavity. That is roughly 22,000 new cases per year if
cancers of the lip are not included. Between 6000 and 7000 deaths per year
occur because of oral cavity cancer. As can be easily seen from the review of the
anatomy and functions of this area, cancers of the oral cavity left untreated can
have devastating effects on critical life functions for people who have this
disease. Similarly, choice of treatment must take into account the potential loss
of function in this area.
Cancers of the oral cavity may involve any single one of these specialized
types of tissue or more than one. As noted, tissues in this area includes bone, teeth,muscle,nerves,a rich supply blood vessels,numerous saliva gland, and the
287
specialized lining called mucosa. Although tumors may arise in any of these types of tissues they are most commonly related to changes in the lining of the
mouth.
The most common cancer of the oral cavity is called squamous cell
carcinoma and arises from the lining of the oral cavity. Over 95 percent of oral
cavity cancers are squamous cell carcinomas and these cancers are further
subdivided by how closely they resemble normal lining cells: well
differentiated, moderately differentiated and poorly differentiated. Other types
of cancers of the oral cavity include cancers of the salivary glands such as
mucoepidermoid carcinoma and adenoid cystic carcinoma, sarcomas (tumors
arising from bone, cartilage, fat, fibrous tissue or muscle), and melanomas. The
pathologist may also described characteristics of the tumor which make it more
concerning such as: deep invasion of the tumor, invasion of nerves, invasion of
the lymph vessels, invasion of blood vessels and the presence of multiple
separate cancers in the area.
Risk Factors
Tobacco and alcohol use are the major risk factors for most cancers of the
head and neck including the oral cavity. Although the most common use of
tobacco in the United States a cigarette smoking, the use of smokeless tobacco,
or chew, is associated with oral cavity cancers. The most common site for oral
cavity cancer in the United States is the tongue. In other regions of the world,
different areas are more commonly affected. In countries such as India, where
the use of a specific type of smokeless tobacco and a substance called beetle nut
is common, the inner cheek area of the oral cavity is most commonly affected.
Although there has been some decrease in the overall numbers of oral cavity
cancers and deaths from the disease noted in the last 20 years, the decrease has
not been dramatic.
Symptoms
There are many symptoms that raise concern for the possibility of the oral
cavity cancer being present. The most common of these is a nonhealing wound
on the tongue, in the floor of mouth or along the inner cheek. These can be painful, but in some cases do not cause significant discomfort. There may be bleeding from the area which occurs in an “on and off” manner. As the lesions increase in size, more symptoms occur. Complaints may include new or increased
pain, pain was swallowing, ear pain, a change in speech, uncoordinated
swallowing, or a lump in the neck. The most important factor to note is that
sores in the mouth, whether they are related to trauma or to a variation of canker
sores, should fully heal within three weeks. If this does not occur attention
should be sought and trained professional should evaluate this region.
Evaluation and Diagnosis
As part of evaluation for a suspicious area in the oral cavity, usually
completed by a specialist in the treatment of diseases of the oral cavity and the
head and neck - such as a Head and Neck Surgeon - a thorough history will be
taken asking for some of the symptoms noted above. The caregiver may also ask
for risk factors including tobacco and alcohol use as well as the family history of
288
cancer. The caregiver will do a thorough physical examination of the area. This
will include not only looking at the area suspicious for cancer, but also feeling
the area with a gloved finger or an instrument. Examination will usually be done
of the entire head and neck region including the throat nose and ears. Particular
attention will be given to feeling the neck to note if there are signs of cancer
spread to lymph nodes in the neck called metastases. Once the clinical
examination is completed, recommendation may be given to obtain a specialized
type of X-ray, such as a CT scan or MRI. One or both of these can may be
necessary at each can provide very specific information concerning the extent of
disease. The physician may also order an X-ray or CT scan of the chest to see if
there is any spread of disease to the lungs, the most common site of spread
outside of the neck.
At this point, a biopsy – a small piece of tissue taken from the suspected
tumor - is often advised. This tissue is sent to a pathologist to define which types
of cells are making up the tumor. With tumors of the oral cavity, biopsies can
often be done safely in the office. The surgeon may also wish to do the biopsy
with the patient under anesthesia. This allows the added benefit of the surgeon
being able to better define the size of the tumor and which other tissues may be
involved. Evaluation of the entire throat, voicebox, esophagus, and windpipe is
also often recommended. The entire procedure is called endoscopy and biopsy.
This is done because between 5 and 15% of individuals who have one cancer of
the mouth, throat or voicebox may also have another tumor present elsewhere in
the head and neck.
Tumor Staging
Once a full examination has been completed as well as the necessary Xrays and biopsies, the tumor is “staged.” Staging is a well-defined method of
describing the exact extent of a specific tumor in an individual patient and then
placing that tumor in a specific category. This not only assists in choosing
treatment options, but also helps predict how successful therapy will be. There
are three categories used to describe the tumor: T (tumor), N (lymph node
involvement), M (metastasis – spread to other areas of the body.) This is called
the TNM classification system. Tumors of the oral cavity are described by their
size. Tumors less than 2 cm are called T1. Tumors greater than 2 cm but less
than 4 cm are called T2. Tumors greater than 4 cm are called T3. Any tumor that
is deeply invading bone, skin or other areas of the head and neck is labeled T4.
Lymph node involvement is labeled as N1 if there is one lymph node less than 3
cm on the same side as the tumor. Lymph node involvement is labeled as N2 if a
single lymph node is greater than 3 cm but less than 6 cm, or is on the opposite
side of the tumor, or if there are more than one lymph node present. If a lymph
node in the neck is greater than 6 cm then it is called N3. The M classification is
M0 if there is no evidence of cancer spread elsewhere in the body or labeled as
positive if there is evidence of cancer spread to tissues such as the lungs, liver,
bones or brain.
Once each part of the TNM classification is completed tumors are then
staged into four separate groups: I, II, III, IV. Stages I and II are usually defined
289
as early-stage tumors, whereas stages III and IV are usually defined as advanced
stage tumors. Treatment is then based on the stage of the tumor, with more
advanced tumors requiring more advanced treatments.
Treatment
The three main tools for treating cancers of the oral cavity are surgery,
radiation therapy, and chemotherapy. For this reason, someone with a cancer of
the oral cavity may also meet a specialist from radiation oncology as well as
medical oncology. In some cases of advanced cancers of the oral cavity, a
specialist in reconstructive surgery may also become involved to assist with
specialized reconstruction should it be required.
In general, Stage I and Stage II cancers require one type of treatment,
either surgery or radiation therapy, to successfully control the cancer. Advanced
Stage III and Stage IV cancers will often require combinations of surgery,
radiation therapy and chemotherapy or even the use of all three.Overall survival
rates for any cancer of the oral cavity are about 70 percent five-year survival for
stage I or II disease. Five-year survival drops to about 50 percent for stage III
cancers and further drops to roughly 35 percent for stage IV cancers.
Reconstruction
If surgery is required as part of the treatment of the cancer with in the oral
cavity, a major emphasis is placed on providing successful reconstruction. This
reconstruction is intended to maintain function as well as appearance.
Reconstruction may be as simple as putting the tongue muscles back together in
the best possible fashion after the removal of the tongue cancer or the placement
of a skin graft to replace the missing oral cavity lining. With more advanced
cancers, more advanced reconstruction is required. In such cases, not only is
new lining of the oral cavity needed in greater amount, but bone – such as the
jawbone - may need to be replaced. In such cases, a surgeon may borrow
replacement tissues from elsewhere in the body. Skin and muscle can be moved
from the chest to re-build the tongue and mouth. Skin can also be moved from
the area of the wrist and used to reline the mouth and read build the tongue.
Bone can be moved, with or without skin, from the lower leg, hip or shoulder
blade and used to rebuild the upper or lower jaw.
Rehabilitation
Following the treatment of cancers in the oral cavity with surgery,
radiation therapy chemotherapy or combinations of these, several important
functions of the oral cavity may be severely affected. These include the
lubrication of the mouth and throat, swallowing without choking on foods or
liquids, speech and movement in areas where surgery has been done. For this
reason, specialists in the rehabilitation of the functions such as speech therapists,
swallow therapists, physical therapists and occupational therapists will often be
very important in the ultimate goal of curing the patient’s cancer while
maintaining an acceptable quality of life. Similarly, specialists in pain
management may also be needed to assist with pain.
Follow-up
290
After treatment of a cancer in the oral cavity has been completed, it will
be important to watch not only the area where the cancer originally began but
also other areas of the body to make sure there are no signs of the tumor coming
back. This is called patient follow-up. The treating physician may request that
the patient be seen every 4 to 6 weeks for the first year after treatment to have
evaluation for possible signs of regrowth of the original tumor. Such evaluation
will include thorough physical exams of the head and neck region: examining
the area of the tumor’s original position and examining the neck closely for
possible spread to lymph nodes. A chest x-ray and blood tests, such as liver
function tests, may also be obtained periodically to check for spread to of cancer
to the lungs or liver. Imaging studies such as a CAT scan or MRI may also be
obtained periodically to check for any changes that may indicate return of the
tumor. These follow-up visits also provide important opportunities for patients
to ask their caregivers specific questions concerning persistent symptoms or
functional limitations relating to their treatment. Follow-up visits also provide
the opportunity for the caregiver to specifically ask questions that may be
suspicious for return of the tumor. Such questions include: New ear pain? New
pain with swallowing? New difficulty with swallowing or speaking? New
weight loss, although you are taking in the same amount of calories? Etc.
As time goes on, the frequency of such follow-up visits will decrease.
Because as time goes on tumors are less and less likely to recur. In general,
about 70 percent of all the tumors that return after treatment will do so within
the first year after the completion of treatment. Ninety percent of the tumors that
return after treatment will do so within the first 18 months after treatment.
Eventually, follow-up visits may be required once or twice a year. During these
visits, the greater concern is not the possibility of the original cancer coming
back, but concern for a possible second cancer developing in the head and neck
region. This is especially concerning in patients who continue to use tobacco
and alcohol after their treatment. If a new cancer (called a second primary
cancer) were to occur, it would be important to identify it while it is small and
often early-stage to achieve the best cure rate with the least invasive means of
treatment. For these reasons, close follow-up after treatment is essential in
patients with cancers of the oral cavity.
Oral Cancer
The mouth and throat This booklet is about cancers that occur in the
mouth (oral cavity) and the part of the throat at the back of the mouth
(oropharynx). The oral cavity and oropharynx have many parts:
Lips
Lining of your cheeks
Salivary glands (glands that make saliva)
Roof of your mouth (hard palate)
Back of your mouth (soft palate and uvula)
Floor of your mouth (area under the tongue)
Gums and teeth
291
Tongue
Tonsils
Understanding cancer Cancer begins in cells, the building blocks that
make up tissues. Tissues make up the organs of the body.
Normally, cells grow and divide to form new cells as the body needs
them. When cells grow old, they die, and new cells take their place.
Sometimes this orderly process goes wrong. New cells form when the
body does not need them, and old cells do not die when they should. These extra
cells can form a mass of tissue called a growth or tumor.
Tumors can be benign or malignant:
Benign tumors are not cancer:
Benign tumors are rarely life-threatening.
Generally, benign tumors can be removed, and they usually do not grow
back.
Cells from benign tumors do not invade the tissues around them. Cells
from benign tumors do not spread to other parts of the body.
Malignant tumors are cancer:
Malignant tumors are generally more serious than benign tumors. They
may be life-threatening.
Malignant tumors often can be removed, but sometimes they grow back.
Cells from malignant tumors can invade and damage nearby tissues and
organs.
Cells from malignant tumors can spread to other parts of the body. The
cells spread by breaking away from the original cancer (primary tumor)
and entering the bloodstream or lymphatic system. They invade other
organs, forming new tumors and damaging these organs. The spread of
cancer is called metastasis.
Oral cancer Oral cancer is part of a group of cancers called head and neck
cancers. Oral cancer can develop in any part of the oral cavity or oropharynx.
Most oral cancers begin in the tongue and in the floor of the mouth. Almost all
oral cancers begin in the flat cells (squamous cells) that cover the surfaces of the
mouth, tongue, and lips. These cancers are called squamous cell carcinomas.
When oral cancer spreads (metastasizes), it usually travels through the
lymphatic system. Cancer cells that enter the lymphatic system are carried along
by lymph, a clear, watery fluid. The cancer cells often appear first in nearby
lymph nodes in the neck.
Cancer cells can also spread to other parts of the neck, the lungs, and
other parts of the body. When this happens, the new tumor has the same kind of
abnormal cells as the primary tumor. For example, if oral cancer spreads to the
lungs, the cancer cells in the lungs are actually oral cancer cells. The disease is
metastatic oral cancer, not lung cancer. It is treated as oral cancer, not lung
cancer. Doctors sometimes call the new tumor "distant" or metastatic disease.
Oral cancer: Who's at risk?
Doctors cannot always explain why one
person develops oral cancer and another does not. However, we do know that
292
this disease is not contagious. You cannot "catch" oral cancer from another
person.
Research has shown that people with certain risk factors are more likely
than others to develop oral cancer. A risk factor is anything that increases your
chance of developing a disease.
The following are risk factors for oral cancer:
Tobacco: Tobacco use accounts for most oral cancers. Smoking cigarettes,
cigars, or pipes; using chewing tobacco; and dipping snuff are all linked to oral
cancer. The use of other tobacco products (such as bidis and kreteks) may also
increase the risk of oral cancer. Heavy smokers who use tobacco for a long time
are most at risk. The risk is even higher for tobacco users who drink alcohol
heavily. In fact, three out of four oral cancers occur in people who use alcohol,
tobacco, or both alcohol and tobacco.
Alcohol: People who drink alcohol are more likely to develop oral cancer
than people who don't drink. The risk increases with the amount of alcohol that a
person consumes. The risk increases even more if the person both drinks alcohol
and uses tobacco.
Sun: Cancer of the lip can be caused by exposure to the sun. Using a
lotion or lip balm that has a sunscreen can reduce the risk. Wearing a hat with a
brim can also block the sun's harmful rays. The risk of cancer of the lip increases
if the person also smokes.
A personal history of head and neck cancer: People who have had head
and neck cancer are at increased risk of developing another primary head and
neck cancer. Smoking increases this risk.
Quitting tobacco reduces the risk of oral cancer. Also, quitting reduces the
chance that a person with oral cancer will get a second cancer in the head and
neck region. People who stop smoking can also reduce their risk of cancer of the
lung, larynx, mouth, pancreas, bladder, and esophagus. There are many
resources to help smokers quit:
The Cancer Information Service at 1-800-4-CANCER can talk with
callers about ways to quit smoking and about groups that offer help to smokers
who want to quit. Groups offer counseling in person or by telephone.
Also, your doctor or dentist can help you find a local smoking cessation
program.
Your doctor can tell you about medicine (bupropion) or about nicotine
replacement therapy, which comes as a patch, gum, lozenges, nasal spray, or
inhaler.
Some studies suggest that not eating enough fruits and vegetables may
increase the chance of getting oral cancer. Scientists also are studying whether
infections with certain viruses (such as the human papillomavirus) are linked to
oral cancer.
If you think you may be at risk, you should discuss this concern with your
doctor or dentist. You may want to ask about an appropriate schedule for
checkups. Your health care team will probably tell you that not using tobacco
and limiting your use of alcohol are the most important things you can do to
293
prevent oral cancers. Also, if you spend a lot of time in the sun, using a lip balm
that contains sunscreen and wearing a hat with a brim will help protect your lips.
What are the symptoms of oral cancer?
Early detection
Your regular checkup is a good time for your dentist or doctor to check
your entire mouth for signs of cancer. Regular checkups can detect the early
stages of oral cancer or conditions that may lead to oral cancer. Ask your doctor
or dentist about checking the tissues in your mouth as part of your routine exam.
Symptoms Common symptoms of oral cancer include:
Patches inside your mouth or on your lips that are white, a mixture of red and
white, or red. White patches (leukoplakia) are the most common. White patches
sometimes become malignant.
Mixed red and white patches (erythroleukoplakia) are more likely than
white patches to become malignant. Red patches (erythroplakia) are brightly
colored, smooth areas that often become malignant.
A sore on your lip or in your mouth that won't heal
Bleeding in your mouth
Loose teeth
Difficulty or pain when swallowing
Difficulty wearing dentures
A lump in your neck
An earache
Anyone with these symptoms should see a doctor or dentist so that any
problem can be diagnosed and treated as early as possible. Most often, these
symptoms do not mean cancer. An infection or another problem can cause the
same symptoms.
Diagnosis of oral cancer
If you have symptoms that suggest oral cancer, the doctor or dentist
checks your mouth and throat for red or white patches, lumps, swelling, or other
problems. This exam includes looking carefully at the roof of the mouth, back of
the throat, and insides of the cheeks and lips. The doctor or dentist also gently
pulls out your tongue so it can be checked on the sides and underneath. The
floor of your mouth and lymph nodes in your neck also are checked.
If an exam shows an abnormal area, a small sample of tissue may be removed. Removing tissue to look for cancer cells is called a biopsy. Usually, a
biopsy is done with local anesthesia. Sometimes, it is done under general anesthesia. A pathologist then looks at the tissue under a microscope to check for
cancer cells. A biopsy is the only sure way to know if the abnormal area is
cancerous.
Treatment for oral cancer
Staging
If the biopsy shows that cancer is present, your doctor needs to know the
stage (extent) of your disease to plan the best treatment. The stage is based on
the size of the tumor, whether the cancer has spread and, if so, to what parts of
the body.
294
Staging may require lab tests. It also may involve endoscopy. The doctor
uses a thin, lighted tube (endoscope) to check your throat, windpipe, and lungs.
The doctor inserts the endoscope through your nose or mouth. Local anesthesia
is used to ease your discomfort and prevent you from gagging. Some people also
may have a mild sedative. Sometimes the doctor uses general anesthesia to put a
person to sleep. This exam may be done in a doctor's office, an outpatient clinic,
or a hospital.
The doctor may order one or more imaging tests to learn whether the
cancer has spread:
Dental x-rays: An x-ray of your entire mouth can show whether cancer
has spread to the jaw.
Chest x-rays: Images of your chest and lungs can show whether cancer
has spread to these areas.
CT scan: An x-ray machine linked to a computer takes a series of detailed
pictures of your body. You may receive an injection of dye. Tumors in the
mouth, throat, neck, or elsewhere in the body show up on the CT scan.
MRI: A powerful magnet linked to a computer is used to make detailed
pictures of your body. The doctor can view these pictures on a monitor and can
print them on film. An MRI can show whether oral cancer has spread.
Treatment Many people with oral cancer want to take an active part in
making decisions about their medical care. It is natural to want to learn all you
can about your disease and your treatment choices. However, shock and stress
after the diagnosis can make it hard to think of everything you want to ask the
doctor. It often helps to make a list of questions before an appointment. To help
remember what the doctor says, you may take notes or ask whether you may use
a tape recorder. You may also want to have a family member or friend with you
when you talk to the doctor—to take part in the discussion, to take notes, or just
to listen.
Your doctor may refer you to a specialist, or you may ask for a referral.
Specialists who treat oral cancer include oral and maxillofacial surgeons,
otolaryngologists (ear, nose, and throat doctors), medical oncologists, radiation
oncologists, and plastic surgeons. You may be referred to a team that includes
specialists in surgery, radiation therapy, or chemotherapy. Other health care
professionals who may work with the specialists as a team include a dentist,
speech pathologist, nutritionist, and mental health counselor.
Getting a second opinion
Before starting treatment, you might want a second opinion about the
diagnosis and the treatment plan. Some insurance companies require a second
opinion; others may cover a second opinion if you or your doctor requests it.
There are a number of ways to find a doctor for a second opinion:
Your doctor may refer you to one or more specialists. At cancer centers, several
specialists often work together as a team.
Preparing for treatment
The choice of treatment depends mainly on your general health, where in
your mouth or oropharynx the cancer began, the size of the tumor, and whether
295
the cancer has spread. Your doctor can describe your treatment choices and the
expected results. You will want to consider how treatment may affect normal
activities such as swallowing and talking, and whether it will change the way
you look. You and your doctor can work together to develop a treatment plan
that meets your needs and personal values.
You do not need to ask all your questions or understand all the answers at
once. You will have other chances to ask your doctor to explain things that are
not clear and to ask for more information.
Methods of treatment
Oral cancer treatment may include surgery, radiation therapy, or
chemotherapy. Some patients have a combination of treatments.
At any stage of disease, people with oral cancer may have treatment to
control pain and other symptoms, to relieve the side effects of therapy, and to
ease emotional and practical problems. This kind of treatment is called
supportive care, symptom management, or palliative care.
Surgery
Surgery to remove the tumor in the mouth or throat is a
common treatment for oral cancer. Sometimes the surgeon also removes lymph
nodes in the neck. Other tissues in the mouth and neck may be removed as well.
Patients may have surgery alone or in combination with radiation therapy.
Radiation therapy
Radiation therapy (also called radiotherapy) is a type of local therapy. It
affects cells only in the treated area. Radiation therapy is used alone for small
tumors or for patients who cannot have surgery. It may be used before surgery to
kill cancer cells and shrink the tumor. It also may be used after surgery to
destroy cancer cells that may remain in the area.
Radiation therapy uses high-energy rays to kill cancer cells. Doctors use
two types of radiation therapy to treat oral cancer:
External radiation: The radiation comes from a machine. Patients go to the
hospital or clinic once or twice a day, generally 5 days a week for several weeks.
Internal radiation (implant radiation): The radiation comes from radioactive
material placed in seeds, needles, or thin plastic tubes put directly in the tissue.
The patient stays in the hospital. The implants remain in place for several days.
Usually they are removed before the patient goes home.
Some people with oral cancer have both kinds of radiation therapy.
Chemotherapy
Chemotherapy uses anticancer drugs to kill cancer
cells. It is called systemic therapy because it enters the bloodstream and can
affect cancer cells throughout the body.
Chemotherapy is usually given by injection. It may be given in an
outpatient part of the hospital, at the doctor's office, or at home. Rarely, a
hospital stay may be needed.
Side effects of treatment for oral cancer
Because treatment often damages healthy cells and tissues, unwanted side
effects are common. These side effects depend mainly on the location of the
tumor and the type and extent of the treatment. Side effects may not be the same
for each person, and they may even change from one treatment session to the
296
next. Before treatment starts, your health care team will explain possible side
effects and suggest ways to help you manage them.
The NCI provides helpful booklets about cancer treatments and coping
with side effects. Booklets such as Radiation Therapy and You, Chemotherapy
and You, and Eating Hints for Cancer Patients may be viewed, downloaded, and
ordered from http://cancer.gov/publications. These materials also may be
ordered by calling the Cancer Information Service at 1-800-4-CANCER.
The National Institute of Dental and Craniofacial Research (NIDCR) also
provides helpful materials. Head and Neck Radiation Treatment and Your
Mouth, Chemotherapy and Your Mouth, and other booklets are available from
NIDCR. See "National Institute of Dental and Craniofacial Research
Information Resources" for a list of publications.
Surgery
It takes time to heal after surgery, and the time needed to
recover is different for each person. You may be uncomfortable for the first few
days after surgery. However, medicine can usually control the pain. Before
surgery, you should discuss the plan for pain relief with your doctor or nurse.
After surgery, your doctor can adjust the plan if you need more pain relief.
It is common to feel tired or weak for a while. Also, surgery may cause
tissues in your face to swell. This swelling usually goes away within a few weeks. However, removing lymph nodes can result in swelling that lasts a long
time.
Surgery to remove a small tumor in the mouth may not cause any lasting
problems. For a larger tumor, however, the surgeon may remove part of the
palate, tongue, or jaw. This surgery may change your ability to chew, swallow,
or talk. Also, your face may look different after surgery. Reconstructive or
plastic surgery may be done to rebuild the bones or tissues of the mouth. (See
"Reconstruction.")
Radiation therapy
Almost all patients who have radiation therapy to the head and neck area
develop oral side effects. That is why it is important to get the mouth in good
condition before cancer treatment begins. Seeing a dentist two weeks before
cancer treatment begins gives the mouth time to heal after dental work.
The side effects of radiation therapy depend mainly on the amount of
radiation given. Some side effects in the mouth go away after radiation
treatment ends, while others last a long time. A few side effects (such as dry
mouth) may never go away.
Radiation therapy may cause some or all of these side effects:
Dry mouth: Dry mouth can make it hard for you to eat, talk, and swallow.
It can also lead to tooth decay. You may find it helpful to drink lots of water,
suck ice chips or sugar-free hard candy, and use a saliva substitute to moisten
your mouth.
Tooth decay:Radiation can cause major tooth decay problems. Good
mouth care can help you keep your teeth and gums healthy and can help you feel
better.
297
Doctors usually suggest that people gently brush their teeth, gums, and
tongue with an extra-soft toothbrush and fluoride toothpaste after every meal
and before bed. If brushing hurts, you can soften the bristles in warm water.
Your dentist may suggest that you use fluoride gel before, during, and after
radiation treatment.
It also helps to rinse your mouth several times a day with a solution made
from 1/4 teaspoon baking soda and 1/8 teaspoon salt in one cup of warm water.
After you rinse with this solution, follow with a plain water rinse.
Sore throat or mouth: Radiation therapy can cause painful ulcers and
inflammation. Your doctor can suggest medicines to help control the pain. Your
doctor also may suggest special rinses to numb the throat and mouth to help
relieve the soreness. If your pain continues, you can ask your doctor about
stronger medicines.
Sore or bleeding gums: It is important to brush and floss teeth gently. You
may want to avoid areas that are sore or bleeding. To protect your gums from
damage, it is a good idea to avoid the use of toothpicks.
Infection: Dry mouth and damage to the lining of the mouth from
radiation therapy can cause infection to develop. It helps to check your mouth
every day for sores or other changes and to tell your doctor or nurse about any
mouth problems.
Delayed healing after dental care: Radiation treatment may make it hard for
tissues in the mouth to heal. It helps to have a thorough dental exam and
complete all needed dental treatment well before radiation therapy begins.
Jaw stiffness: Radiation can affect the chewing muscles and make it
difficult for you to open your mouth. You can prevent or reduce jaw stiffness by
exercising your jaw muscles. Health care providers often suggest opening and
closing the mouth as far as possible (without causing pain) 20 times in a row, 3
times a day.
Denture problems: Radiation therapy can change the tissues in your mouth so
that dentures do not fit anymore. Because of soreness and dry mouth, some
people may not be able to wear dentures for as long as one year after radiation
therapy. After the tissues heal completely and your mouth is no longer sore,
your dentist may need to refit or replace your dentures.
Changes in the sense of taste and smell: During radiation therapy, food
may taste or smell different.
Changes in voice quality: Your voice may be weak at the end of the day.
It may also be affected by changes in the weather. Radiation directed at the neck
may cause your larynx to swell, causing voice changes and the feeling of a lump
in your throat. Your doctor may suggest medicine to reduce this swelling.
Changes in the thyroid: Radiation treatment can affect your thyroid (an
organ in your neck beneath the voice box). If your thyroid does not make
enough thyroid hormone, you may feel tired, gain weight, feel cold, and have
dry skin and hair. Your doctor can check the level of thyroid hormone with a
blood test. If the level is low, you may need to take thyroid hormone pills.
298
Skin changes in the treated area: The skin in the treated area may become
red or dry. Good skin care is important at this time. It is helpful to expose this
area to the air while protecting it from the sun. Also, avoid wearing clothes that
rub the treated area, and do not shave the treated area. You should not use
lotions or creams in the treated area without your doctor's advice.
Fatigue: You may become very tired, especially in the later weeks of
radiation therapy. Resting is important, but doctors usually advise their patients
to stay as active as they can.
Although the side effects of radiation therapy can be distressing, your
doctor can usually treat or control them. It helps to report any problems that you
are having so that your doctor can work with you to relieve them.
Chemotherapy
Chemotherapy and radiation therapy can cause some of
the same side effects, including painful mouth and gums, dry mouth, infection,
and changes in taste. Some anticancer drugs can also cause bleeding in the
mouth and a deep pain that feels like a toothache. The problems you have
depend on the type and amount of anticancer drugs you receive, and how your
body reacts to them. You may have these problems only during treatment or for
a short time after treatment ends.
Generally, anticancer drugs affect cells that divide rapidly. In addition to
cancer cells, these rapidly dividing cells include the following:
Blood cells: These cells fight infection, help your blood to clot, and carry
oxygen to all parts of the body. When drugs affect your blood cells, you are
more likely to get infections, bruise or bleed easily, and feel very weak and
tired.
Cells in hair roots: Chemotherapy can lead to hair loss. The hair grows
back, but sometimes the new hair is somewhat different in color and texture.
Cells that line the digestive tract: Chemotherapy can cause poor appetite,
nausea and vomiting, diarrhea, or mouth and lip sores. Many of these side
effects can be controlled with drugs. Nutrition Eating well during cancer
treatment means getting enough calories and protein to prevent weight loss,
regain strength, and rebuild healthy tissues. But eating well may be difficult
after treatment for oral cancer. Some people with cancer find it hard to eat
because they lose their appetite. They may not feel like eating because they are
uncomfortable or tired. A dry or sore mouth or changes in smell and taste also
may make eating difficult.
If your mouth is dry, you may find that soft foods moistened with sauces
or gravies are easier to eat. Thick soups, puddings, and milkshakes often are
easier to swallow. Nurses and dietitians can help you choose the right foods.
Also, the National Cancer Institute booklet Eating Hints for Cancer Patients
contains many useful ideas and recipes. The "National Cancer Institute
Information Resources" section tells how to get this publication.
After surgery or radiation therapy for oral cancer, some people need a
feeding tube. A feeding tube is a flexible plastic tube that is passed into the
stomach through an incision in the abdomen. In almost all cases, the tube is
temporary. Most people gradually return to a regular diet.
299
To protect your mouth during cancer treatment, it helps to avoid:
Sharp, crunchy foods like taco chips
Foods that are hot, spicy, or high in acid like citrus fruits and juices
Sugary foods that can cause cavities
Alcoholic drinks
Reconstruction
Some people with oral cancer may need to have plastic
or reconstructive surgery to rebuild the bones or tissues of the mouth. Research
has led to many advances in the way bones and tissues can be replaced.
Some people may need dental implants. Or they may need to have grafts
(tissue moved from another part of the body). Skin, muscle, and bone can be
moved to the oral cavity from the chest, arm, or leg. The plastic surgeon uses
this tissue for repair.
If you are thinking about reconstruction, you may wish to consult with a
plastic or reconstructive surgeon before your treatment begins. You can have
reconstructive surgery at the same time as you have the cancer removed, or you
can have it later on. Talk with your doctor about which approach is right for
you.
Rehabilitation
The health care team will help you return to normal
activities as soon as possible. The goals of rehabilitation depend on the extent of
the disease and type of treatment. Rehabilitation may include being fitted with a
dental prosthesis (an artificial dental device) and having dental implants. It also
may involve speech therapy, dietary counseling, or other services.
Sometimes surgery to rebuild the bones or tissues of the mouth is not
possible. A dentist with special training (a prosthodontist) may be able to make
you a prosthesis to help you eat and talk normally. You may need special
training to learn to use it.
If oral cancer or its treatment leads to problems with talking, speech
therapy will generally begin as soon as possible. A speech therapist may see
you in the hospital to plan therapy and teach speech exercises. Often speech
therapy continues after you return home.
Follow-up care for oral cancer
Follow-up care after treatment for oral cancer is important. Even when the
cancer seems to have been completely removed or destroyed, the disease
sometimes returns because undetected cancer cells remained in the body after
treatment. The doctor monitors your recovery and checks for recurrence of
cancer. Checkups help ensure that any changes in your health are noted. Your
doctor will probably encourage you to inspect your mouth regularly and
continue to have exams when you visit your dentist. It is important to report any
changes in your mouth right away.
Checkups include exams of the mouth, throat, and neck. From time to
time, your doctor may do a complete physical exam, order blood tests, and take
x-rays.
People who have had oral cancer have a chance of developing a new
cancer in the mouth, throat, or other areas of the head and neck. This is
especially true for those who use tobacco or who drink alcohol heavily. Doctors
300
strongly urge their patients to stop using tobacco and drinking to cut down the
risk of a new cancer and other health problems.
The NCI has prepared a booklet for people who have completed their
treatment to help answer questions about follow-up care and other concerns.
Facing Forward Series: Life After Cancer Treatment provides tips for making
the best use of medical visits. It describes how to talk to your health care team
about creating a plan of action for recovery and future health.
Support for people with oral cancer
Living with a serious disease such as oral cancer is not easy. You may
worry about caring for your family, keeping your job, or continuing daily
activities. You may have concerns about treatments and managing side effects,
hospital stays, and medical bills. Doctors, nurses, and other members of the
health care team can answer your questions about treatment, working, or other
activities. Meeting with a social worker, counselor, or member of the clergy can
be helpful if you want to talk about your feelings or discuss your concerns.
Often, a social worker can suggest resources for financial aid, transportation,
home care, or emotional support.
Support groups also can help. In these groups, patients or their family
members meet with other patients or their families to share what they have
learned about coping with the disease and the effects of treatment. Groups may
offer support in person, over the telephone, or on the Internet. You may want to
talk with a member of your health care team about finding a support group. The
NCI's fact sheets "Cancer Support Groups: Questions and Answers" and
"National Organizations That Offer Services to People With Cancer and Their
Families" tell how to find a support group. See "National Cancer Institute
Information Resources" for ordering information.
The Cancer Information Service can provide information to help patients and
their families locate programs, services, and publications.
The promise of cancer research
Doctors all over the country are conducting many types of clinical trials.
These are research studies in which people volunteer to take part. In clinical
trials, doctors are testing new ways to treat oral cancer. Research has already led
to advances, and researchers continue to search for more effective approaches.
People who join clinical trials may be among the first to benefit if a new
approach is shown to be effective. And if participants do not benefit directly,
they still make an important contribution to medical science by helping doctors
learn more about the disease and how to control it. Although clinical trials may
pose some risks, researchers do all they can to protect their patients.
Researchers are testing anticancer drugs and combinations of drugs. They
are studying radiation therapy combined with drugs and other treatments. They
also are testing drugs that prevent or reduce the side effects of radiation therapy.
301
PLASTIC AND RECONSTRUCTIVE SURGERY OF THE FACE
Cosmetic surgery
(Redirected from Cosmetic Surgery) This article or section needs to be
wikified to meet Wikipedia's quality standards.
Please help improve this article with relevant internal links. (March 2008)
Cosmetic surgery, as defined by the American Board of Cosmetic
Surgery, is a subspecialty of medicine and surgery that uniquely restricts itself to
the enhancement of appearance through surgical and medical techniques. It is
specifically concerned with maintaining normal appearance, restoring it, or
enhancing it beyond the average level toward some aesthetic ideal. Cosmetic
surgery is a multi-disciplinary and comprehensive approach directed to all areas
of the head, neck and body.
Special skill and knowledge are essential and specialists in cosmetic surgery are competent in the anatomy, physiology, pathology and basic sciences.
The educational profile of this specialty is unique in that it begins with a fully
trained and certified physician. Through continued post-residency education, training and experience, cosmetic surgery is taught and learned across traditional
disciplinary boundaries.The subspecialty fully incorporates the participation and
knowledge from all contributing disciplines to attain a high level of skill and
understanding. Contributing disciplines include dermatology, general surgery,
plastic surgery, otolaryngology, maxillofacial surgery, oculoplastic surgery, and
others.
The cosmetic surgeon offers specialized expertise in patient education and
counseling, procedural skills, and the early recognition and treatment of
complications. As a specialty, cosmetic surgeons have enhanced the knowledge
and training of fellow physicians and directly benefitted society through
educational publications, scientific journals and in the development of safe and
innovative techniques.
Competency in cosmetic surgery implies a combination of knowledge,
surgical judgment, technical expertise and ethics in order to achieve the goal of
providing aesthetic improvement.
Costs. Cosmetic surgery is generally not something that is covered by
health insurance. The cost can range from a few hundred dollars to a few thousand dollars depending upon what procedure the patient is having performed.
The cost of plastic surgery has now become affordable to the average citizen
through the availability of finance companies. Finance Companies such as
Capital One, CareCredit, and DoctorsSayYes.net make operations affordable by
loaning the money necessary to the patient for their procedures. An estimated
$14 billion is spent on cosmetic medical procedures last year, of that $1 billion
was financed. Financing a cosmetic procedure allows a patient to proceed as
soon as they are ready and not have to wait and save the money.
The average cost for some of the more popular procedures are:
Breast Lift - $4,258.00
Collagen Injection - $333.00
Facelift - $6,298.00
302
Laser Hair Removal - $347.00
Liposuction - $2,979.00
Rhinoplasty (Nose Job) - $4,188.00
Tummy Tuck - $5,232.00
Botox Injections - $382.00
What makes a good or bad candidate? Before a patient undergoes any
type of surgical procedure they should be well informed of the benefits and
risks. A patient should consult their current physician before attempting any type
of cosmetic surgery to make sure they are in good health. One or more of the
following conditions is reason to consult a current physician:
1. Heavy Smoking/Drinking
2. Diabetes
3. High Blood Pressure
4. A Bleeding Disorder
5. Heart or Lung Disease
6. Obesity
7. Severe Allergies
8. High Cholesterol
9. Arthritis
Cosmetic Surgery not only alters a patient physically, but also
emotionally. The surgeries can boost self-esteem if a patient’s appearance
changes for the better or lower self-esteem if a patient is unsatisfied with the
results. Self confidence is built in people after going under the knife. Although
family support is recommended, a patient needs to accept the new image on their
own to uplift self confidence. Being strong in handling critical comments from
family and friends is essential to out do the criticism. If a patient has extremely
high expectations they may not be the best candidates. Even though results can
be highly predicted, a surgeon cannot promise perfect results.
Patient’s who are considering some form of cosmetic surgery will go
through a consultation with a doctor previous to the surgery to determine
whether or not they are considering the surgery for the right reasons. Doctors
sometimes recommend psychological counseling. This helps to recognize the
patients reasons for undergoing surgery and to ensure that the reasons are not
emotion related. Good candidates might fall under more than one of the
following categories:
1. Within 30% of their ideal weight
2. Non-smoker
3. Low Stress Levels
4. Maintain a Healthy Lifestyle
Risks and Complications
Just like any form of surgery a patient can have a reaction to the
anesthesia or sedation that is used. Some of these complications may include:
1. Abnormal heart rhythm
2. Airway Obstruction
3. Blood Clots
303
4. Brain Damage
5. Death
6. Heart Attack
7. Malignant Hyperthermia
8. Nerve Damage
9. Stroke
10. Temporary Paralysis
These complications are not things that apply strictly to cosmetic surgery.
Surgical risks that do apply to the cosmetic procedure may include:
1. Nausea, Dizziness, Pain
2. Numbness and Tingling
3. Seroma
4. Collection of Blood Beneath Closed Incision
5. Skin Breakdown
6. Bleeding
7. Infection At The Site
8. Lumpy Appearance
9. Drop In Body Temperature
A patient’s expectations are also a risk to consider. Satisfaction with
results cannot be guaranteed.
Procedures
Facelift:
Technically known as rhytidectomy, a facelift is a surgical procedure to
improve visible signs of aging in the face and neck. A facelift is designed to
correct all aging features, restoring a more youthful, rested appearance with
uplifted contours and improved tone in facial skin and underlying muscle.
Eyelid Surgery: Cosmetic eyelid surgery, called blepharoplasty, is a
surgical procedure to improve the appearance of the upper eyelids, lower
eyelids, or both, restoring firmness to the area surrounding the eyes and making
one look more rested and alert.
Nose Surgery: Also known as rhinoplasty, surgery of the nose improves
the appearance and proportion of your nose, enhancing facial harmony and self
confidence. Surgery of the nose may also correct impaired breathing caused by
structural defects in the nose.
Brow Lift: If one has expression lines or other signs of aging in the
forehead and brow region which they find bothersome, a brow lift (also called a
forehead lift) may be the right choice. A brow lift is designed to correct all of
these aging features, restoring a more youthful, rested appearance with uplifted
contours and improved tone in facial skin and underlying muscle.
Facial Implants: If one would like to change the contours of their face,
they might want to consider implants. It can improve proportion and profiles and
correct imbalance caused by injury or hereditary traits. Appropriately sized and
shaped implants most commonly in the cheek, chin, jaw or nasal region will be
carefully selected and placed.
304
Injectable Fillers: If one would like to restore facial contours, or reduce
the appearance of lines and creases, injection therapy with soft tissue fillers may
be the answer.
Skin Resurfacing: If considering improving the surface and appearance
of the skin, reducing fine lines, surface irregularities such as scarring, uneven
pigmentation and sun damage, then skin resurfacing is an option.
Breast Augmentation: Also known as augmentation mammaplasty, the
procedure involves using implants to fulfill one’s desire for fuller breasts or to
restore breast volume lost after weight reduction or pregnancy. Implants also
may be used to reconstruct a breast after mastectomy or injury. Implants may
need to be replaced: It’s important to know that breast implants are not designed
to last a lifetime. A patient should plan for an annual examination by your
plastic surgeon to see if the implants need to be replaced.
Breast Lift: Also known as mastopexy, a breast lift raises and firms the
breasts by removing excess skin and tightening the surrounding tissue to reshape
and support the new breast contour. Sometimes the areola becomes enlarged
over time, and a breast lift will reduce this as well.
Gynecomastia: Enlarged male breasts, also known as gynecomastia, can
cause emotional discomfort and impair self confidence. Gynecomastia can be
surgically treated by removing excess fat, glandular tissue and/or skin. The
result is a better proportioned, more masculine-contoured upper body and the
freedom and self confidence to lead an active life.
Tummy Tuck: Also known as abdominoplasty, a tummy tuck removes
excess fat and skin, and in some cases restores weakened or separated muscles.
This creates an abdominal profile that is smoother and firmer, often enhancing
body image and confidence.
Spider Veins: Also known as sclerotherapy, this cosmetic procedure reduces or eliminates surface vessels or spider veins, commonly on the face and legs.
Liposuction: Despite good health and a reasonable level of fitness, some
people may still have a body with disproportionate contours due to localized fat
deposits. These areas may be due to family traits rather than a lack of weight
control or fitness. Liposuction slims and reshapes specific areas of the body by
removing excess fat deposits, improving body contours and proportion, and
ultimately enhancing self-image.
Choosing A Surgeon Before any patient undergoes an elective surgical
procedure, they may also want to ask their surgeon how many procedures of this
type he/she has performed. It is also important to feel comfortable around one’s
surgeon. A patient is also going to want to ask questions and be familiar with the
surgery being performed.
Clefts of the Lip and Palate
Each year approximately 227,500 or 7 percent of births in the United
States are affected by birth defects of the head and face. The most common of
these are clefts of the lip and palate which occur once in every 700 births. Clefts
occur in infants of all races with a 2:1 male to female ratio. The incidence of
305
clefts is highest in the Asian population and lowest in African Americans. Of all
orofacial clefts, 21 percent present as cleft lip only (unilateral and bilateral), 46
percent present as cleft lip and palate, while the remaining 33 percent have cleft
palate alone.
What is a cleft? A cleft is a division or separation of parts of the lip or
roof of the mouth that is formed during the early months of development of the
unborn child. All of the parts of the lip or roof of the mouth are present; they
simply failed to fuse in a normal way. Surgical intervention is necessary to align
the parts and join them. Often the bones of upper jaw (maxilla) and/or the upper
gum are affected. A cleft lip can be incomplete with a variable degree of
notching of the lip, or complete, extending through the lip and into the nose.
Clefts of the palate can vary in severity. Some may involve just the uvula
and the soft palate. These are incomplete clefts of the palate. Others extend the
length of the palate and are complete clefts. They may involve one side of the
palate (unilateral) or both sides (bilateral).
Etiology: The exact cause of lip and palatal clefting is not known, but
most experts feel that it is due to both genetic and environmental factors. Clefts
are associated with abnormalities in the genes which may be a result of
inheritance or from a spontaneous mutation during fetal development. We
recommend genetics counseling to discuss causes of the cleft and the recurrence
risk factors.
Team Assessment: Children born with clefts should be carefully assessed
by the craniofacial team in order to detect potentially serious abnormalities that
can be associated with clefting. There are over 150 syndromes that include cleft
lip or palate in their differential diagnosis. Generally, clefting is the only
congenital abnormality that the child has, but nearly 15 percent of all cleft lip or
palate patients present clinically with multiple problems.
The team concept allows a systematic, comprehensive treatment plan to
be developed and allows the team members to work together to identify
problems before they become significant. The most common specialities
involved in the care of a child with a cleft are: plastic surgery, otolaryngology,
dentistry, audiology, speech pathology, genetics and pediatrics. Once a complete
assessment of the child with a cleft has been performed, a plan for treatment can
be outlined.
Feeding an infant is important not only in providing nourishment, it also
provides an intimacy and closeness for both the parent and the child. Infants
with a cleft of the lip or of the soft palate seldom have problems with feeding
either by bottle or breast.
In babies with clefts of the hard palate, the opening in the roof of the
mouth often causes difficulty in creating adequate pressure on the nipple, thus
creating an inability to suck well enough to get adequate nourishment. Feeding
the infant takes patience and practice. At our center we recommend the use of a
soft squeezable plastic bottle like Mead Johnson with an orthodontic nipple such
as Nuk. You can increase the flow by gently squeezing or putting pressure on
the bottle. It is important to feed the infant before he/she becomes too hungry.
306
Position the infant in an upright position with the head tilted back slightly. This
position allows the milk to flow down into the throat and less into the nose. Infants with clefts do swallow more air and need to be burped more frequently. At
first, it may take extra time, but this will steadily decrease . Feeding time of the
newborn varies from 20-30 minutes. When feeding takes longer than 45 minutes, the infant may be burning up calories necessary to gain weight. If this occurs
the feeding consultant should be contacted to help with the feeding technique.
Breast feeding the newborn with a cleft of the hard palate is often
unsuccessful. Generally the infant cannot produce enough negative pressure to
obtain ample breast milk to provide adequate nourishment. Using a breast pump
to extract the milk and feeding the infant breast milk from a squeezable bottle is
recommended.
Cleft Lip Repair - The objective in repairing the lip is to close the cleft to
create a pleasing face that will develop normally with minimal scarring. Closure
of the lip is performed by the plastic surgeon when the baby is approximately 3
months of age and weighs at least 10 pounds. When there is involvement of the
alveolus and palate, an orthodontic appliance may be placed in the maxillary
segments as the first procedure. This is performed by the team dentist as an
outpatient surgical procedure. The appliance is used to align the alveolus so that
it can be repaired (gingivoperiosteoplasty) at the time of the lip repair or lip
adhesion. This improves nasal support on the cleft side and creates a tunnel that
should develop bone, closing the cleft. If the alveolus is not closed in infancy,
then the alveolar ridges will be orthodontically aligned and a bone graft
performed to stabilize the maxilla (5-10 years of age). Correction of the nasal
deformity is usually performed at the time of lip repair. Additional procedures
may be necessary to enhance the appearance of either the lip or nose.
A unilateral cleft lip results from failure of the union of the maxillary and
median nasal processes, thus creating a split or cleft in the lip on either the left
or right side. It may be just a notching of the lip or extend completely through
the lip into the nose and palate. A number of procedures have been described to
repair the unilateral cleft lip. The procedure used at our Center is the Millard
rotation advancement technique. The procedure is designed to reconstruct the
lip, muscle, oral mucosa, and to reposition the nose. It is performed under
general anesthesia with surgery lasting 2-3 hours and a hospital stay of 2-4 days.
Special considerations are necessary for feeding and positioning the infant
postoperatively. The baby's elbows are restrained from bending to prevent
him/her from disrupting the nose or lip. Positioning the child in an infant seat
keeps him/her from rolling over and injuring the lip or nose. Pacifiers and
nipples are not allowed. The baby is fed with a special syringe feeder with a soft
tube. It takes approximately 3 weeks for the wound to gain enough strength to
discontinue the above precautions. The lip scar is initially red and swollen, but it
begins to mature and improve in appearance in six-twelve months.
The bilateral cleft lip involves separation of the lip along philtral lines,
isolating the central segment (prolabium). Fifteen percent of children born with
cleft lips have bilateral clefts. The associated nasal deformity is usually more
307
severe than the unilateral cleft due to a very short columella and flaring of both
nostrils. Surgical correction of the bilateral cleft lip is usually performed in one
procedure at three months of age; however, the procedure may be staged,
closing one cleft at a time. Rotation of the nostrils to a more normal position is
performed in the first procedure. A second procedure is performed by 2-3 years
of age to lengthen the columella. Patients with complete bilateral cleft lips
frequently require additional procedures to enhance the appearance of the lip
and nose. Performed under general anesthesia, the operation generally requires
2-3 hours. A hospital stay of 2-4 days should be expected. Feeding, positioning
and elbow restraints are the same as those for repair of the unilateral cleft lip.
The objective of cleft palate surgery is to close the palate to restore
normal function to eating and drinking and to enhance the development of
normal speech.
Clefts of the palate can occur as isolated deformities or in combination
with a cleft of the lip. Cleft palates result from failure of fusion of the embryonic
facial processes resulting in a fissure through the palate. This may be complete
(extending through the hard and soft palates) or any degree of incomplete
(partial cleft). The palate forms the roof of the oral cavity and the floor of the
nose; thus, a cleft causes a free communication between these two cavities. As a
result, treatment of palatal clefts is complex because of potential problems with
feeding, speech, middle ear infections, occlusion and jaw alignment.
Surgical treatment of the cleft palate is best accomplished in one surgical
procedure before the child reaches 12-14 months of age. The cleft palate is
surgically closed by elevating two muscoperiosteal flaps. The levator muscles
are elevated, redirected and repaired; and a three layer closure of nasal mucosa,
muscle and oral mucosa accomplished. Surgery under general anesthesia usually
lasts about 2 hours. Special precautions as those after the repair of the cleft lip
are necessary for 2-3 weeks. We prefer that the child be weaned from the bottle
and pacifier prior to the palatal repair. No hard or crunchy foods are allowed for
3 weeks post operatively.
Cleft Palate Repair: Closure of cleft palate with pushback palatoplasty.
A) Two mucoperiosteal flaps are outlined. B) Flaps are elevated off the hard
palate. C,D) The abnormal levator muscle insertion to the hard palate is
identified and cut free. E) The nasal lining is closed as a separate layer and the
levator muscle reapproximated. F) The palatial mucoperiosteal flaps are closed
in a V-Y fashion.
Approximately 70-80 percent of all cleft palate patients will develop
velopharyngeal competence after palate closure and thus the potential for normal
speech. The remaining 20-30 percent will require speech therapy and/or an
additional surgical procedure called a pharyngeal flap. To correct persistent
hypernasal speech, this procedure involves raising a flap of tissue from the
posterior pharynx and inserting it into the soft palate. This flap is indicated when
the repaired palate is too short or the muscles do not function properly, causing a
persistent hypernasal speech. The procedure is performed usually after the age
308
of 4-5 when speech and velopharyngeal competence can be thoroughly assessed
and before the child begins school.
Pharyngeal Flap: The pharyngeal flap procedure for hypernasal speech. A
superiorly based flap of tissue is raised from the the posterior pharynx and
sutured to the soft palate thereby decreasing the amount of air through the nose.
Lateral ports or holes are left so that the nose will not be obstructed.
Late Cleft Treatment: The Craniofacial Center can also help those
individuals that have grown up without access to a comprehensive, coordinated
team approach. For adults with speech problems, the previously mentioned
pharyngeal flap, combined with an intensive regimen of speech therapy, can
produce significant improvements. Orthognatic surgery is available to patients
with deformities of the jaws to improve their appearance as well as to correct
dental occlusion. For soft tissue revision of a severely tightened or notched
upper lip, an Abbe flap is the surgical option. This procedure is usually indicated
in bilateral cleft patients who have a short or deficient columella and a tightened
upper lip. This operation can add fullness to the upper lip as well as lengthen the
columella. A number of additional surgical therapies, similar to the ones
described, are available to patients who desire further improvements.
Hearing: Children with cleft palate have a higher incidence of hearing
problems. The Eustachian tube connects the middle ear space to the back of the
throat. It normally opens and shuts to relieve pressure that builds up behind the
ear drum. If the Eustachian tube does not open, then the pressure increases until
mucus or "fluid" accumulates behind the eardrum. The muscles responsible for
opening the Eustachian tube do not function as well in children with cleft palates
resulting in more frequent problems with fluid, otitis media and ear infections
which can be very painful. Because of this problem, it is important to have the
infant's hearing tested during the first few months. If hearing is impaired by fluid
buildup or unequal pressure, it may be necessary for the otolaryngologist to
place pressure equalizing (PE) tubes. Tubes are often placed at the time of the
lip or palate surgery. It is crucial that children with cleft palates have regular
hearing tests to monitor middle ear problems that could alter the development of
normal hearing as well as speech. As the child grows, the frequency of ear
infections and fluid in the ears seem to decrease.
Speech: Speech development in children with cleft lip only should be
normal. The unrepaired cleft palate causes speech to sound hypernasal because
air passes through the nose while talking. Most speech sounds require the nose
to be closed off from the mouth. Cleft palate surgery usually remedies the
problem, but speech therapy is still recommended. Approximately 20-30 percent
of cleft palate patients will have velopharyngeal incompetence or hypernasal
speech after surgery, and may require a pharyngeal flap to correct it around the
age of 4-5 years.
Dental: Clefts of the palate generally have an effect on dental
development. In the area of the cleft, teeth often erupt in a crooked position with
extra teeth or missing teeth being common in the cleft area. Radiographs are
often taken to determine the exact position of the teeth. Dental problems have an
309
effect on speech, chewing, appearance and frequently require orthodontic
treatment. Early orthodontic intervention may require a palatal expansion device
with further alignment of the dental arches. Later treatment after the primary
teeth have erupted can begin at 10-12 years of age. Orthognathic surgery may be
indicated if a malocclusion develops due to abnormal growth of the maxilla.
This syndrome was described in 1923 by Pierre Robin in which he
described airway obstruction associated with glossoptosis and hypoplasia of the
mandible. Today this syndrome is characterized by retrognathia or micrognathia,
glossoptosis, and airway obstruction. An incomplete cleft of the palate is
associated with the syndrome in approximately 50% of these patients.
In patients with micrognathia (small jaw) or retrognathia, the chin is
posteriorly displaced causing the tongue to fall backward toward the posterior
pharyngeal wall. This results in obstruction of the airway on inspiration. Crying
or straining by these children can often keep the airway open. However, when
the child relaxes or falls asleep, airway obstruction occurs. Due to these
respiratory problems, feeding may become very difficult. This can lead to a
sequence of events: glossoptosis, airway obstruction, crying or straining with
increased energy expenditure and decreased oral intake. This vicious cycle of
events if untreated can led to exhaustion, cardiac failure, and ultimately death.
Treatment of this syndrome can be divided into conservative therapy
versus surgical intervention. The majority of these patients can be managed by
placing the infant in the prone position until adequate growth of the jaw occurs.
This causes the jaw and the tongue to fall forward opening the airway. If this
type of treatment fails the infant should then be considered for a tongue-lip
adhesion (a procedure to pull the tongue forward) or a tracheostomy.
In children with severe underdevelopment of the lower jaw, a new
technique called mandibular bone expansion is now available. This technique
also called distraction osteogenesis involves placement of an expansion device
that is turned daily to slowly lengthen the jaw. An external incision is required
to make a surgical cut through the jaw bone with placement of pins that are
secured to the expansion device. Once the amount of expansion of the bone has
been obtained (4-5 weeks) the device is then kept in place until the bone gap
heals with new bone formation
(8 weeks). This technique can be performed at a very early age which is a
significant advantage over the traditional technique of lower jaw lengthening.
Ear Reconstruction
Reconstruction of the ear is one of the most challenging problems facing a
reconstructive surgeon as it demands precise technique combined with artistic
creativity. Microtia is a congenital deformity of the external ear where the
auricle (the external ear) is severely deformed. There may be a spectrum of
external ear deformities with various degrees of involvement of the middle and
inner ear. This type of ear deformity is commonly seen in patients with
hemifacial microsomia and Treacher-Collins syndrome.
310
Psychological effects of an ear deformity play a significant role in timing
of reconstruction. Most surgeons prefer to initiate treatment when the patient is
between 5 and 7 years of age since this early intervention will reduce anxiety as
a result of peer pressure. This also allows for sufficient rib growth to provide the
quantity of cartilage needed by the surgeon for adequate framework fabrication.
Surgery at this time can give a more consistent result than earlier intervention
due to the fact that the child has had a chance to grow, thus making it easier for
the surgeon to balance the size and shape of the reconstructed ear to the child's
normal ear.
The treatment of microtia involves surgical reconstruction of the external
ear framework. Ear reconstruction requires a carefully planned, staged
reconstruction that involves 3-4 operative procedures. The first procedure
involves the construction of the cartilage framework for the ear. Under general
anesthesia, donor cartilage for the frame is obtained en bloc from the rib area
contralateral to the ear being reconstructed to take full advantage of the
cartilage's natural curvature. Working from pre-surgical templates that have
been drawn as well as from photographs, the surgeon then carves the cartilage
into its new shape and carefully positions the graft into position. The overlying
skin then redrapes to the newly carved cartilage framework. Subsequent
operations are required to rotate the lobule and to elevate the framework into its
final position.
It is of significance to note that if there is normal hearing in one ear,
surgery to improve hearing in the abnormal ear is not recommended. Almost
90% of all patients with microtia have only unilateral involvement and quickly
adjust to this condition following birth. Potential gains from working on the
middle ear are outweighed by the inherent risks of the surgery itself. Therefore,
middle ear surgery should be performed only on the true bilateral microtia
patient or the patient with significant hearing loss in both ears.
The traumatic amputation of an ear is another circumstance in which this
type of staged cartilage reconstruction can be effectively used. The amount of
ear loss determines the types and stages of reconstruction needed. If only a small
part of the ear is lost from trauma or tumor resection, then helical or rim advancement flaps may be used to reconstruct this portion. If larger sections are lost
then a staged reconstruction is necessary. Effective ear reconstruction is dependent upon meticulous surgical technique and careful preoperative planning.
Encephalocele
Congenital nasal encephaloceles are complex problems that are best
treated by the combined efforts of a neurosurgeon and a plastic surgeon. An
encephalocele is the herniation of the brain through a congenital or traumatic
opening in the cranium. Alterations and distortions of the surrounding facial
structures, such as deformities of the naso-orbital skeleton due to an absence or
separation of bone in the midline of the face, are complications of
encephaloceles due to their position and size. Comprehensive treatment includes
resecting the encephalocele, repairing the fibrous covering of the brain, repairing
311
any bony defects, and reconstructing a more normal soft tissue facial
appearance. With recent advances in diagnostic and surgical techniques, it is
possible to perform a thorough preoperative evaluation and to treat the lesion
with definitive one-stage reconstruction at the time of excision.
(The nasofrontal encephalocele bone defect is shown with osteotomies for
mobilization of the medical orbital walls illustrated. The orbital walls are
centrally mobilized and then stabilized and the remaining defects bone grafted.)
Orbital hypertelorism represents an increased interorbital distance and is
most commonly associated with craniofacial dysostosis (ApertХs and
CrouzonХs diseases), encephaloceles, facial clefts, and fronto-nasal dysplasias.
The treatment of moderate to severe deformities involves surgery to reduce the
interorbital distance and to correct any nasal abnormalities by way of an
intracranial surgical approach that releases the bony orbits of the eyes and
repositions them closer together. Inlay bone grafts, secured in place with
miniplate fixation, are then placed to provide structural support and to fill the
spaces left by moving the orbits. The ideal timing for this surgery is between
two and five years of age in order for the psychological trauma involved with
the deformity to be minimized while maximizing the ophthalmological benefits.
In cases where the deformity from hypertelorism is less severe, the surgery can
be performed using an extracranial approach to achieve orbital rearrangement.
However, it is generally agreed that using the intracranial technique constitutes a
more consistent and safer method of correcting the malformation.
Enophthalmos
Enophthalmos can be defined as the relative recession (backward or
downward displacement) of the globe into the bony orbit. The three basic
structures that determine globe position are the bony orbits, the ligament system
and the orbital fat. Displacement of the orbital walls with enlargement of the
bony orbit may be the major components in the production of enophthalmos in
orbital fractures. Post-traumatic enophthalmos is frequently seen and is the
result of disruption of the bony orbit and ligament system with displacement of
the orbital soft tissue. This presents clinically as a sunken appearance to the eye
with pseudoptosis and deepening of the supratarsal fold. Treatment involves
reconstruction of the bony orbit with restoration of bony orbital volume and
repositioning of the globe. The use of craniofacial techniques allows this to be
accomplished with minimal complications.
Exophthalmos
Exophthalmos is an abnormal prominence or protrusion of the eyeball,
most frequently seen in patients with Grave's disease (hyperthyroidism). As with
enophthalmos, surgical correction is frequently necessary to achieve the desired
aesthetic result. The extent of the deformity dictates the surgeon's choice of
treatment options. Although severe exophthalmos may present as a surgical
emergency in which vision is threatened, moderate exophthalmos can also be
312
distressing to the patient - the wide-eyed stare, lid retraction, and proptosis are at
best unsightly and at worst psychologically handicapping. By utilizing
craniofacial approaches and techniques, excellent aesthetic results can be safely
obtained. Mild to moderate cases are repaired by removing the floor and lateral
wall of the orbit to allow for tissue decompression (removing compressive
pressure on the eyeball itself), while severe cases necessitate a more aggressive
approach including multi-wall (lateral, medial, and inferior) orbital osteotomies.
This functionally enlarges the bony orbit, allowing the globe to assume a more
normal posterior position.
The surgical technique of facial bipartition involves vertical splitting or
separation of the facial skeleton into two segments. The procedure has been used
to successfully correct hypertelorism with widening and leveling of the lower
maxilla. The facial bipartition concept can also be designed to provide a facial
advancement in addition to enlargement of the maxilla and medial rotation of
the orbits. This procedure offers new possibilities and can be applied to correct a
number of deformities in various syndromes in a one-stage procedure. With
appropriate indications and careful technique, this procedure can produce
dramatic results.
Monobloc Advancement
Infants born with faciocraniosynostosis may have severe airway problems,
increased intracranial pressure, vision-threatening proptosis and a failure to
thrive. Such life-threatening problems may be treated with a one-stage
procedure known as a monobloc advancement. This single phase operation
releases the stenosis and advances the forehead and facial bones en bloc to a
more anterior position. This has the goal of establishing normal function and
appearance as early as possible. We feel this procedure has increased risks of
infection due to the likely communication between the nasal and intracranial
cavities and therefore use it cautiously for such indications as airway
compromise or vision-threatening proptosis.
Nasal Reconstruction
A properly proportioned, well placed nose can have a dramatic impact on
one's facial appearance. Nasal surgery involves a wide spectrum of procedures
ranging from cosmetic rhinoplasty to total nasal reconstruction. Basic
rhinoplasty surgery involves the correction or reshaping of existing nasal
structures, whereas more extensive cases will require that the craniofacial
surgeon actually construct a part of the nose that may be missing or badly
misshapened due to disease or trauma. Meticulous attention to detail when
repairing or reconstructing the nasal lining, skeletal support, or skin covering is
essential to obtain a structure that is fully functional as well as pleasing to the
eye.
When large defects of the nose are present from tumor resection or
trauma, flap tissue provides the best aesthetic coverage. The most common flap
used for major nasal reconstruction is the forehead flap. This reliable flap can
313
supply a large area of skin with good color match making it suitable for partial
or total nasal reconstruction.
Reconstruction of the nasal skeletal framework is frequently necessary in
patients with congenital or traumatic deformities. This support is best obtained
using bone or cartilage. Outer table calvarial bone grafts harvested from the
parietal area of the skull make excellent cantilever bone struts for support of the
nasal dorsum. These grafts can be rigidly fixed with lag screws to provide good
stability and dorsal contour. Additional techniques are available to provide nasal
support such as the L-shaped grafts and columella struts. The tip of the nose is
best supported with cartilage grafts. Careful attention to detail and planning is
necessary in these procedures to create a structure that is both functional and
aesthetically pleasing.
Orthognathic Surgery
Orthognathic surgery refers to the surgical repositioning of the maxilla,
mandible, and the dentoalveolar segments to achieve facial and occlusal balance.
One or more segments of the jaw(s) can be simultaneously repositioned to treat
various types of malocclusions and jaw deformities.
Preoperative diagnosis and planning for patients with jaw asymmetries
and deformities includes a photographic analysis and a complete orthognathic
work-up involving cephalometric and panorex radiographs, dental impressions,
and models. This is done by the Pedodontist/Orthodontist in coordination with
the craniofacial surgeon. All findings are analyzed and pre-surgical model
surgery performed to ascertain the feasibility of various treatment options.
Additionally, computer analysis is done pre-surgically by the craniofacial
surgeon to simulate surgical results, thereby facilitating proper planning of the
case. Computer analysis provides the craniofacial team with visual information
and numerical data that is a compilation of many time-consuming calculations
such as those used in various cephalometric analyses (Steiner, Ricketts, or
Jarabak-Bjork).
Usually, pre-surgical orthodontics are necessary to straighten the teeth and
align the arches so that a stable occlusion can be obtained post-operatively,
while orthodontics following surgery are frequently required to revise minor
occlusal discrepancies. Orthognathic surgery is often delayed until after all of
the permanent teeth have erupted unless medical conditions necessitate that the
surgery be performed earlier. In adult patients, orthognathic surgery can be
combined with soft tissue contouring to improve the aesthetic results.
Maxillary advancement is a type of orthognathic surgery that may be
necessary to improve the facial contour and normalize dental occlusion when
there is a relative deficiency of the midface region. This is done by surgically
moving the maxilla with sophisticated bone mobilization techniques and fixing
it securely into place. For most patients, the use of screws and miniplates have
replaced wiring of the bone and teeth required to hold the jaw stable. Inlay bone
grafts can be utilized for space maintenance and secured with screw and plate
314
fixation, while onlay bone grafting is used to augment the bony skeleton and
improve facial soft tissue contour.
Depending on the soft tissue profile of the face or the severity of an
occlusal discrepancy, problems with the lower face may require surgery on the
mandible. This can be done in conjunction with or separate from maxillary
surgery. The mandible can be advanced, set back, tilted or augmented with bone
grafts. A combination of these procedures may be necessary. Pre-operative
planning is crucial to the success of the procedure and evaluates the surgical and
orthodontic options. The surgeon chooses the type of mandibular surgery based
on his experience, evaluation of the photographic and cephalometric analysis,
and model surgery. Following any significant surgical movement of the
mandible, fixation may be accomplished with miniplates and screws or with a
combination of interosseous wires and intermaxillary fixation (IMF). Rigid
fixation (screws and plates) has the advantage of needing limited or no IMF.
However, if interosseous wiring is used, IMF is maintained for approximately
six weeks. Nutritionally balanced, blenderized diets are important for proper
healing in the patient in IMF.
The chin is an important component of the facial profile as well as the
aesthetic balance. The position and projection of the chin should be evaluated in
patients considering orthognathic and facial soft tissue contouring procedures.
Photographic and cephalometric analysis help determine the amount of change
necessary to obtain a well balanced face. The chin can be augmented with such
alloplastic materials as silicone, polyethylene or hydroxyapatite. However, most
craniofacial surgeons prefer a sliding horizontal osteotomy genioplasty. This
procedure tends to give a more natural contour to the chin and avoids the risk of
extrusion that goes along with alloplastic implants.
Craniomaxillofacial Trauma
Millions of people sustain trauma to the head and face resulting in complex
fractures which, if not correctly diagnosed and treated, may cause permanent
functional and cosmetic deformities. During the past decade, advances in
radiographic procedures, the utilization of craniofacial surgical techniques, and
the advent of rigid miniplate fixation have tremendously improved the
functional and aesthetic results in facial fracture management.
The accurate diagnosis of facial fractures has been greatly improved by
the addition of two- and three-dimensional CT scans which have replaced the
plain radiographs for the diagnosis of many types of fractures. The threedimensional reconstructions have enhanced preoperative bone analysis and
planning by providing a life-like simulation of the fractures.
In acute trauma cases, the goal of reconstruction is a one-stage repair
which has been made possible by the application of craniofacial techniques.
Delayed treatment has been replaced by early or immediate surgical treatment
and stabilization of small bone fragments augmented by bone grafts and
miniplate fixation. These recent advances have allowed surgeons to approach
and often reach the goal of restoring preinjury facial appearance and function
while at the same time minimizing revisional surgery.
315
Without treatment in a timely manner, many individuals will develop
future problems, the severity and consequences of which can be much greater
than if the injury had been immediately repaired. However, modern craniofacial
surgical techniques can now offer hope for patients with pre-existing posttraumatic facial deformities despite considerable delays between injury,
diagnosis, and treatment. These innovative techniques establish a higher
standard of care for the management of facial injuries.
Test questions.
1. What is the name of disease shown clinically in localized change of color, a
softening of hard substancies of tooth and formation of defect:
A. Caries
B. Pulpitis
C. Periodontitis
D. Hypopasia of enamels
E. Teeth of the artist
2. With increase of the contents of fluoride in drinking water up to 2 mg/l it is
observed defeats of a teeth:
A. Caries
B. Pulpitis
C. Fluorosis
D. Marginal Periodontites
E. Apical Periodontitis
3. At initial caries it is observed:
A. A cavity of a chewing surface of tooth
B. Chalklike spot without defect of a tooth
C. Chalklike spot with defect of a tooth
D. A cavity up to enamelo-dentine boundary
E. Changes are not present
4. The permanent dentition of upper and lower jaws consists of
A. 20 teeth
B. 16 teeth
C. 32 teeth
D. 16-24 teeth
E. 12-20 teeth
5. At chronic granolomatous periodontitis on the roentgenogram it is observed:
A. The center of destruction in alveolar process
B. Destruction of bony tissue in the area of periodontium in the form of a flame
C. Resorption of part of root of the tooth
316
D. Fibrous degeneration
E. Center of destruction in the area of root apex of rounded shape
6. Differential diagnostics of acute pulpitis is with....
A. Caries
B. Periodontitis
C. Neuralgia of trigeminal nerve
D. Apical periodontitis
E. Fluorosis
7. The firm formation covering the surface of tooth, mainly its neck, and not
removing by a tooth-brush refers to....
A. Dental plaque
B. Dental calculosis
C. Plaque from coffee
D Fluorosis
E. Wedgeshape defects
8. The inflammatory process arising in tissues, surrounding a root of tooth,
refers to apical....
A. Pulpitis
B. Caries
C. Periodontitis
D. Stomatitis
E. Wedgeshape defect
9. The differential diagnosis of caries of stage of a spot should be done with...
A. Hyperasthesia
B. Partial pulpitis
C. Wedgeshape defect
D. Hypoplasia and fluorosis
E. Attriction of enamel
10. The acute apical periodontitis develops more often as a result of
complication of
A. Deep caries
B. Generalized periodontitis
C. Acute purulent pulpitis
D. Hyperasthesia
E Insufficiency of fluorde in drinking water
11. Resorption of alveolar process is a characteristic radiological symptom of:
A. Stomatitis
B. Caries
C. Marginal periodontitis
317
D. Apical periodontitis
E. Pulpitis
12. Characteristic clinical symptoms of acute pulpitis
A. Permanent shoot pain
B. Shoot spontaneous pain, amplifying at night
C. A pain during eating meal
D. A constantly aching pain
E. Feeling of the growing tooth
13. Treatment of chronic granulomatous periodontitis of intact tooth is…
A. Extraction
B. Resection of apex of a root
C. Replantation of teeth
D. Resection of apex of a root, Retrosealing of the canal
E. Transplantation of a tooth
14. Treatment of chronic granulating periodontitis:
A. Resection of apex of a root
B. Extraction of a tooth
C. Hemisection of tooth
D. Sealing
E. Medicamentous processing of cavity of a tooth
15. For what disease it is characteristic mobility of some teeth:
A. Odontogenic phlegmon
B. Acute odontogenic periostitis
C. Acute odontogenic osteomyelitis
D. Sharp apical periodontitis
E. Adenophlegmon
16. What is the treatment of acute odontogenic osteomyelitis?
A. Resection of pathological changes of bony tissue
B. Conservative treatment
C. Osteoperforations and intraosseous lavage
D. Extraction of a "causal" tooth
E. Osteoperforations and intraosseous lavage, decortication, extraction of a
"causal" tooth
17. At the patient bilateral exophthalmos, edema and hyperemia of skin of
cheeks, forehead, chemosis of conjunctiva, mydriasis, headache, rise in
temperature of a body. The given clinical picture is characteristic for:
A. Phlegmons of an orbit
B. Mediastinitis
318
C. Iridocyclitis
D. Thrombosis of cavernous sinus
E. Thrombophlebitis of angular vein
18. Acute odontogenic osteomyelitis is necessary to differentiate from...
A. Odontoma
B. Cementoma
C. Odontogenic phlegmon of massetericoparotid region
D. Acute pericoronitis
E. Acute periostitis
19. Volume of the surgical help at acute purulent periostitis:
A. Extraoral incision and drainage of soft tissues
B. Incision of mucous membrane of gum at causal tooth
C. Extraction of "causal tooth ” and periostotomy on transitive fold
D. Puncture of periosteal abscess
E. Resection of apex of root of "causal tooth ”
20. Moreoften the hard chancre is localized on:
A. Cheeks
B. Nose
C. Lobule of auricle
D. Tongue
E. Red margin of lips
21. The main symptom of ankylosis of TMJ is:
A. Disorder of swallowing
B. Numerous caries
C. Severe pain symptom
D. Noise in ears
E. Limit of mobility of the mandible
22. Method of prevention of intracranial complications at thrombophlebitis of
angular vein of the face is:
A. Prescription of direct anticoagulants
B. Phlebotomy
C. Prescription of indirect anticoagulants
D. Electrophoresis of 10 % iodine of kalium
E. Revision of the primary focus of infection
23. Pain at swallowing are observed at phlegmon of:
A. Pterygopalatine fossa
B. Parapharyngeal spaces
C. Buccal region
319
D. Mental region
E. Massetericoparotid region
24. Which nerve is blocked in infraorbital anesthesia?
A.
Nasopalatine n.
B.
Greater palatine n.
C. Posterior superior alveolar n.
D. Anterior and middle superior alveolar nn.
E. Inferior alveolar n.
25. Hard and soft fibroma is more often located on the mucous membrane of:
A. Cheeks
B. Lips
C. Floor of the mouth
D. Muscular tissue
E. Tongue
26. What is the local complication appearing after extraction of upper molar
tooth, in case of which the patient feels the air passing the extracted tooth
socket?
A. Osteomyelitis
B. Perforation of maxillary sinus
C. Phlebitis
D. Periostitis
E. Mediasinitis
27. What is the surgical method of treatment of acute purulent pericoronitis
A. Curettage of socket
B. Resection of root apex
C. Radical sinusotomy
D. Resection of hood (gum over erupting tooth)
E. Extraction of neighbouring teeth
28. Location of trigger areas in neuralgia of 2nd branch of trigeminal nerve is on
the:
A. Region of chin
B. Lower lip
C. Temporal region
D. Infraorbital region
E. It is absent
29. What is the most favourable period for primary surgical debriment of wound
of face?
A. 1st period - 48 hours after injury
B. 2nd period – from 3rd day after injury
320
C. 3rd period – granulation of wound
D. 4th period – epithelization and scar formation
E. Period of 1st 50 minuts
30. Synonym of Le Fort 2 fracture is:
A. Fracture of nasal bones
B. Fracture of zygomatic bone
C. Subbasal fracture
D. Pyramidal suborbital fracture
E. Fracture of alveolar process
Test keys
№
1
2
3
4
5
6
7
8
9
10
Correct
answer
A
C
B
C
E
C
B
C
D
C
№
11
12
13
14
15
16
17
18
19
20
Correct
answer
C
B
D
B
C
E
D
E
C
E
№
21
22
23
24
25
26
27
28
29
30
Correct
answer
E
B
B
D
A
B
D
D
A
D
321
List of abbreviations
ABCDE - airway, breathing, circulation, disability, exposure
AIDS – acquired immunodeficiency syndrome
ANF - antinuclear factor
ANUG - acute necrotizing ulcerative gingivitis
ASA - anterior superior alveolar
ASIF - Association for the Study of Internal Fixation
ATLS - American Trauma Life Support
BIPP - bismuth-iodoform-paraffin paste
CA-MRSA - community-acquired Methicillin-resistant Staphylococcus aureus
CGRP - calcitonin gene-related peptide
CMC - Chronic mucocutaneous candidosis
CMV – cytomegalovirus
COX-2 - Cyclo-oxygenase-2
CSF – cerebrospinal fluid
CT scan – computed tomography scanning
DNA – desoxyribonucleic acid
DPT - dental panoramic tomography
ECG – Electrocardiography
E coli - Escherichia coli
ESR - erythrocyte sedimentation rate
FDA - Food and Drug Administration
FMF - Familial Mediterranean fever
FOM – floor of oral mucosa
FTA-ABS - fluorescent treponemal antibody absorption
GA – General anesthesia
GFR – gnathofacial region
GeN - Geniculate neuralgia
GPO - Gross Periostitis Ossificans
GSE - gluten-sensitive enteropathy
HHV - human herpes virus
HIDS - hyperimmunoglobulinemia D and periodic fever syndrome
HIV – human immunodeficiency virus
HLA - human leukocyte antigen
HSV - herpes simplex virus
IBD - inflammatory bowel disease
IMF – Intermaxillary fixation
LCDCP - low-contact dynamic compression plate
MDR-TB – multidrug - resistant tubercle bacillus
MMF - maxillomandibular fixation
MRI – magnetic resonance imaging
MS - multiple sclerosis
MSA - middle superior alveolar
MVAs – motor vehicle accidents
MVD- Microvascular decompression
322
NICE - National Institute for Clinical Excellence
NK cells - natural-killer cells
NSAID's - non steroidal anti inflammatory drugs
OA – Osteoarthritis
OAF - oroantral fistula
OKC - odontogenic keratocyst
O.p - odontogenic periostitis
ORIF - open reduction and internal fixation
PAS - p-aminosalicylic acid
PBCTN - percutaneous balloon compression of the trigeminal nerve
PCR - polymerase chain reaction
PHN - Postherpetic neuralgia
PO - Periostitis ossificans
PSA - posterior superior alveolar
PSRTR - percutaneous stereotactic radiofrequency thermal rhizotomy
RAU - Recurrent aphthous ulcers
RBC – red blood cells
S aureus - Staphylococcus aureus
SAPHO - Synovitis, Acne, Pustulosis, Hyperkeratosis and Ostitis
SLE - Systemic lupus erythematosus
S.O.D - superoxide dismutase
TENS - Transcutaneous electrical nerve stimulation
TMJ - temporomandibular joint
TN - trigeminal neuralgia
TNF-alpha – tumor necrosis factor-alpha
TPHA - T pallidum hemagglutination
TPPA - T pallidum particle agglutination
TRAPS - TNF-alpha receptor associated periodic fever syndrome
VZV - varicella-zoster virus
WBC – white blood cells
ZMC – zygomaticomaxillary complex
323
Bibliography
1. Irene R.Woodall, Bonnie R.Dafoe, Nancy Stutsman Young [et.al].
Comprehensive Denlal Hygiene Care. – 3rd Ed. – St.Louis: The
C.V.Mosby Company, 1989. – 809p.
2. Bailey H.Demonstrations of physical signs in clinical surgery. –
12ed.– Baltimore: The Williams and Wilkins Company, 1954. – 456p.
3. Bailey & Love’s short practice of surgery/ Edited by R.S.G.Russell,
N.S.Williams, C.J.K.Bulstrode.- 23rd ed. – London: Arnold. V.1. –
2003 – 1348p.
4. Shenoy K.R. Manipal manual of surgery/K.R.Shenoy. – 2nd ed. – New
Delhi, Bangalore: CBS publishers and distributors, 2005. – 828p.
5. Principles of surgery: 2V./Editor-in-Chief S.I.Schwartz. – 7 ed. – New
York: Mc Graw – Hill, 1999. – V.1. – 1326 p, V2. – 947p.
6. Susan Standring, Gray’s Anatomy 39 Ed.
7. Paul Coulthard, Keith Horner, Philip Sloan, Elizabeth D. Theaker
Master Dentistry. Oral and Maxillofacial Surgery,Radiology, Pathology
and Oral Medicine. – 1st ed. – Edinburgh: Churchill Livingstone, 2003.
V.1. – 267p.
8. Robert W.Dolan, Facial Dentistry. Facial Plastic Reconstructive and
Trauma Surgery.
9. Робустова Т.Ф. Хирургическая стоматология, Москва 2005.
324
Отпечатано в типографии КГМА
г. Караганда, ул. Гоголя,40
Объем 20.3 уч. - печ. л.
Тираж 100 экз.
325
326