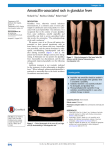
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Apo-Amoxi
Survey
Document related concepts
Transcript
A New Zealand Data Sheet New APO-AMOXI APOTEX NZ LTD Amoxicillin trihydrate trihydrate equivalent to Amoxicillin 250mg and 500mg capsules Presentation APO-AMOXI 250mg capsules), imprinted imprinted 'APO 250'. Each Each APO-AMOXI 250mg capsules capsules are are red red and and gold gold (size 22 capsules), capsule contains amoxicillin amoxicillin trihydrate trihydrate equivalent equivalent to to 250mg 250mg of amoxicillin, capsule contains amoxicillin, and typically typically weighs weighs 376mg. APO-AMOXI 500mg capsules), imprinted imprinted 'APO 500'. Each Each APO-AMOXI 500mg capsules capsules are are red red and and gold gold (size 00 capsules), capsule contains amoxicillin, and typically typically weighs weighs capsule contains amoxicillin amoxicillin trihydrate trihydrate equivalent equivalent to to 500mg 500mg of amoxicillin, 735mg. Indications Treatment of infection indicated in in the the treatment treatment of of infections infections due due to susceptible organisms. APO-AMOXI isisindicated APO-AMOXI may useful in in instituting instituting therapy therapy prior prior to to bacteriology; bacteriology; however however bacteriological bacteriological APO-AMOXI may be useful studies to determine determine the causative causative organisms organisms and their sensitivity sensitivity to amoxicillin amoxicillin should should be studies and their performed. Prophylaxis for endocarditis APO-AMOXI may be used for the prevention of bacteraemia, associated with procedures such as dental extraction, in patients at risk of developing bacterial endocarditis. Dosage and Administration This product is not able to deliver all approved dose regimens. Upper respiratory tract infections, Genito-urinary tract infections, skin and soft tissue infections For upper upper respiratory respiratory tract tract infections infections due duetotostreptococci, streptococci,pneumococci, pneumococci,non-penicillinasenon-penicillinaseFor producing staphylococci and H. influenzae) or Genito-Urinary Tract Infections (due to Escherichia coli, Proteus Proteus mirabilis mirabilis and Streptococcus Streptococcus faecalis faecalis or Skin Skin and and Soft Soft Tissue Tissue Infections Infections due due to coil, Escherichia coil: coli: streptococci, sensitive staphylococci and Escherichia Adults: 250 mg every every 8 hours. hours. Children Children (under (under 20 kg): kg): 25 25 mg/kg/day mg/kg/day in in equally equally divided divided doses doses Adults: every 8 hours. severe infections infections or those caused caused by less susceptible susceptible organisms, hours for In severe organisms, 500 500 mg mg every every 88 hours adults and 50 mg/kg/day in equally divided doses every 8 hours for children may be needed. Lower respiratory tract infections For lower lower respiratory respiratory tract tract infections infections (due (duetotostreptococci, streptococci,pneumococci, pneumococci, non-penicillinase non-penicillinase For influenzae: producing staphylococci and H. influenzae: Adults: 500 mg every every 8 hours. hours. Children Children (under (under 20 kg): kg): 50 50 mg/kg/day mg/kg/day in in equally equally divided divided doses doses Adults: every 8 hours. High dosage therapy The maximum recommended oral dosage 6 g daily in divided doses. An adult dosage of 3 g twice daily is recommended recommended in appropriate cases treatment of severe severe or or recurrent recurrent purulent purulent daily in appropriate cases for for the treatment infection of the respiratory tract. Please refer to Medsafe website (www.medsafe.govt.nz) for the most recent datasheet Page 1 of 13 A APOTEX NZ LTD APO-AMOXI Amoxicillin trihydrate equivalent to toAmoxicillin Amoxicillin 250mg 250mg and and 500mg 500mgcapsules capsules Amoxicillin trihydrate equivalent Prophylaxis of Endocarditis - Dental Procedures Prophylaxis for patients undergoing undergoing extraction, Prophylaxis extraction, scaling scaling or surgery involving gingival tissues who the previous previous month. Patients with prosthetic heart valves should have not received a penicillin in the be referred to hospital (see below). Patient not having a general anaesthetic Adults – hour before before procedure. hours later Adults —33 gg orally, orally, 11 hour procedure. AA second second dose dose may may be be given given 66 hours later if considered necessary. considered necessary. Children Children under under 10 10 -- half half the the adult adult dose. dose. Children Children under under 55 - quarter adult dose. Patients having a general anaesthetic, oral antibiotics considered to be appropriate Adults -- initially initially 3 orally 44 hours hours prior prior to to anaesthesia anaesthesia followed orally (or (or 1 g Adults 3 gg orally followedby by 33 g orally amoxicillin/ampicillin IM amoxicillin/ampicillin IM ifif the the dose dose is not tolerated) 6 hours after the initial dose. Children under 10 - half adult dose. Children under 5 - quarter adult dose. Patient having general anaesthesia, oral antibiotics not appropriate Adults— – 1 g amoxicillin IM immediately before induction with 500 mg orally 6 hours later. Children Adults under 10 - half adult dose. Note: Note: If prophylaxis prophylaxis with with amoxicillin amoxicillin is given given twice twice within within one one month, month, emergence emergence of resistant resistant streptococci is unlikely to be aaproblem. problem. Alternatively, Alternatively, antibiotics antibiotics are are recommended recommended if more more streptococci is unlikely to be frequent prophylaxis is required, or the patient has received a course of treatment with a penicillin during the previous month. Patients for whom referral to hospital is recommended Patients to be given a general anaesthetic who have been given a penicillin in the previous month. Patients to be given a general anaesthetic who have a prosthetic heart valve. Patients who have had one or more attacks of endocarditis. Adults with 120 120 mg mg gentamicin IM immediately prior to Adults - Initially Initially 1 g amoxicillin/ampicillin amoxicillin/ampicillin with gentamicin IM immediately prior anaesthesia (if given) or 15 minutes prior to dental procedure, followed by 500 mg APO-AMOXI APO-AMOXI anaesthesia orally, 6 hours later. Children under 10 - the dose of amoxicillin should be half the adult dose. The dose of gentamicin should be 2 mg/kg. Note: Amoxicillin Amoxicillin and Note: and gentamicin gentamicin should should not not be be mixed mixed in the same syringe. Please consult the appropriate Data Sheet for parenteral amoxicillin and gentamicin. Urethritis (due to Neisseria gonorrhoeae) single dose. dose. Cases Cases of gonorrhoea gonorrhoea with a suspected suspected lesion of syphilis should have Adults: 3 g as single dark field examinations before receiving amoxicillin and monthly serological serological tests tests for a minimum of four months. Lower urinary tract infections coli, Proteus mirabilis, For acute, uncomplicated lower urinary tract infections (due to Escherichia coil, Streptococcus faecalis, non-penicillinase producing staphylococci): Adults: 3 g as a single dose. Please refer to Medsafe website (www.medsafe.govt.nz) for the most recent datasheet Page 2 of 13 A APOTEX NZ LTD APO-AMOXI Amoxicillin trihydrate equivalent to toAmoxicillin Amoxicillin 250mg 250mg and and 500mg 500mgcapsules capsules Amoxicillin trihydrate equivalent children's dose is intended for individuals whose NOTE: The children's whose weight weight will not cause dosage to be recommended for adults. Children weighing calculated greater than that recommended weighing more than 20 kg should be dosed according to the adult recommendations. should be be recognised recognised that that in in the thetreatment treatment ofofchronic chronicurinary urinary tract tractinfections, infections, frequent frequent It should bacteriological and appraisals are necessary. necessary. Smaller doses than than those those recommended recommended bacteriological and clinical clinical appraisals above should not be used. In stubborn infections, therapy therapy may be required required for several several weeks. It necessary to continue continue clinical clinical and/or and/or bacteriological bacteriological follow-up may be necessary follow-up for several months after cessation of therapy. Treatment duration Treatment should should be continued continued for for a minimum minimum of 48 to 72 hours beyond the time that the patient becomes asymptomatic or evidence of bacterial eradication has been obtained. It is recommended that there be at least 10 days treatment for any infection caused by haemolytic streptococci to prevent the occurrence of rheumatic fever or glomerulonephritis. Impaired renal function renal impairment impairment the excretion of amoxicillin amoxicillin will delayed. Depending Depending on degree of In renal the excretion will be delayed. on the the degree impairment, itit may necessary to reduce reduce the total total daily daily dosage. dosage. No No dosage dosage adjustment adjustment is impairment, may be necessary required in patients with a creatinine clearance > 30 ml/min. The maximum recommended dose in patients with creatinine clearance between 10 and 30 ml/min is 500 mg twice daily. The maximum recommended dose in patients with a creatinine clearance < 10 ml/min is 500 mg/day. patients receiving receiving peritoneal peritoneal dialysis, dialysis, the maximum maximum recommended recommended dose 500 mg/day. mg/day. In patients dose in in 500 Amoxicillin may be removed from the circulation by haemodialysis. Renal impairment in children under 40 kg Creatinine clearance >30 ml/min: No adjustment necessary Creatinine clearance mg/kg give give twice twice daily daily (maximum (maximum 500 500 mg/twice mg/twice Creatinine clearance 10 10 to to 30 ml/min: 15 mg/kg daily) Creatinine clearance <10 ml/min: 15 mg/kg given as a single daily dose (maximum 500 mg) In the majority of cases, parenteral therapy will be preferred. Contraindications Amoxicillin is a penicillin and should not be given to patients patients with a history history of of hypersensitivity hypersensitivity to Amoxicillin beta-lactam antibiotics (e.g. penicillins, cephalosporins). contraindicated in patients Amoxicillin is contraindicated patients who have had previous previous experience experience of of a major allergy or anaphylaxis to a cephalosporin or penicillin. Hypersensitivity to any of the excipients. Warnings and Precautions Warnings Serious and occasionally occasionally fatal hypersensitivity hypersensitivity reactions reported in Serious reactions (anaphylaxis) (anaphylaxis) have have been been reported patients receiving beta-lactam antibiotics. Before initiating therapy with amoxicillin, careful enquiry should be made made concerning concerning previous previous hypersensitivity hypersensitivity reactions reactions to to penicillins, penicillins, cephalosporins. cephalosporins. should Cross-sensitivity between Cross-sensitivity between penicillins penicillins and cephalosporins cephalosporins is well documented. Patients should be told about about the potential potential occurrence occurrence of allergic reactions told of allergic reactions and and instructed instructed to to report report them. them. IfIf an Please refer to Medsafe website (www.medsafe.govt.nz) for the most recent datasheet Page 3 of 13 A APOTEX NZ LTD APO-AMOXI equivalent to toAmoxicillin Amoxicillin 250mg 250mg and and 500mg 500mgcapsules capsules Amoxicillin trihydrate trihydrate equivalent allergic reaction reaction occurs, amoxicillin should be discontinued discontinued and and appropriate appropriate alternative alternative therapy allergic instituted. Serious Serious anaphylactic anaphylactic reactions require immediate immediate emergency emergency treatment treatment with with instituted. reactions may may require adrenaline or epinephrine. epinephrine. Oxygen, Oxygen, intravenous intravenous steroids steroids and and airway airway management, management, including including adrenaline intubation, may also be required. Amoxicillin should with caution caution to patients patients with with lymphatic lymphatic leukaemia leukaemia as they are are Amoxicillin should be be given given with as they susceptible to amoxicillin induced skin rashes. Amoxycillin should not be used for the treatment of bacterial bacterial infections infections in in patients patients with with viral infections, infections, presenting presenting with with sore throat, throat, pharyngitis pharyngitis or infectious mononucleosis, as a high incidence of amoxycillin induced erythematous (morbilliform) rashes have been associated with glandular fever in patients receiving amoxicillin. As with with any any potent potent drug, drug, periodic periodic assessment assessment of of renal, renal, hepatic hepatic and andhaematopoietic haematopoietic function function should be made during prolonged therapy. Prolonged use may occasionally result in overgrowth non-susceptible organisms. superinfection with mycotic or bacterial bacterial of non-susceptible organisms. The The possibility possibility of of superinfection with mycotic pathogens should be particularly particularly considered. considered. If If superinfection superinfection occurs occurs (usually (usually involving involving pathogens should be Aerobacter, Pseudomonas or Candida) discontinue amoxicillin and/or initiate appropriate therapy. Pseudomembranous colitis should be borne in mind if severe persistent diarrhoea occurs (in most cases caused caused by Clostridium Clostridium difficile) In this this case case Amoxicillin Amoxicillin should should be discontinued discontinued and cases and an adequate therapy has to be started. The use of antiperistaltics is contraindicated. Abnormal prolongation been reported reported rarely rarely in in patients patients receiving receiving Abnormal prolongationof of prothrombin prothrombintime time has has been amoxicillin and oral anticoagulants. anticoagulants. Appropriate Appropriate monitoring monitoring should undertaken when when amoxicillin and oral should be undertaken anticoagulation treatment the anticoagulant anticoagulant adjusted anticoagulation treatment is is prescribed prescribed concurrently concurrently and and the the dose of the as necessary. high doses, doses, adequate adequate fluid intake intake and and urinary urinary output output must must be be maintained maintained to minimise minimise the At high possibility of amoxicillin crystalluria. Precaution should be taken in premature children and during neonatal period: renal, hepatic and haematological functions should be monitored. with other other beta-lactams, beta-lactams, the the blood blood formula formula should should be be checked checked regularly regularly during during high-dose high-dose As with therapy. High dose therapy therapy with with beta-lactams beta-lactams for patients patients with with renal renal insufficiency insufficiency or or seizures seizures history, history, High treated epilepsy and meningeal affection, could exceptionally lead to seizures. The occurrence occurrence of a generalized erythema with fever and pustules at the beginning of treatment should make make suspect suspect aa generalized generalized acute acute exanthematic exanthematic pustulosis; pustulosis; this this necessitates necessitates the the should interruption of therapy and contraindicated any further administration of amoxicillin. Amoxicillin should experienced symptoms of allergy Amoxicillin should be given with caution to patients who have experienced associated with a cephalosporin or penicillin. hypernatraemia. Use of a Massive doses of amoxicillin can cause hypokalaemia and sometimes hypernatraemia. potassium-sparing diuretic treatment for more potassium-sparing diuretic may may be be helpful. helpful. In patients undergoing high-dose treatment than 5 days, electrolyte balance, blood counts and renal functions should be monitored. Precautions Dosage should adjusted in patients with with renal renal impairment impairment (refer (refer to Dosage Dosage and and Dosage should be be adjusted in patients administration). Please refer to Medsafe website (www.medsafe.govt.nz) for the most recent datasheet Page 4 of 13 A APOTEX NZ LTD APO-AMOXI equivalent to toAmoxicillin Amoxicillin 250mg 250mg and and 500mg 500mgcapsules capsules Amoxicillin trihydrate trihydrate equivalent Following single dose therapy of acute lower urinary tract infections, the urine should be cultured. positive culture may be evidence of a complicated complicated or upper urinary tract infection, and higher A positive dose or prolonged course of treatment may be appropriate. Following administrationofof ampicillin ampicillin to to pregnant women aa transient Following administration pregnant women transient decrease decrease in plasma plasma concentration of total conjugated estriol, estriol-glucuronide, conjugated estrone and estradiol has been noted. This effect may also occur with amoxicillin. patients with reduced reduced urine urine output output crystalluria crystalluria has been been observed observed very very rarely, rarely, predominantly predominantly In patients parenteral therapy. with parenteral therapy. During During the the administration administration of of high high doses doses of of amoxicillin, amoxicillin, itit is advisable to maintain adequate possibility of amoxicillin adequate fluid fluid intake intake and urinary output output in order to reduce the possibility crystalluria (refer to Overdosage). The presence of high urinary concentrations of amoxicillin can cause precipitation precipitation of product in urinary urinary catheters. catheters. Therefore, Therefore, catheters catheters should should be visually visually cause of the product inspected at intervals. gastrointestinal disturbances disturbances with diarrhoea and vomiting should Patients suffering from severe gastrointestinal with diarrhoea not be be treated treated with with APO-AMOXI, APO-AMOXI, due the risk risk of of reduced reduced absorption. absorption. In these cases cases a not due to to the In these parenteral treatment with amoxicillin is advisable. APO-AMOXI should be used with caution in patients with allergic diathesis and asthma. Precautions should be taken for children, premature infants and during the neonatal period, renal, hepatic and haematological functions should be monitored. Use in Pregnancy Category A Assigned Category by the the Australian Australian Drug Drug Evaluation Evaluation Committee. Committee. This This category category includes includes Assigned Category A A by medicines which large number number of of pregnant pregnant women women and and women women of medicines which have have been been taken taken by by a large childbearing age without any proven increase in the frequency of malformations or other direct or amoxicillin for use in indirect harmful harmful effects effects on the foetus having having been been observed. observed. The The safety safety of amoxicillin human pregnancy pregnancy has been established established by well well controlled controlled studies studies in in pregnant pregnant women. women. human has not not been Reproduction studies studies have have been been performed performed in in mice mice and and rats rats at doses doses up to ten times the human dose and these studies have revealed no evidence of impaired fertility or harm to the foetus due amoxicillin. Amoxicillin Amoxicillin may pregnancy when the the potential potential benefits benefits outweigh outweigh the to amoxicillin. may be used in pregnancy potential risks associated with treatment. Use in labour and delivery Oral ampicillin class antibiotics antibiotics are generally generally poorly absorbed absorbed during during labour. labour. Studies Studies in guinea guinea Oral ampicillin pigs have have shown shown that that intravenous intravenous administration administration of ampicillin decreased decreased the uterine tone, tone, pigs of ampicillin the uterine frequency and duration of contractions. However, it is not known whether the use of amoxicillin in humans during delivery has immediate immediate or delayed delayed adverse adverse effects effects on the the foetus, foetus, humans during labour labour or or delivery prolongs the duration of labour or increases the likelihood that forceps delivery or other obstetrical intervention or resuscitation of the newborn will be necessary. Use in lactation Residual amoxicillin may be present in breast milk at levels corresponding to approximately 0.7% the maternal maternal dose. dose. Penicillins Penicillins are are considered considered to to be be compatible compatible with with breastfeeding breastfeeding although although of the sensitisation. So far no there are theoretical risks of alterations to infant bowel flora and allergic sensitisation. detrimental effects for the breast-fed infant have been reported after taking amoxicillin. Amoxicillin used during during breast-feeding. breast-feeding. However, breast-feeding must be stopped stopped ifif gastrointestinal gastrointestinal can be used disorders (diarrhoea, candidosis or skin rash) occur in the new born. Please refer to Medsafe website (www.medsafe.govt.nz) for the most recent datasheet Page 5 of 13 A APOTEX NZ LTD APO-AMOXI Amoxicillin trihydrate equivalent to toAmoxicillin Amoxicillin 250mg 250mg and and 500mg 500mgcapsules capsules Amoxicillin trihydrate equivalent Trace quantities potential for hypersensitivity hypersensitivity quantities of penicillin can be detected in breast milk with the potential reactions (e.g. drug rashes) rashes) or gastrointestinal gastrointestinal disorders candidosis) in reactions (e.g. drug disorders (e.g. (e.g. diarrhea diarrhea or or candidosis) in the breast-fed infant. Consequently, breastfeeding might have to be discontinued. Effects on ability to drive and use machines This medicine medicine is is presumed presumed to to be be safe safe or unlikely unlikely to to produce produce and and effect effect on on the the ability ability to to drive or use machinery. During treatment treatment with with amoxicillin, amoxicillin, undesirable undesirable effects occur (e.g. (e.g. allergic allergic reactions, reactions, During effects may may occur dizziness, convulsions) and use use machines. machines. Patients Patients dizziness, convulsions)which which may may influence influence the the ability ability to to drive and should be cautious when driving or operating machinery. Adverse Effects transitory nature. Side-effects, as with other penicillins, are uncommon uncommon and mainly of a mild and transitory The majority majority of the side-effects side-effects listed amoxicillin and may occur when listed below below are not unique to amoxicillin using other penicillins. Undesirable effects classified systematically frequency according following Undesirable effects are are classified systematically and and by by frequency according to to the following convention: very common (above 1 in 10); common (from 1 in 100 to 1 in 10); uncommon (from 1 in 1000 to 1 in 100; rare (from 1 in 10,000 to 1 in 1,000); very rare (below 1 in 10,000). otherwise stated, Unless otherwise stated, the the frequency frequency of of adverse adverse events events has has been been derived derived from from more more than 30 years of post-marketing reports. Haemic and the lymphatic system disorders Very rare Reactions such as as anaemia, anaemia, thrombocytopenia, thrombocytopenia, thrombocytopenic thrombocytopenic purpura, purpura, eosinophilia eosinophilia and Reactions leucopenia (including severe neutropenia or agranulocytosis), have been reported during therapy with other penicillins. penicillins. All were reversible reversible on discontinuation discontinuation of believed to be with of therapy therapy and and are are believed hypersensitivity phenomena. Prolongation Prolongation of bleeding bleeding time and prothrombin time have also been reported rarely (refer to Warnings and precautions). Immune system disorders Very rare with other other antibiotics, antibiotics, severe severe allergic allergic reactions, reactions, including including angioneurotic angioneurotic oedema, oedema, anaphylaxis anaphylaxis As with precautions), serum sickness and allergic vasculitis. If aa hypersensitivity hypersensitivity (refer to Warnings and precautions), reaction is reported, the treatment must be discontinued. (see also Skin and subcutaneous tissue disorders). Infections and infestations Uncommon Prolonged and repeated use of the preparation can result in superinfections and colonisation with resistant organisms or yeasts such as oral and vaginal candidiasis. Gastrointestinal disorders Common Gastric complaints, nausea, appetite, flatulence, flatulence, soft soft stools, stools, diarrhoea, diarrhoea, enanthemas enanthemas Gastric complaints, nausea, loss loss of appetite, (particularly in region of the the mouth), mouth), dry dry mouth, mouth, taste taste disturbances. disturbances. These These effects effects on the (particularly in the the region gastrointestinal system frequently disappear treatment or gastrointestinal system are are mostly mostly mild mild and frequently disappear either either during during the treatment Please refer to Medsafe website (www.medsafe.govt.nz) for the most recent datasheet Page 6 of 13 A APOTEX NZ LTD APO-AMOXI Amoxicillin trihydrate equivalent to toAmoxicillin Amoxicillin 250mg 250mg and and 500mg 500mgcapsules capsules Amoxicillin trihydrate equivalent very soon after completion completion of therapy. therapy. The occurrence occurrence of effects can generally generally be very of these side effects reduced by taking amoxicillin during meals. Uncommon Vomiting. Rare Superficial discoloration oral suspension suspension is used). Usually Usually the the Superficial discolorationofofthe the teeth teeth (especially (especiallyifif aa oral is used). discoloration can be removed by teeth brushing. Very rare Mucocutaneous candidiasis. Antibiotic associated colitis including pseudomembranous colitis and haemorrhagic colitis. persistent diarrhoea rare possibility possibility of haemorrhagic colitis. IfIf severe severe and and persistent diarrhoea occurs, occurs, the the very very rare pseudomembranous colitis pseudomembranous colitis should should be considered. considered. The administration administration of anti-peristaltic agents is contraindicated. Development of a black hairy tongue. General disorders and administration site conditions Rare Drug fever Hepatobiliary disorders Rare Hepatitis and cholestatic jaundice. Uncommon Moderate and Moderate and transient transient increase increase of of liver liver enzymes. enzymes. The The significance significance of of aa rise rise in liver enzymes is unclear Nervous system disorders Rare Hyperkinesia, dizziness Hyperkinesia, dizziness and and convulsions. convulsions. Convulsions Convulsions may may occur occur in patients with impaired renal function, epilepsy meningitis, or in those receiving high doses. Skin and subcutaneous tissue disorders Common Cutaneous reactions exanthema, pruritus, pruritus, urticaria, urticaria, erythematous erythematous maculopapular rash; Cutaneous reactions such as exanthema, morbilliform exanthema days after after commencement commencement of therapy. therapy. The the typical morbilliform exanthema occurs occurs 55 to 11 days immediate appearance of urticaria indicates an allergic reaction to amoxicillin and therapy should therefore be discontinued. Rare Skin reactions reactions such such as as Angioneurotic Angioneurotic oedema oedema (Quincke's (Quincke's oedema oedema ,, erythema erythema multiforme multiforme Skin exudativum, exsudativum, exsudativum, acute generalized pustulosis, pustulosis, Lyell's Lyell’s syndrome, syndrome, Stevens-Johnson Stevens-Johnson exudativum, acute generalized syndrome, toxic epidermal necrolysis, necrolysis, bullous dermatitis and acute acute generalised generalised syndrome, toxic epidermal bullous and exfoliative dermatitis exanthematous pustulosis (see also Immune system disorders). Please refer to Medsafe website (www.medsafe.govt.nz) for the most recent datasheet Page 7 of 13 A APOTEX NZ LTD APO-AMOXI APO-AMOXI equivalent to toAmoxicillin Amoxicillin 250mg 250mg and and 500mg 500mgcapsules capsules Amoxicillin trihydrate trihydrate equivalent Renal and urinary tract disorders Rare Interstitial nephritis, crystalluria (refer to Overdosage) The incidence of these adverse adverse events derived from clinical studies The incidence of these events was was derived from clinical studies involving involving aa total total of approximately 6,000 adult and paediatric patients taking amoxicillin. Reporting of suspected adverse reactions Reporting suspected suspected adverse adverse reactions reactions after after authorisation authorisation of of the medicine is important. It allows continued monitoring benefit/risk balance balance of the the medicine. medicine. Healthcare Healthcare professionals professionals are continued monitoring of of the benefit/risk asked to report any suspected adverse reactions https://nzphvc.otago.ac.nz/reporting/. Interactions Medicines and other pharmacologically active substances Concurrent administration amoxicillin can increase the the Concurrent administrationofof allopurinol allopurinol during during treatment treatment with with amoxicillin can increase likelihood of allergic skin reactions. Concomitant administration coumarin, may increase Concomitant administration of of amoxicillin amoxicillin and and anticoagulants, anticoagulants, such such as as coumarin, the incidence of bleeding due to prolongation of prothrombin time. Appropriate monitoring should undertaken when when anticoagulation anticoagulation treatment be undertaken treatment is is prescribed prescribed concurrently concurrentlyand and the the dose dose of of the anticoagulant adjusted showing an increase of oral anticoagulant adjustedas as necessary. necessary.AA large large number number of of cases cases showing an increase of oral anticoagulant activity reported in patients patients receiving receiving antibiotics. antibiotics. The infectious infectious and and anticoagulant activity has has been been reported inflammatory context, factors. In these inflammatory context, age age and and the the general general status status of of the patient patient appear appear as as risk factors. circumstances, itit is difficult to know the part part of of the the responsibility responsibility between between the infectious infectious disease circumstances, treatment in the the occurrence occurrence of INR INR disorders. disorders. However, However, some classes of antibiotics antibiotics are and its treatment more involved, involved, notably notably fluoroquinolones, fluoroquinolones, macrolides, macrolides, cyclines, cyclines, cotrimoxazole cotrimoxazole and some more and some cephalosporins There possibility that that the the bactericidal bactericidal action action of amoxicillin amoxicillin could could be antagonised antagonised on There is aa possibility on coadministration with bacteriostatic agents such as as macrolides, macrolides, tetracyclines, tetracyclines, sulphonamides sulphonamides or administration with bacteriostatic agents such chloramphenicol. is possible possible on on concurrent concurrent administration administration with amoxicillin. An increase in the absorption of digoxin is A dose adjustment of digoxin may be necessary Interaction between amoxicillin and methotrexate leading to methotrexate toxicity has been reported. Serum methotrexate levels should be closely monitored monitored in patients patients who who receive receive amoxicillin amoxicillin and andmethotrexate methotrexate simultaneously. simultaneously. Amoxicillin Amoxicillin decreases the renal clearance of methotrexate, methotrexate, probably competition at the common tubular decreases probably by competition secretion system. Probenecid decreases Probenecid decreases the the renal tubular secretion of amoxicillin. Concurrent use with amoxicillin may result in increased and prolonged levels of amoxicillin in serum and bile. Administration of estrogens and and Administration of amoxicillin amoxicillin can can transiently transiently decrease decreasethe the plasma plasma level level of estrogens progesterone, and may reduce the efficacy of oral contraceptives. It is therefore recommended to take supplemental non-hormonal contraceptive measures. Forced diuresis reduction in in blood bloodconcentrations concentrations by by increased increased elimination elimination of Forced diuresis leads leads to to aa reduction amoxicillin. Please refer to Medsafe website (www.medsafe.govt.nz) for the most recent datasheet Page 8 of 13 A APOTEX NZ LTD APO-AMOXI Amoxicillin trihydrate equivalent to toAmoxicillin Amoxicillin 250mg 250mg and and 500mg 500mgcapsules capsules Amoxicillin trihydrate equivalent is recommended recommended that that when when testing testing for the the presence presence of of glucose glucose in in urine urine during during amoxicillin amoxicillin It is treatment, enzymatic the high high urinary urinary treatment, enzymaticglucose glucoseoxidase oxidasemethods methodsshould shouldbebeused. used.Due Due to to the concentrations of amoxicillin, false positive readings are common with chemical methods. The occurrence of diarrhoea may impair the absorption absorption of other other medicines medicines consequently consequently limiting their efficacy. Amoxicillin may decrease the amount of urinary estriol in pregnant women. At high concentrations, amoxicillin may diminish the results of serum glycaemia levels Amoxicillin may interfere with protein testing when colorimetric methods are used Abnormal laboratory test results At high high risk risk concentrations, concentrations, amoxicillin amoxicillin may diminish glycaemia levels. diminish the the results results of serum glycaemia levels. It is recommended that when testing for the presence of glucose in urine during amoxicillin treatment, enzymatic glucose urinary concentrations concentrations of enzymatic glucose oxidase oxidase methods methods should should be be used. used. Due Due to the high urinary amoxicillin, false positive readings are common with chemical methods. Amoxicillin may interfere with protein testing when colorimetric methods are used. Penicillins may interfere with: - Urinary glucose test - Coomb’s Coomb's tests tests - Tests for urinary or serum proteins - Tests which use bacteria e.g. Guthrie test. Overdosage Signs and symptoms Cases of overdosage overdosage with with amoxicillin amoxicillin are are usually usuallyasymptomatic. asymptomatic. Gastrointestinal Gastrointestinal disturbances disturbances Cases such as nausea, nausea, vomiting vomiting and and diarrhoea diarrhoea and and symptoms symptoms of of fluid-electrolyte fluid-electrolyte imbalance imbalance may such may be evident. In patients with severely impaired evident. impaired renal renal function, function, large large overdoses overdoses can can result result in in signs signs of administration of high doses of amoxicillin, amoxicillin, renal toxicity and crystalluria is possible. During the administration adequate fluid urinary output output must must be maintained maintained to minimise minimise the possibility possibility of adequate fluid intake intake and and urinary amoxicillin crystalluria. Management There no specific specific antidote antidote for for an an overdose overdose of ofamoxicillin. amoxicillin. Treatment Treatment consists consists primarily primarily of There is no administration of gastric lavage is usually usually not necessary), necessary), or symptomatic symptomatic administration of activated activated charcoal charcoal (a gastric supportive measures. and electrolyte electrolyte and supportive measures. Particular Particular attention attention should should be be directed directed to to the the water and balance of the patient. Amoxicillin can be removed from the circulation by haemodialysis. For advice on the management of overdose, please contact the National Poisons Centre on 0800 POISON (0800 764766). Further Information Actions Amoxicillin is an aminobenzyl aminobenzyl penicillin bactericidal action inhibition of the Amoxicillin penicillin that that has has a bactericidal action due due to to its inhibition bactericidal effect against many Gram-positive and synthesis of the bacterial cell wall. It exerts a bactericidal Gram-negative effective against against beta-lactamase beta-lactamase producing producing Gram-negative microorganisms. microorganisms. Amoxicillin Amoxicillin isis not effective organisms. Please refer to Medsafe website (www.medsafe.govt.nz) for the most recent datasheet Page 9 of 13 A APOTEX NZ LTD APO-AMOXI equivalent to toAmoxicillin Amoxicillin 250mg 250mg and and 500mg 500mgcapsules capsules Amoxicillin trihydrate trihydrate equivalent Pharmacotherapeutic group J01CA04 JO1CA04 –—Penicillins Penicillinswith withextended extendedspectrum, spectrum, amoxicillin. amoxicillin. Mechanism of action Beta-lactam antibiotic. Pharmacodynamic effects Inhibition of bacterial cell wall synthesis. Antibiotic class Amoxicillin is a semi-synthetic aminopenicillin of the beta-lactam group of antibiotics. Antibiotic nature and mode of action Amoxicillin has a broad spectrum of antibacterial activity against many Gram-positive and Grammicroorganisms, acting biosynthesis of cell wall wall mucopeptide. mucopeptide. negative microorganisms, acting through through the the inhibition inhibition of biosynthesis Amoxicillin Amoxicillin is active active in vitro vitro against against beta-lactamase beta-lactamase negative mirabilis, and negative strains strains of Proteus mirabilis, Haemophilus Haemophilus influenza. influenza. In vitro studies studies have also demonstrated demonstrated activity activity against against most most strains strains of alpha- and and beta-haemolytic beta-haemolytic streptococci. streptococci. Streptococcus Streptococcus pneumoniae, pneumoniae, and beta-lactamase beta-lactamase alphanegative strains strains of of staphylococci, staphylococci, Neisseria Neisseria gonorrhoeae, gonorrhoeae, Neisseria Neisseria meningitidis meningitidis and negative Enterococcus faecalis. faecalis. However, However, some organisms are are sensitive sensitive to amoxicillin amoxicillin only Enterococcus some of of the organisms only at concentrations achieved in the urine. Strains of gonococci which are relatively resistant to benzyl penicillin may also be resistant to amoxicillin. Amoxicillin is susceptible to degradation by beta-lactamases and therefore it is ineffective against bacteria which which produce produce these enzymes enzymes particularly particularly resistant bacteria resistant staphylococci, staphylococci, which which now now have have a high prevalence. prevalence. All strains of Pseudomonas, Pseudomonas, Klebsiella Klebsiella and Enterobacter, indole indole positive positive high All strains Proteus, Serratia Serratia marcescens, marcescens, Citrobacter, penicillinase producing producing N. gonorrhoeae gonorrhoeae and Citrobacter, penicillinase penicillinase producing H. influenzae influenzae are also also resistant. resistant. Escherichia coli isolates are becoming becoming increasingly resistant to amoxicillin in vitro due to the presence of penicillinase-producing strains. Susceptibility The prevalence prevalence of resistance may geographically and selected species and of resistance may vary geographically and with with time for selected local information information on resistance is desirable, desirable, particularly particularly when when treating treating severe severe infections. infections. As local on resistance necessary, expert prevalence of resistance resistance is such that necessary, expert advice advice should should be be sought sought when when the local prevalence the utility of the agent in at least some types of infections is questionable. Breakpoints The MIC breakpoints breakpoints for susceptible susceptible organisms organisms vary vary according according to to species. species. Enterobacteriaceae Enterobacteriaceae amoxicillin and resistant are considered susceptible susceptible when when inhibited inhibited at at NMT NMT 8 mcg/ml amoxicillin resistant at NLT 32 mcg/ml. From NCCLS NCCLS recommendations recommendations and using using NCCLS-specified NCCLS-specified methods, methods, M. catarrhalis catarrhalis (betaFrom influenzae (beta-lactamase (beta-lactamase negative) susceptible at lactamase negative) and H. influenzae negative) are considered susceptible NMT 1 mcg/ml mcg/ml and resistant resistant at NLT 4 mcg/ml; mcg/ml; Str. pneumoniae pneumoniae are considered susceptible susceptible to amoxicillin at MIC NMT 2 mcg/ml and resistant at NLT 8 mcg/ml. Susceptibility data Strains of the the following following named named organisms organisms are generally generally sensitive bactericidal action action of Strains sensitive to to the bactericidal amoxicillin in vitro. Susceptible Gram-positive Gram-positive aerobes aerobes include: include: Enterococcus Enterococcus faecalis faecalis (Note (Note 2), 2), Streptococcus Susceptible pneumoniae (Notes 1, 3), Streptococcus pyogenes (Notes 1, 3), Streptococcus viridans (Note 2), Page 10 of 13 Please refer to Medsafe website (www.medsafe.govt.nz) for the the most mostrecent recentdatasheet datasheet Page A APOTEX NZ LTD APO-AMOXI equivalent to toAmoxicillin Amoxicillin 250mg 250mg and and 500mg 500mgcapsules capsules Amoxicillin trihydrate trihydrate equivalent Streptococcus agalactiae, agalactiae, Streptococcus Streptococcus bovis, bovis, Staphylococcus Staphylococcus aureus aureus (penicillin (penicillin sensitive), sensitive), Streptococcus Corynebacterium species (Note 2), Bacillus anthracis, Listeria monocytogenes. Susceptible Gram-negative Gram-negative aerobes aerobes include: include: Haemophilus Haemophilus influenzae influenzae (Note (Note 3), Haemophilus Susceptible parainfluenzae (Note 3), Escherichia coli (Note 3), Proteus mirabilis, Salmonella species (Note 2), Shigella species (Note 2), Bordetella pertussis, Brucella species (Note 1), Neisseria gonorrhoeae (Note 2), Neisseria meningitidis (Note 1), Pasteurella septica, Helicobacter pylori, Leptospira spp, Vibrio Cholerae Susceptible anaerobes anaerobes include: include: Bacteroides melaninogenicus melaninogenicus (Note (Note 2), Clostridium species, Susceptible Fusobacterium spp. (Note 2), Peptostreptococci Other susceptible organisms include Borrelia burgdorferi. Note 1: No beta-lactamase producers have as yet been reported for these bacterial species. Note 2: Inconstantly Inconstantly susceptible; susceptible; susceptibility absence of 2: susceptibilityisis therefore therefore unpredictable unpredictableinin the the absence susceptibility testing. susceptibility testing. Note Note 3: Clinical efficacy has been demonstrated for susceptible isolates in approved clinical indications. Resistance Bacteria may be resistant resistant to amoxicillin amoxicillin due due to to production production of of beta-lactamases beta-lactamases which which hydrolyse hydrolyse Bacteria aminopenicillins, due to alteration in penicillin-binding proteins, due to impermeability to the drug, due to to drug drug efflux efflux pumps. pumps. One One or or more more of of these these mechanisms mechanisms may co-exist co-exist in the the same same or due organism, leading unpredictable cross-resistance cross-resistance to other beta-lactams beta-lactams and to organism, leading to a variable and unpredictable antibacterial drugs of other classes. Resistant Gram-positive aerobes include: Staphylococcus (beta-lactamase producing strains). Resistant Gram-negative Gram-negative aerobes include: include: Acinetobacter spp., Citrobacter spp., Enterobacter Resistant Moraxella catarrhalis catarrhalis (non-susceptible (non-susceptible isolates), isolates), Proteus spp. spp. (indole (indole spp., Klebsiella spp., Moraxella positive), Proteus vulgaris, Providencia spp., Pseudomonas spp., Serratia spp. Resistant anaerobes include: Bacteroides fragilis. Other resistant organisms include: Chlamydia, Mycoplasma, Rickettsia Pharmacokinetics Absorption Amoxicillin is stable in the presence of gastric acid and rapidly absorbed from the gut to an extent to 93%. 93%. Absorption Absorption is independent independent of food intake. intake. Peak of 72 to Peak blood blood levels levels are are achieved achieved 11 to 2 hours after after administration. administration. After 500 mg doses doses of of amoxicillin, amoxicillin, average average peak peak serum serum hours After 250 250 and and 500 concentrations of 5.2 mcg/ml and 8.3 mcg/ml respectively have been reported. Distribution Amoxicillin is not highly protein bound. Approximately 18% of total plasma drug content is bound to protein. protein. Amoxicillin Amoxicillin diffuses tissues and fluids, fluids, including including sputum and diffuses readily readily into into most body tissues brain and and spinal spinal fluid. fluid. Inflammation Inflammation generally increases the permeability of the saliva but not the brain meninges to penicillins and this may apply to amoxicillin. Amoxicillin diffuses across the placenta and a small percentage is excreted into the breast milk. Biotransformation Page 11 of 13 Please refer to Medsafe website (www.medsafe.govt.nz) for for the themost mostrecent recentdatasheet datasheet Page A APOTEX NZ LTD APO-AMOXI equivalent to toAmoxicillin Amoxicillin 250mg 250mg and and 500mg 500mgcapsules capsules Amoxicillin trihydrate trihydrate equivalent Amoxicillin is where itit exists exists in in aahigh highconcentration. concentration. Amoxicillin Amoxicillin is Amoxicillin is excreted excreted mainly mainly via the urine where partly excreted urine as the the inactive inactive penicilloic penicilloic acid quantities equivalent also partly excreted in in the urine acid in quantities equivalent to to 10 to 25% of of the the initial initial dose. dose. Small Small amounts amounts of the drug drug are are also also excreted excreted in in faeces faeces and and bile. bile. 25% of the Concentrations in the bile may vary and are are dependent dependentupon upon normal normalbiliary biliary function. function. Elimination Approximately 60 70% of of amoxicillin amoxicillin is excreted excreted unchanged unchanged in urine urine during during the first first 6 hours hours Approximately 60 to to 70% after administration administration ofof aa standard standard dose. elimination half life is is approximately approximately 1 hour. hour. after dose. The elimination half life Concurrent Concurrent administration administration of of probenecid probenecid delays delays amoxicillin amoxicillin excretion. excretion. In In patients patients with with end-stage substance is haemodialysable renal failure, the half-life half-life ranges ranges between 5 to 20 hours. The substance Chemical Structure H N \ , o List of excipients Each capsule capsule contains contains the the following following excipients: Each Croscarmellose sodium, Silicon dioxide, dioxide, Stearic acid and gelatin capsule shell. The capsule capsule shell shell also also contains contains the the following following colourants: colourants: Bluish and Titanium dioxide. Allura red, Brilliant Blue, Quinoline yellow, Sunset yellow, Eosine I Bluish Pharmaceutical Precautions Shelf-Life Shelf life: 3 years from the date of manufacture. Special Precautions for Storage °C Store at or below 25 25°C Protect from heat light and moisture. Package Quantities APO-AMOXI 250 mg capsules: Bottles of 28, 100 and 500 capsules. APO-AMOXI 500 mg capsules: Bottles of 28, 100 and 500 capsules Not all strengths and packs sizes may be available. Medicine Schedule Prescription Prescription Medicine Page 12 of 13 Please refer to Medsafe website (www.medsafe.govt.nz) for for the themost mostrecent recentdatasheet datasheet Page A APOTEX NZ LTD APO-AMOXI equivalent to toAmoxicillin Amoxicillin 250mg 250mg and and 500mg 500mgcapsules capsules Amoxicillin trihydrate trihydrate equivalent Sponsor Details Apotex NZ Ltd 32 Hillside Road Glenfield Private Bag Private Bag 102-995 102-995 North Shore Mail Centre Auckland Telephone: Telephone: (09) 444 2073 Fax: Fax: (09) (09) 444 2951 Date of Preparation 2016 15 April April 2016 refer to Medsafe website website (www.medsafe.govt.nz) for datasheet Page 13 13 of 13 Please refer for the themost mostrecent recent datasheet Page