* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Anticoagulation Therapy in the CICU
Survey
Document related concepts
Remote ischemic conditioning wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Saturated fat and cardiovascular disease wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Cardiovascular disease wikipedia , lookup
Cardiac surgery wikipedia , lookup
Drug-eluting stent wikipedia , lookup
Antihypertensive drug wikipedia , lookup
Jatene procedure wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
History of invasive and interventional cardiology wikipedia , lookup
Coronary artery disease wikipedia , lookup
Discovery and development of direct thrombin inhibitors wikipedia , lookup
Transcript
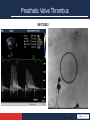
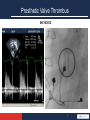
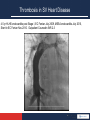
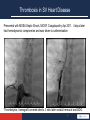
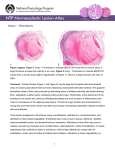
Anticoagulation Therapy in the CICU: What, When, Why? Therese M. Giglia, MD December 14, 2014 Propensity to Coagulopathy Virchow’s triad: factors that predispose to thrombosis (1856) Anesth Analg 2012;114:275–85 Propensity to Coagulopathy CPB and Mechanical Support Inflammation and Blood Stream Infection Thrombosis in Pediatric and Congenital Heart Disease Fibrinolysis Coagulation Propensity to Coagulopathy Special Concerns in Cyanotic Heart Disease: • More than stroke and pulmonary embolus • Potentially lethal complications of – MBTS occlusion – Fontan obstruction • Loss of arterial and/or venous access for necessary diagnostic and therapeutic cardiac catheterizations • Loss of major veins may make bidirectional Glenn or Fontan impossible Scope of the Problem • Overall incidence and prevalence unclear • Associated with high mortality and morbidity • Involves many areas of pediatric and congenital HD – Most Studied: KD, post-cardiac catheterization, ECMO – Recommendations extrapolated from adult guidelines: prosthetic valves, arrhythmias, cardiomyopathy, pulmonary embolus, pulmonary hypertension, VADs, stroke – Of significant clinical concern but with a paucity of data: early postoperative thrombosis, single ventricle population, infant prosthetic valves, VADs in children, stroke in children with heart disease Anticoagulation in the CICU: what, when why? Overview 1. What is available to prevent and treat thrombosis 2. When and Why (Monagle 2012, Giglia 2013, McCrindle 2014) • • • • • Early postoperative thrombosis Single ventricle population VADs (Moffett 2014) Valves (Nishimura 2014, Giglia 2012) Stroke (Roach 2008, Sinclair 2014) 3. CHOP Initiative on Thrombosis Prevention and Treatment Anticoagulation in the CICU: what, when why? What is available to prevent and treat thrombosis: Pharmacotherapy: • Anticoagulants • Antiplatelet agents • Thrombolytics Anticoagulation in the CICU: what, when why? What is available to prevent and treat thrombosis: 1. Pharmacotherapy 2. Avoidance of risk factors 3. Suspicion and surveillance for new thrombosis 4. Collaboration and communication re thrombosis Rx (CICU, cardiology, surgery, hematology, nursing, pharmacy) 5. Plan for and follow-up of each thrombotic event Anticoagulation in the CICU: what, when why? Pharmacotherapy Anticoagulants: fibrin, venous clots • UFH – IV, short half-life (1-2 hrs), binds to AT and potentiates AT’s anticoagulate effect 1000X, challenge in neonates who have low AT, interacts with other plasma proteins high inter and intrapatient dose response, full reversal with protamine, osteoporosis in adults • LMWH – subQ, longer half-life 3-6 hrs, neonates and infants require higher doses secondary to increased volume of distribution and increased clearance and decreased AT, cleared by kidneys, 70% reversed by protamine, less osteoporosis in adults, most widely studies anticoagulant in children Anticoagulation in the CICU: what, when why? Pharmacotherapy Anticoagulants • Fondaparinux – subQ, synthetic analog, longer half life (17hrs), no reversal agent, renal clearance, reports of successful off-label use in HIT • Warfarin – oral, inhibits regeneration of Vit K needed for the synthesis of clotting factors II, V, VII, X, C, S, Z, dosing difficult in infants; genetic polymorphisms (metabolism, sensitivity), interaction with many drugs, sensitivity to dietary Vit K, reversal with Vit K, initial procoagulant effect • Direct Thrombin Inhibitors (Bivalirudin Argatroban Lepirudin) – IV, direct/specific/reversible inhibitors of thrombin, half-life 25-60 min, excreted by liver (bivalirudin/argatroban), kidney (lepirudin), antibodies/anaphylaxis reported, approved in adults for HIT and coronary thrombosis, trials in MCS Anticoagulation in the CICU: what, when why? Novel Oral Anticoagulants • Rivaroxaban, Apixiban: direct Factor Xa inhibitors • Dabigitran: direct thrombin inhibitor • compared to warfarin; • wide therapeutic window • limited drug-drug interactions • rapid onset • shorter half-life • routine monitoring not necessary in adults (unclear in children) • no reversal agents (dabigitran partially removed with dialysis) • approved in adults for stroke prevention from atrial fibrillation, treatment of acute DVT, secondary prevention of DVT post elective knee and hip surgery. PE (Apixiban). Not approved for mechanical valves. Ongoing pediatric trials. (www.clinicaltrials.gov), Anticoagulation in the CICU: what, when why? Pharmacotherapy Antiplatelet Agents: (plaque, arterial clots) • Aspirin oral/rectal, irreversibly inhibits platelet aggregation, hepatically metabolized/ renally excreted, no reversal agents, plt aggregation panels/mapping techniques available, usefulness in children not established, ASA resistance reported, 7 days needed for full plt regeneration after DC • Clopidogrel – oral, prevents platelet activation and therefore aggregation, plt aggregation assays (TEG/PM, VerifyNow, Plateworks) available but not validated in children. • Dipyridamole – oral/IV, plt inhibition via cAMP, PGE2 release causes vasodilation (coronary), hepatically metabolized and excreted in feces • Abcximab IV, binds to platelet IIb/IIIa receptors preventing aggregation Anticoagulation in the CICU: what, when why? Pharmacotherapy Thrombolytic Agents: • Tissue-type plasminogen activator (t-PA) – biosynthetic (recombinant DNA origin), only agents available in US alteplase (TPA), recteplase (RPA) and tenecteplase (TNK), initiates local fibrinolysis by binding to fibrin converting entrapped plasminogen to plasmin that intern degrades fibrinogen, usually used concomitantly with low-dose UFH, may require plasminogen supplementation, not reversible but short half-life, pharmacokinetics not established in infants and children/but long experience Several modes of delivery: • Low dose systemic • High dose systemic • Catheter directed • Pharmacomechanical Thrombolysis (Angiojet, Trellis device) Prosthetic Valve Thrombus 08/17/2012 Prosthetic Valve Thrombus 08/19/2012 Anticoagulation in the CICU: what, when why? Early Postoperative Thrombosis: Population age Incidence Associations Comments Giglia 2001 pediatric 3.6% (1930 operations) Age < 1y, CPB Median age 2.6m, medial time from OR 3 w, CL-DVT, SV Manlhiot 2011 pediatric 11% (1542 operations) <31d, sat<85%, prev thrombus, TP, DCA, ECMO, more days w CVL Thrombus assoc w longer LOS, cardiac arrest, reintervention, mortality Hanson* 2012 *VTE only pediatric 3.8% (1070 operations) SV physiology, cyanosis, more days w CVL 37% assoc w CVL, 66% receiving anticoagulation at dx Emani 2014 neonates 20% (100 neonates) SV physiology, higher mortality (15% cw 0%), preop hypercoag All received UFH 10U/kg/h X4d Anticoagulation in the CICU: what, when why? Early Postoperative Thrombosis Should we anticoagulate prophylactically? • PROTEKT trial 2003: no benefit to LMWH in preventing CVL-DVT in general pediatric population (CHD in 23%LMWH/19% std care) • Schroeder 2010: uFH (10U/kg/hr) not protective against CVL-DVT infants after cardiac surgery What can we do? • Recognize the potential triggers of thrombosis • Minimize these risk factors whenever possible • Recognize the signs of perioperative thrombosis. Be suspicious in high-risk patients and proceed to imaging studies as clinically indicated • Treat arterial, venous and intracardiac thrombi as per guidelines • Follow-up plan for each thrombotic event (inpatient and outpatient) • No data for overall prophylaxis. Define high risk groups. Thrombosis in SV Heart Disease 4.5 yr HLHS endocarditis post Stage I, EC Fontan July 2009, MSSA endocarditis July 2010, Stent in EC Fontan Nov 2010. Outpatient Coumadin INR 2-3 Thrombosis in SV Heart Disease Presented with MSSA Septic Shock, MOSF, Coagulopathy Apr 2011. 4 days later had hemodynamic compromise and was taken to catheterization Thrombolysis, homograft covered stents, 3 wks later conduit removal and BDG Anticoagulation in the CICU: what, when why? Single Ventricle Heart Disease: • BTS occlusion post stage I and thrombosis post Fontan well described • Coagulation abnormalities documented in HLHS across all three stages (Odegard 2002, 2009) • Manlhiot 2012-cross-sectional analysis across 3 stages – Children at each stage are at increased risk – Combined over all three stages, 51% of survivors had one or more thrombotic events – Early post-operative peak in thrombosis after each stage followed by lower but constant risk • Second peak in patients after the Fontan (5-15 yrs) Thrombosis in SV Heart Disease enoxaparin Stage I 40% incidence TE Enox assoc w dec risk BDG 28% incidence TE Enox assoc w dec risk Fontan 79% 5-yr freedom from TE 94% on Rx , 77% warfarin Warfarin assoc w dec risk Manlhiot 2012 Anticoagulation in the CICU: what, when why? • • • • • Prevention and Treatment of Thrombosis in Pediatric and Congenital Heart Disease A Scientific Statement From the American Heart Association (Giglia 2013) Aspirin is recommended therapy for the prevention of long-term shunt thrombosis in infants and children (Class I; Level of Evidence B). Antiplatelet therapy may be reasonable in infants and children after a superior cavopulmonary anastomosis (Class IIb; Level of Evidence C). Antiplatelet therapy is reasonable after the Fontan procedure (Class IIa; Level of Evidence C). Warfarin or LMWH may be reasonable for 3 to 12 months after the Fontan procedure (Class IIb; Level of Evidence C). Warfarin may be reasonable after the Fontan procedure for patients with anatomic or hemodynamic risk factors (Class IIb; Level of Evidence C). – flow stasis, ventricular dysfunction, arrhythmias, bilateral bidirectional cavopulmonary anastomoses, hypoplastic cardiac chambers with flow stasis, presence of a blind ended pulmonary artery stump, Kawashima connection, history of previous thrombosis, proteinlosing enteropathy, prolonged pleural effusions, and prolonged immobilization. Anticoagulation in the CICU: what, when why? • Risk may change over time necessitating repeated clinical screening (Kaulitz 2005) • An increase in the magnitude of antithrombotic therapy may be warranted if anatomic and/or hemodynamic risk factors become present. • Certain patients appear to be “clotters” and their propensity for thrombosis may warrant a higher level of surveillance and possibly a higher level of prophylaxis • Understanding of risk factors, patients at particular individual risk (“clotters”), alternative agents, as well as alternative management strategies are essential. Chair’s Initiative on Thrombosis Prevention and Treatment • Multidisciplinary (Card, Heme, Surg, Nursing, Pharmacy) initiative to define the incidence and complications of thrombosis in cardiac patients (CICU, CCU) and to improve both inpatient and outpatient anticoagulation monitoring and thrombosis therapy • Dedicated NP • Weekly Cardiac Thrombosis Rounds • Red Cap database to track all inpatient thrombi using PC4 for denominator data • Pathways for femoral arterial clots post cath (Glatz 2014) and acute catheter-related VTE • Pilot initiative using Epic to optimize management of anticoagulation of cardiac center outpatients Anticoagulation in the CICU: what, when why? Prevent, Find, Treat, Follow the Clots Prosthetic Valve Thrombus M C-L 7 yr SLL, DORV, VSD, PS, Ebstein’s anom TV • 26 w gestation • BTS (10/2006) • Senning/Rastelli (LV to Ao, homograft RV to PAs) 10/2008 – Chylothorax, TD ligation • Subaortic resection >> CHB>>>pacemaker – Arrest during cath>>>ECMO – VSD closure w Amplatzer on ECMO – MVR 21 mm St Jude • MV thrombus 2009 to OR in extremis for thrombectomy • 6/30/2012 heparin bridge for PM revision – 5/31/2012 MV mean gradient 5 mm Hg