* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Carboxylic Acids Theory Sheet
Survey
Document related concepts
Transcript
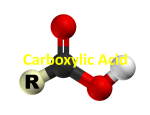
LA.4.1Carboxylic Acids Key Points Carboxylic acids are compounds with the formula of that illustrated in Figure 1 and the general formula R-COOH where R is part of a larger organic molecule e.g.CH3, C2H5, C6H5. To name carboxylic acids you must look at the alkyl chain, take its prefix and add “oic acid” to it. CH3CH2COOH is PROPANOIC ACID. Salts of Carboxylic Acids Carboxylic acids are more acidic than alcohols as their conjugate base, the carboxylate ion, is resonance stabilised (see Figure 2). This anion then can form a salt ionically bonding with a metal such as sodium (see Figure 3). Figure 1 The general formula of a carboxylic acid. Figure 2 The stabilised carboxylate ion. Figure 3 The salt, sodium ethanoate. Physical Properties of Carboxylic Acids The boiling point and solubility of carboxylic acids is related to their ability to form hydrogen bonds. Carboxylic acids are polar and can act as both hydrogen bond donors through the hydroxyl group and hydrogen bond acceptors through the carbonyl. Boiling Point Solubility Carboxylic acids have a higher boiling point than the corresponding alcohol due their ability to dimerise through hydrogen bonds which is illustrated in Figure 4. This dimerization causes the size of the molecule to double and the van der Waals’ interactions to increase. For boiling to occur either the dimer must break first or the whole dimer must be vaporised requires more energy than the monomer. In the presence of water carboxylic acids won’t dimerise but will form hydrogen bonds with water instead. Smaller carboxylic acids (up to 5 carbons) are soluble in water but the solubility decreases rapidly with size. This is due to the hydrophobic nature of alkly chains. EHANOIC ACID is considerably more soluble than HEPTANOIC ACID due the difference in chain length Reactions of Carboxylic Acids 1. Carboxylic acids react with alcohols to give ESTERS. e.g. CH3COOH + CH3CH2OH CH3COOCH2CH3 + H2O 2. Carboxylic acids react with metals and bases to give a METAL CARBOXYLATE SALT. e.g. CH3COOH + Na CH3COOH + NaOH CH3COO-Na+ + H2 CH3COO-Na+ + H2O 3. Carboxylic acids react with amines to form AMIDES. e.g. 1) 2CH3COOH + (NH4)2CO3 2CH3COONH4 + H2O + CO2 2) CH3COONH4 Heat CH3CONH2 + H2O 4. Carboxylic acids can be reduced to give ALCOHOLS. e.g. CH3COOH + 4[H] CH3CH2OH + H2 (H= LiAlH4, a strong reducing agent.) 5. Carboxylic acids can be used to synthesis ACYL CHLORIDES. e.g. CH3COOH + PCl5 CH3COOH + SOCl2 CH3COCl + POCl3 + H2O CH3COCl + SO2 + H2O 6. Carboxylic acids can be used to synthesis ACID ANHDRIDES. e.g. 2 CH3COOH (CH3CO)2 + H2O Esterification: The Mechanism Designed by Leticia Bonita Prince Newcastle University 4th Year MChem Student