* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download HYBRID CARDIOVASCULAR SIMULATOR PERFORMANCE
Heart failure wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Saturated fat and cardiovascular disease wikipedia , lookup
Myocardial infarction wikipedia , lookup
Aortic stenosis wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Artificial heart valve wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Cardiovascular disease wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
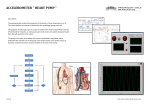
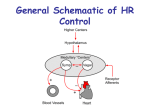
HYBRID CARDIOVASCULAR SIMULATOR PERFORMANCE EVALUATION Jeison Fonseca1,2,3, Bruno Utiyama1,4, Aron Andrade1,3, Beatriz Uebelhart1,4, Juliana Leme1, Cibele Silva1, José F. Biscegli1, Julio Lucchi2,3 1 2 Department of Bioengineering, Institute Dante Pazzanese of Cardiology, Sao Paulo (SP), Brazil Department of Electronic and Computer Engineering, Technological Institute of Aeronautics, Sao Jose dos Campos (SP), Brazil 3 Department of Electrical Engineering, University São Judas Tadeu, Sao Paulo (SP), Brazil 4 Department of Mechanical Engineering, University of Campinas, Campinas (SP), Brazil E-mail: [email protected] Background: A mock circulatory system (MCS) is an equipment capable to simulate the cardiovascular system. MCS main goal is to perform simulation of physiological circulatory conditions and to minimize number of “in vivo” animal tests. Hybrid Cardiovascular Simulator (HCS) is a system composed by two sections: 1) numeric section: comprising seven compartments – right heart, lungs, head, arms, trunk, legs and vena cava; 2) physical section: a hydraulic apparatus consisting of an electromechanical pump – as left heart, compliance chamber - simulating arteries and a proportional hydraulic valve – simulating Systemic Vascular Resistance (SVR). Purpose: This paper presents a performance evaluation of our HCS in mimicking different human circulatory conditions. Methods: LabVIEW software is used in the numerical section, for physical section control and for parameters visualization. An acquisition board is connected to physical section as interface between the computer and hydraulic system. Baroreflex is simulated by changing left pump frequency and SVR. HCS evaluation was made with physiological and pathological conditions, and also, pathological condition with Left Ventricular Assist Device (LVAD). Results: The results demonstrate HCS ability to reproduce different circulatory conditions and their response to LVAD actuation. Keywords: Mock Circulatory System, Hybrid Cardiovascular Simulator, Left Ventricular Assist Device, LabVIEW. 1. INTRODUCTION Cardiovascular simulators development has grown mainly because of the necessity to decrease the number of in vivo tests to evaluate ventricular assist devices (VADs) (Patel, 2003). Simulators of the cardiovascular system are not intentioned to replace in vivo tests, because in these experiments, some physiological conditions are evaluated which will not be possible in the simulation environment. However, the use of cardiovascular simulation in a controlled environment with a reliable simulation tool, turn possible that many variables can be observed and controlled. Also, the execution of some tests is easier, and sometimes possible, to be accomplished than an in vivo test. In the literature, there are many examples of cardiovascular computer simulators which are mathematical or electrical analog models of the cardiovascular system (Avanzolini, 1991). The goal of a computer cardiovascular simulator, modeled by electrical analog is to evaluate VAD performance where it is possible to change cardiovascular parameters such resistances, compliances and elastances in order to observe the support provided by VADs in these situations. Numeric simulators are low cost, have high flexibility to allow modifications, and also are possible to evaluate various physiological signals. Although these simulators show reliable and flexible methods to reproduce the cardiovascular system, in order to evaluate VADs is necessary to model the device to be studied. The use of VAD model interacting to computer cardiovascular model, give us an approximation of real entire system behavior. Although, is much better if rather than use a VAD model, we could connect a real device directly to the simulator. On the other hand, physical simulators has advantage in connecting real devices under evaluation, however if system modifications are necessary, sometimes it needs to be entirely disassembled. Also, it demands high cost (Felipini, 2008), (Legendre, 2008). In this context we assembled a Hybrid Cardiovascular Simulator, which combines the positive characteristics of numeric and physical simulators (Fonseca, 2010). HCS provides a flexible system where it is possible to connect a LVAD evaluating its control and performance in different cannulation sites. HCS is composed by two sections, Fig. 1: 1) physical section: left ventricle (LV), aorta compliance, systemic vascular resistance; 2) numeric model section concentrates lumped parameters of right heart, pulmonary circulation and systemic circulation. Also, in numeric section the coronary circulation and brachial shunt is modeled. Figure 1. Hybrid Cardiovascular Simulator diagram. 2. MATERIALS AND METHODS 2.1 Description of the assembled HCS Figure 2 shows the assembled system. A brushless direct current motor was used to pump the fluid. A mechanism composed of a roller screw and a pusher plate changes the rotational movement of the rotor to linear movement of screw. Inside the pumping compartment there is a mechanical valve prosthesis to drive the flow. A polyurethane diaphragm is used like an interface between the pusher plate and the fluid. This electromechanical set is driven by a computer through an electronic controller. From the reservoir, modeling the left atria passively, the fluid passes to a pumping chamber through a mechanical valve (like the mitral valve).The chamber is passively filled by a diaphragm displacement. Pumping phase occurs depending on synchronism signal provided by computer through electronic interface. When that happens, the electronic driver turns the motor on in order to eject the fluid starting the systolic phase of the left ventricle. The fluid goes to the arterial system through another mechanical valve (aortic valve). Closed to ventricle outflow, the fluid flow passes through the adjustable compliance chamber to the systemic system where there is a proportional valve (EPV-375B, Hass Manufacturing Co., New York, USA), controlled by computer, to adjust the peripheral resistance. After that, the fluid returns to the reservoir. Figure 2. Hybrid Cardiovascular Simulator. [1] Reservoir (left atrium); [2] Pumping chamber (left ventricle); [3] Pumping-chamber internal pressure probe. [4] Systemic Vascular Resistance; [5] Adjustable compliance chamber; [6] Systemic pressure sensor; [7] Mechanical valves; [8] LVAD Cannulation Sites. The acquired signals from physical section of HCS are: left atrial pressure (pre load), intraventricular pressure, systemic pressure, systemic flow and diaphragm displacement. Pressure signals are acquired by TrueWave pressure transducers (Edwards Lifesciences, Irvine, CA, USA) and amplified by an electronic module assembled in our laboratories. An ultrasound flowmeter (HT110,Transonic, Ithaca, NY, USA) was used for the acquisition of the systemic flow. A linear variable differential transformer (LVDT) (DC-SE 1000, Schaevitz Sensors, Hampton, VA, USA) was used for diaphragm displacement acquisition to measure the instantaneous volume. All signals are sent to an acquisition board (USB-6229, National Instruments, Austin, TX, USA), which is connected to a microcomputer PC by USB interface. An interface programmed in LabVIEW shows the results. Another interface between the computer and the physical apparatus (PCI-6036E, National Instruments, Austin, TX, USA) drives the events: systole/diastole; motor speed; air volume inside compliance chamber; proportional valve aperture and left ventricle preload. The baroreflex was accomplished by numerical section measuring the arterial mean pressure and actuating in the motor speed controlling the heart rate. Also, this mechanism actuates in the proportional valve to adjust the systemic vascular resistance (SVR). Both adjusts are realized in according to a table (Lucchi, 1999). 2.2 Evaluation of HCS In order to evaluate the HCS, some tests were performed. Firstly, LV preload was changed (10, 15 and 20mmHg). After that, the ventricle contractility was modified (0.6, 1.0 and 1.6mL/mmHg) keeping a fixed preload (Ferrari, 1994). HCS assessment with LVAD connected was performed: a rotary pump was connected through LV to aorta (LV apex to aorta); a pulsatile pump was connected between LV chamber to aorta (LV apex to aorta). In both cases previous descripted, the LV contractility was kept in 1 mL/mmHg simulating a LV disease. 3. RESULTS AND DISCUSSION Figure 3 shows the LV cardiac cycle (pressure vs volume loop) in three different situations: (a) displacements when atrial pressure changes (10, 15, 20 mmHg) with constant arterial pressure and contractility. Is possible to observe that the end systolic volume remains approximately unchanged (110mL) while end diastolic volume changes; (b) changes in contractility state (1/Ees=0.66, 1, 1.66 mL/mmHg), where Ees is the end systolic elastance, with fixed atrial pressure. It shows the change in the end systolic volume while end diastolic volume remains unchanged; (c) effect on the cardiac cycle of the rest volume variations, contractility remains unchanged and its volume shifts according to the rest volume variations – arterial pressure remains constant. The use of pressure vs volume loop has been emphasized, because it makes possible the evaluation of the left cardiac pump (Li, 2004). (a) (b) (c) Figure 3. Experimental results obtained by HCS. (a) displacements of the work cycle for three different values of atrial pressure (10, 15 and 20 mmHg) at fixed heart contractility and arterial pressure; (b) three different values of heart contractility (1/Ees = 0.6, 1 and 1.6 mL/mmHg) and fixed atrial pressure; (c) displacements of the work cycle for three different values of ventricular rest volume (49, 59 and 69mL) and fixed heart contractility and atrial pressure. Figure 4 shows the HCS behavior when a rotary blood pump is connected between LV chamber and aorta. This test was performed at cardiac disease situation which the LV contractility was kept at 1/Ees=1mL/mmHg – in a healthy LV, 1/Ees 0.26 mL/mmHg. Firstly, the pump speed was kept at 900 rpm in order to not allow backflow into LV, thus a step increasing the speed to 1800 rpm was applied as shown in Fig. 4(a). As result, heart rate decreased from 120bpm to 75bpm, Fig. 4 (b). (a) (b) Figure 4. HCS connected to rotary LVAD between LV chamber and aorta. (a) LV pressure and aortic pressure; (b) After speed step, heart rate is automatically decreased by baroreflex actuation. In Fig. 5, an electromechanical pulsatile blood pump as LVAD was connected between LV chamber and aorta of HCS. The LV contractility was kept at 1/Ees=1mL/mmHg. Figure 5(a) show the aortic and LV pressures and Figure 5(b) the heart rate behavior when the LVAD was turned on. (a) (b) Figure 5. Pulsatile LVAD between LV chamber and aorta connected to HCS. (a) LV pressure and aortic pressure; (b) After LVAD turned on, heart rate was decreased. 4. CONCLUSION HCS test results showed in Fig. (3) prove that it follows the Frank Starling law. Also, comparing to Ferrari studies (Ferrari, 1994), the obtained HCS behavior are similar to that showed in the article. HCS behavior when connected to a rotary or pulsatile LVAD is similar to simulations using electrical analog model presented by Lucchi (1999). Also, HCS demonstrated that a rotary pump as LVAD, Fig. (4), can help the diseased heart, however the pump needs to have a careful rotational speed control to avoid natural heart injuries. Application of an electromechanical pulsatile pump as LVAD, Fig. (5), runs synchronized to HCS, decreasing the left ventricle afterload helping to maintain physiological mean aortic pressure. Also, in case of LVAD failure, the pulsatile device rather than continuous pump do not provoke natural heart acceleration. ACKNOWLEDGMENTS The authors would like to thanks the Adib Jatene Foundation (FAJ), Heart Hospital (HCor) and FAPESP for partially supporting this research. REFERENCES Avanzolini, G., Cappello, A. (1991), “Computer simulation of the controlled cardiovascular system: application to cardiac assistance evaluation”. IEEE Comput. Cardiol., 693–696. Felipini, C.L., Andrade, A., Lucchi, J.C., Fonseca, J., Nicolosi, D.E.C. (2008), “An electro-fluid-dynamic simulator for the cardiovascular system”. Artifial Organs, 32, 349–354. Ferrari, G., De Lazzari, C., Mimmo, R., Ambrosi, D., Tosti, G. (1994), “Mock circulatory system for in vitro reproduction of the left ventricle, the arterial tree and their interaction with a left ventricular assist device”. Journal of Medical Engineering & Technology, 18(3), 87-95. Fonseca, J.W.G., Andrade, A.J.P., Legendre, D.F., et al. (2010), “Pressure versus volume loop assessment for a physical cardiovascular simulator (PCS) system”. ASAIO Journal, 56, 126. Legendre, D., Fonseca, J., Andrade, A., et al. (2008), “Mock circulatory system for the evaluation of left ventricular assist devices, endoluminal prostheses, and vascular diseases”. Artificial Organs, 32, 461–467. Li, JK-J. (2004), “Dynamics of the Cardiovascular System”, Series on Engineering & Biomedical Engineering, Vol. 1,World Scientific, EUA. Lucchi, J.C. (1999), “Simulação Elétrica Aplicada à Investigação Hemodinâmica da Assistência Ventricular” Ph.D. Thesis, Instituto Tecnológico de Aeronáutica – ITA, São José dos Campos. Patel, S., Allaire, P.E., Wood, H.G., Adams, J.M., Olsen, D.B. (2003), “Design and construction of a mock human circulatory system”, in Summer Bioengineering Conference, Sonesta Beach Resort, Florida, 965–966.