* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download vancomycin (van-koe-mye-sin) - DavisPlus
Survey
Document related concepts
Transcript
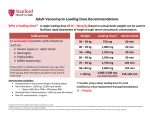
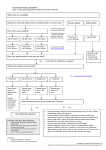
Name /bks_53161_deglins_md_disk/vancomycin 02/17/2014 11:31AM vancomycin (van-koe-mye-sin) min); Hearing impairment; Intestinal obstruction or inflammation (qsystemic absorption when given orally); OB, Lactation: Safety not established. Vancocin Classification Therapeutic: anti-infectives Pregnancy Category C Adverse Reactions/Side Effects EENT: ototoxicity. CV: hypotension. GI: nausea, vomiting. GU: nephrotoxicity. Derm: rashes. Hemat: eosinophilia, leukopenia. Local: phlebitis. MS: back and neck pain. Misc: hypersensitivity reactions including ANAPHYLAXIS, chills, fever, “red Indications IV: Treatment of potentially life-threatening infections when less toxic anti-infectives are contraindicated. Particularly useful in staphylococcal infections, including: Endocarditis, Meningitis, Osteomyelitis, Pneumonia, Septicemia, Soft-tissue infections in patients who have allergies to penicillin or its derivatives or when sensitivity testing demonstrates resistance to methicillin. PO Treatment of staphylococcal enterocolitis or diarrhea due to Clostridium difficile. IV: Part of endocarditis prophylaxis in high-risk patients who are allergic to penicillin. Action Binds to bacterial cell wall, resulting in cell death. Therapeutic Effects: Bactericidal action against susceptible organisms. Spectrum: Active against gram-positive pathogens, including: Staphylococci (including methicillin-resistant strains of Staphylococcus aureus), Group A beta-hemolytic streptococci, Streptococcus pneumoniae, Corynebacterium, Clostridium difficile, Enterococcus faecalis, Enterococcus faecium. Pharmacokinetics Absorption: Poorly absorbed from the GI tract. Distribution: Widely distributed. Some penetration (20– 30%) of CSF; crosses placenta. Metabolism and Excretion: Oral doses excreted primarily in the feces; IV vancomycin eliminated almost entirely by the kidneys. Half-life: Neonates: 6– 10 hr; Children 3 mo– 3 yr: 4 hr; Children ⬎3 yr: 2– 2.3 hr; Adults: 5– 8 hr (qin renal impairment). TIME/ACTION PROFILE (blood levels) IV ONSET PEAK DURATION rapid end of infusion 12–24 hr ⫽ Canadian drug name. pg 1 # 1 Contraindications/Precautions Contraindicated in: Hypersensitivity. Use Cautiously in: Renal impairment (dosage reduction required if CCr ⱕ80 mL/ 1 ROUTE Plate # 0-Composite ⫽ Genetic Implication. man” syndrome (with rapid infusion), superinfection. Interactions Drug-Drug: May cause additive ototoxicity and nephrotoxicity with other ototoxic and nephrotoxic drugs (aspirin, aminoglycosides, cyclosporine, cisplatin, loop diuretics). May enhance neuromuscular blockade from nondepolarizing neuromuscular blocking agents.qrisk of histamine flush when used with general anesthetics in children. Route/Dosage Serious Systemic Infections IV (Adults): 500 mg q 6 hr or 1 g q 12 hr (up to 4 g/day). IV (Children ⬎1 mo): 40 mg/kg/day divided q 6– 8 hr Staphylococcal CNS infection— 60 mg/kg/day divided q 6 hr, maximum dose: 1 g/dose. IV (Neonates 1 wk– 1 mo): ⬍1200 g: 15 mg/kg/day q 24 hr. 1200– 2000 g: 10– 15 mg/kg/dose q 8– 12 hr. ⬎2000 g: 15– 20 mg/kg/dose q 8 hr. IV (Neonates ⬍1 wk): ⬍1200 g: 15 mg/kg/day q 24 hr. 1200– 2000 g: 10– 15 mg/ kg/dose q 12– 18 hr. ⬎2000 g: 10– 15 mg/kg/dose q 8– 12 hr. IT (Adults): 20 mg/day. IT (Children): 5– 20 mg/day. IT (Neonates): 5– 10 mg/day. Endocarditis Prophylaxis in Penicillin-Allergic Patients IV (Adults and Adolescents): 1-g single dose 1-hr preprocedure. IV (Children): 20-mg/kg single dose 1-hr preprocedure. Diarrhea Due to C. difficile PO (Adults): 125 mg q 6 hr for 10 days. PO (Children): 40 mg/kg/day divided into 3 or 4 doses for 7– 10 days (not to exceed 2 g/day). CAPITALS indicate life-threatening, underlines indicate most frequent. Strikethrough ⫽ Discontinued. PDF Page #1 Name /bks_53161_deglins_md_disk/vancomycin 02/17/2014 11:31AM Plate # 0-Composite pg 2 # 2 2 ● Lab Test Considerations: Monitor for casts, albumin, or cells in the urine or Staphylococcal Enterocolitis ● May cause increased BUN levels. ● Toxicity and Overdose: Trough concentrations should not exceed 10 mcg/mL decreased specific gravity, CBC, and renal function periodically during therapy. PO (Adults): 500– 2000 mg/day in 3– 4 divided doses for 7– 10 days. PO (Children): 40 mg/kg/day in 3– 4 divided doses for 7– 10 days (not to exceed 2 g/day). Renal Impairment IV (Adults): An initial loading dose of 750 mg– 1 g (not less than 15 mg/kg); serum level monitoring is optimal for choosing maintenance dose in patients with renal impairment; these guidelines may be helpful. CCr 50– 80 mL/min— 1 g q 1– 3 days; CCr 10– 50 mL/min— 1 g q 3– 7 days; CCr ⬍10 mL/min— 1 g q 7– 14 days. NURSING IMPLICATIONS Assessment ● Assess patient for infection (vital signs; appearance of wound, sputum, urine, and stool; WBC) at beginning of and throughout therapy. ● Obtain specimens for culture and sensitivity prior to initiating therapy. First dose may be given before receiving results. ● Monitor IV site closely. Vancomycin is irritating to tissues and causes necrosis and ● ● ● Potential Nursing Diagnoses Risk for infection (Indications) Disturbed sensory perception (auditory) (Side Effects) Implementation ● Vancomicin must be given orally for treatment of staphylococcal enterocolitis and C. difficile-associated diarrhea. Orally administered vancomicin is not effective for other types of infections. ● PO: Use calibrated measuring device for liquid preparations. IV dose form may be diluted in 30 mL of water for oral or nasogastric tube administration. Resulting solution has bitter, unpleasant taste. May mix with a flavoring syrup to mask taste. Stable for 14 days if refrigerated. IV Administration severe pain with extravasation. Rotate infusion site. ● pH: 2.5– 5.0. ● Intermittent Infusion: Diluent: To reconstitute, add 10 mL of sterile water for prior to and throughout therapy in patients with borderline renal function or those ⬎60 yr of age. Prompt recognition and intervention are essential in preventing permanent damage. Monitor intake and output ratios and daily weight. Cloudy or pink urine may be a sign of nephrotoxicity. Assess patient for signs of superinfection (black, furry overgrowth on tongue; vaginal itching or discharge; loose or foul-smelling stools). Report occurrence. Observe patient for signs and symptoms of anaphylaxis (rash, pruritus, laryngeal edema, wheezing). Discontinue drug and notify health care professional immediately if these problems occur. Keep epinephrine, an antihistamine, and resuscitation equipment close by in case of an anaphylactic reaction. Pseudomembranous Colitis: Assess bowel status (bowel sounds, frequency and consistency of stools, presence of blood in stools) throughout therapy. injection to 500-mg vial or 20 mL of sterile water for injection to 1-g vial for a concentration of 50 mg/mL. Dilute further with at least 100 mL of 0.9% NaCl, D5W, D5/0.9% NaCl, or LR for every 500 mg of vancomycin being administered. Reconstituted vials stable for 14 days if refrigerated. Infusion is stable for 96 hr if refrigerated. Concentration: ⱕ5 mg/mL. Rate: Infuse over at least 60 min (90 min for doses ⬎1 g). Do not administer rapidly or as a bolus, to minimize risk of thrombophlebitis, hypotension, and “red-man (neck)” syndrome (sudden, severe hypotension; flushing and/or maculopapular rash of face, neck, chest, and upper extremities). May need to slow infusion further to 1.5– 2 hr if red-man syndrome occurs. ● IT: Diluent: Dilute with preservative-free NS. Concentration: 1– 5 mg/mL. Rate: Directly instill into ventricular cerebrospinal fluid. ● Y-Site Compatibility: acetylcysteine, acyclovir, aldesleukin, alemtuzumab, alfentanil, allopurinol, alprostadil, amifostine, amikacin, amiodarone, amsacrine, anidulafungin, argatroban, ascorbic acid, atracurium, atropine, azithromycin, benztropine, bleomycin, bumetanide, buprenorphine, butorphanol, calcium ● Monitor BP throughout IV infusion. ● Evaluate eighth cranial nerve function by audiometry and serum vancomycin levels ● (mild-moderate infections) or 15– 20 mcg/mL (for severe infections). 䉷 2015 F.A. Davis Company CONTINUED PDF Page #2 Name /bks_53161_deglins_md_disk/vancomycin 02/17/2014 11:31AM Plate # 0-Composite pg 3 # 3 3 Patient/Family Teaching CONTINUED vancomycin ● Instruct patient to report signs of hypersensitivity, tinnitus, vertigo, or hearing loss. ● Advise patient to notify health care professional if no improvement is seen in a few ● Advise patients on oral vancomycin to take as directed. Take missed doses as soon as remembered unless almost time for next dose; do not double dose. chloride, calcium gluconate, carboplatin, carmustine, caspofungin, chlorpromazine, ciprofloxacin, cisatracurium, cisplatin, clindamycin, cyanocobalamin, cyclophosphamide, cyclosporine, cytarabine, dactinomycin, dexamethasone, dexmedetomidine, dexrazoxane, digoxin, diltiazem, diphenhydramine, dobutamine, docetaxel, dolasetron, dopamine, doripenem, doxacurium, doxapram, doxorubicin hydrochloride, doxorubicin liposome, doxycycline, enalaprilat, ephedrine, epinephrine, epirubicin, eptifibatide, ertapenem, erythromycin, esmolol, etoposide, etoposide phosphate, famotidine, fenoldopam, fentanyl, filgrastim, fluconazole, fludarabine, folic acid, gemcitabine, gentamicin, glycopyrrolate, granisetron, hetastarch, hydromorphone, ifosfamide, insulin, irinotecan, isoproterenol, ketamine, labetalol, levofloxacin, lidocaine, linezolid, lorazepam, magnesium sulfate, mannitol, mechlorethamine, melphalan, meperidine, meropenem, metaraminol, methyldopate, metoclopramide, metoprolol, metronidazole, midazolam, milrinone, mitoxantrone, morphine, multivitamins, mycophenolate, nalbuphine, naloxone, nesiritide, nicardipine, nitroglycerin, nitroprusside, norepinephrine, octreotide, ondansetron, oxacillin, oxaliplatin, oxytocin, paclitaxel, palonosetron, pamidronate, pancuronium, papaverine, pemetrexed, penicillin G, pentamidine, pentazocine, pentobarbital, perphenazine, phenobarbital, phentolamine, phenylephrine, phytonadione, potassium acetate, potassium chloride, procainamide, prochlorperazine, promethazine, propranolol, protamine, pyridoxime, ranitidine, remifentanil, rifampin, sodium acetate, sodium bicarbonate, sodium citrate, succinylcholine, sufentanil, tacrolimus, teniposide, thiamine, thiotepa, tigecycline, tirofiban, tobramycin, tolazoline, trastuzumab, vasopressin, vecuronium, verapamil, vinblastine, vincristine, vinorelbine, voriconazole, zidovudine, zolendronic acid. ● Y-Site Incompatibility: albumin, aminophylline, amphotericin B cholesteryl, amphotericin B colloidal, amphotericin lipid complex, amphotericin B liposome, azathioprine, bivalirudin, cefoperazone, chloramphenicol, dantrolene, daptomycin, diazepam, diazoxide, epoetin alfa, fluorouracil, furosemide, ganciclovir, heparin, ibuprofen, idarubicin, indomethacin, ketorolac, leucovorin calcium, methylprednisolone, mitomycin, moxifloxacin, phenytoin, rituximab, streptokinase, trimethoprim/sulfamethoxazole, valproate sodium. ⫽ Canadian drug name. ⫽ Genetic Implication. days. ● Patients with a history of rheumatic heart disease or valve replacement need to be taught importance of using antimicrobial prophylaxis prior to invasive dental or medical procedures. ● Advise female patient to notify health care professional if pregnancy is planned or suspected or if breast feeding. Evaluation/Desired Outcomes ● Resolution of signs and symptoms of infection. Length of time for complete resolu- tion depends on organism and site of infection. ● Endocarditis prophylaxis. Why was this drug prescribed for your patient? CAPITALS indicate life-threatening, underlines indicate most frequent. Strikethrough ⫽ Discontinued. PDF Page #3