* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Ch 5 HEAT IN CHEMICAL REACTIONS Chemical reactions and the
Survey
Document related concepts
Water splitting wikipedia , lookup
Thermodynamics wikipedia , lookup
Chemical equilibrium wikipedia , lookup
Electrolysis of water wikipedia , lookup
Chemical reaction wikipedia , lookup
Solar air conditioning wikipedia , lookup
Marcus theory wikipedia , lookup
Bioorthogonal chemistry wikipedia , lookup
Heat transfer wikipedia , lookup
George S. Hammond wikipedia , lookup
Stoichiometry wikipedia , lookup
Energy applications of nanotechnology wikipedia , lookup
Transcript
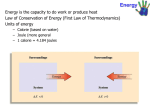
Ch 5 HEAT IN CHEMICAL REACTIONS Chemical reactions and the associated energy transfers are extremely important to our lives. Our bodies metabolism, photosynthesis, fuels, batteries Energy: the ability to do work or produce heat Kinetic energy: energy of motion KE = ½ mv2 KE = kinetic energy, Joules m = mass, kg v = velocity, m/s As velocity increases, KE increases Particles in motion have kinetic energy Potential energy: stored energy (or energy an object has due to its position relative to another object) Includes energy stored in chemical bonds. Chemical potential energy: energy stored in a substance electrostatic potential energy: is important in chemistry. It is the energy resulting from the interactions of charged particles. Thermochemistry: the study of changes in heat in chemical reactions. Heat: the flow of energy from a warmer object to a cooler object When studying chemical reactions we consider the energy in the reactants and the products. This is our “system”. The container and everything else around is the “surroundings”. a. Closed systems (what we will be considering): in which the system can exchange energy but not matter with the surroundings. The energy exchanged with the surroundings can be in the form of heat or work. b. Open systems: can exchange matter and energy with the surroundings. c. Isolated systems: cannot exchange matter or energy with the surroundings. First Law of Thermodynamics Law of Conservation of Energy: energy can be converted from one form to another or from one object to another, but it cannot be created or destroyed. Internal energy, E, of a system includes motion of the particles (kinetic energy) and the potential energy stored in bonds. ∆E = Efinal - Einitial Einitial = energy of reactants Efinal = energy of the products If ∆E is positive, the system has gained energy from the surroundings If ∆E is negative, the system has lost energy to the surroundings A closed system can exchange energy with its surroundings as either heat or work. ∆Esystem = q + w q = heat (energy added or lost) W = work Signs for ∆E, q, and w and the meaning q w ∆E + system gains heat + work done on system + net gain of E by system - (neg) system loses heat - work done by system - net loss of energy by system Problems: A. In a sample reaction, A and B are gases that combine to form C, a solid. A(g) + B(g) C(s). The system loses 1150 J heat to the surroundings The surroundings do 480 J work on system. What is the change in the internal energy of the system? ∆Esystem = q + w = -1150 J + 480 J = -670J The system has lost energy to the surroundings B. Calculate the ∆E for the process in which a system absorbs 140J from the surroundings and does 85J work on the surroundings. ∆Esystem = q + w = 140J -85J = 55J Heat units: 1. calorie (cal) – the amount of heat needed to increase the temp of 1 g of water 1 0C. 2. Calorie – a kilocalorie, 1000 calories, used for nutrition 3. Joule (J) – SI unit of energy and heat 1kJ = 1000J 1 J = 0.02390 cal 1 cal = 4.184 J Almost all chemical reactions absorb or release energy in the form of heat. Energy must be input to break bonds and energy is often released when new bonds are formed (due to more favorable electron configurations) 1. Exothermic reactions: release heat to surroundings container feels warm heat is a product and is written into the chemical equation as a quantity of Joules (J), or kilojoules (kJ). C3H8 + 5O2 3CO2 + 4H2O + 2043kJ In exothermic reactions the energy required to break bonds in the reactants is less than the energy released when new bonds form in the products. This extra energy is released to the surroundings. 2. Endothermic reactions: absorb heat from the surroundings container will feel cold input heat is written as a reactant C + 2H2O + 113 kJ CO2 + 2H2 In endothermic reactions the energy needed to break bonds is greater than the energy released when bonds are formed in the products. Summary: Energy input to break bonds vs Exothermic lesser Endothemic greater Energy released when bonds are formed in products greater lesser State functions: a property of the system that is determined by the systems conditions or state, for example by its pressure or temperature. Energy of system is a state function. It has a fixed value depending on the temperature and/or pressure. ∆E depends only on the final overall change in energy, not any of the up and down changes that occurred between the initial and the final conditions. CALORIMETRY – the study of heat flow and heat measurement Heat capacity: the amount of heat needed to raise the temp. of an object 1oC (or K) The greater the heat capacity, the greater the heat required to change the temperature of the object. Heat capacity depends both on the type and quantity of matter in an object. Molar heat capacity: the heat required to increase the temperature of 1 mole of a substance 1 oC (or K) Specific Heat - the amount of heat needed to raise the temperature of 1 g of any substance by 1oC (or K) varies with substance, see table pg 176 Water has a high specific heat (it takes a lot of energy to raise the temp of water compared to other types of matter) q = m C ∆T q = heat absorbed or released by a substance m = mass of substance, grams C = specific heat of substance T = Tfinal - Tinitial (either oC or K) a –q value means heat is released a + q value means heat is absorbed (exothermic) (endothermic) Problems: A. How much heat is absorbed by 5000g concrete heats up from 19oC to 25pC, given Cconcrete = 0.84J/goC? m = 5000g ∆T = 25-19 = 6oC C = 0.84 J/goC need to solve for q in q = mC∆T q = 5000 x 0.84 x 6 q = 25200 J or 25.2 kJ B. How much heat is released by the 5000g piece of concrete when it cools from 74oC to 40oC at night? m = 5000g C = 0.84 J/goC ∆T = 40-74 = -34oC need to solve for q in q = mC∆T q = 5000 x 0.84 x -34 q = -142800 J or -142.8 kJ (the neg sign reminds you the heat is released) C. How much heat is needed to warm 250g water from 22oC to 98oC? What is the molar heat capacity of water? m = 250 g C = 4.184 J/goC ∆T = 98-22=76 q = mC∆T = 250 * 4.184* 76 = 79J Molar heat capacity is for 1 mole of water or 18 g water We know Cwater = 4.184 J/gOC so re-write as a fraction 4.184 J * 18 g g oC 1 mole = 75.2J/mole oC HW Calorimetry problems 4 – 7, 9,10,12 Constant Pressure Calorimeter: a well- insulated container filled with a known mass of water, used to measure heat absorbed or released by a “reacting system”. Also known as a “coffee cup calorimeter” The water temp changes as the reacting system absorbs or releases energy used by food scientists to determine Calories in food qrxn = heat transfer of the reaction qsur = heat change by the surroundings (the water) qrxn = - qsur they are equal in magnitude, just opposite in sign because the heat lost in a rxn = heat gain of the water OR the heat gained in the rxn = heat loss of the water) Since q = mC ∆T, mrxn Crxn ∆T rxn = - msur Csur ∆T sur qrxn = - msur Csur ∆T sur Use this equation when we are solving for heat change of a substance by measuring heat change of its surroundings (usually water in a calorimeter). Note that if you mix two quantities of solutions or dissolve a substance in water, the mass you use must be the mass of the total solution. Example: A calorimeter contains 125 g water at 25.6oC. A 50.0 g piece of metal at 115oC is put into the water. The temp. of the water stabilizes at 29.3oC. Calculate the specific heat of the metal, and identify the metal using the table of specific heats. qrxn = - qsur think qmetal = - qwater substitute mC∆T for each side: mC∆T metal = - mC∆T water Look up specific heat of water = 4.184 J/goC Write in values for variables and solve for Cmetal (50) C (29.3 – 115) = - (125) (4.184) (29.3-25.6) -4285C = -1935.1 C = 0.452 J/goC The metal must be iron. Problems A. When a student mixes 50 mL of 1.0M HCl and 50mL of 1.0 M NaOH in a constant pressure calorimeter, the temperature of the resultant solution increases from 21oC to 27.3oC. Calculate the enthalpy (heat) change in J/mol HCl? In kJ/mol HCl assuming the total volume of the solution is 100mL and the density is 1.0g/mL. The Cwater = 4.184 J/g oC. ** Under constant pressure we can assume enthalpy = q qrxn = - mC∆T qrxn = ? m = we can solve for using density and volume Cwater = 4.184 J/g oC (given) ∆T = 27.3 – 21 = 6.5 Solve for mass : density = mass/vol 1 = mass/100 ml, so mass = 100g Plug values into equation: qrxn = - 100 * 4.184* 6.5 = - 2717 J Now divide by 1000 for kJ -2717J * 1kJ / 1000 J = 2.715 kJ The last thing is to solve for a per mole basis. How many moles of HCl do we start with? 50 mL * 1L * 1 mole HCl = 0.05 moles 1 1000mL 1 L Now divide kJ by moles - 2.715 kJ = -54 kJ/mol 0.05 mol Note that the negative sign tells you the rxn is exothermic. Bomb Calorimeter: a constant volume calorimeter often used in combustion reactions b/c it can withstand high pressure. The sample is put in a sealed container the ( “bomb”) which is then placed in a precise amount of water. The heat released is absorbed by the water and the calorimeter, so we must include a value for the heat capacity of this calorimeter in calculations. More precise than a “coffee cup calorimeter”. Equation for constant volume (Bomb) calorimetry problems. qrxn = - Ccal ∆T qrxn = heat change of rxn Ccal = specific heat of the calorimeter ∆T = Tfinal - Tinitial in oC or K Problem : The combustion of liquid rocket fuel is shown in the following equation. When 4.00 g of CH6N2 is combusted in a bomb calorimeter, the temp increases from 25 oC to 39.50oC. The Ccal is 7.794 kJ/oC. Calculate the heat of reaction for the combustion of 1 mole of CH6N2. 2CH6N2 + 5 O2 2N2 + 2CO2 + 6H2O qrxn = - Ccal ∆T = - (7.794) ( 39.50 – 25) = -113 kJ This is per 4 grams of CH6N2 , so we need to convert it to per mole by writing kJ / g, then using conversion factors to get it per mole. - 113 kJ x 46 4g 1 mol = -1299.5 kJ/mol Group practice: A 0.5865 g sample of lactic acid, HC3H5O3 is burned in a bomb calorimeter whose heat capacity is 4.812 kJ/°C. The temp increases from 23.10 °C to 24.95°C. Calculate the heat of combustion for lactic acid a. per gram b. per mole HW Problems 19 - 21 Enthalpy , H, is the energy (heat) in a substance at a constant pressure. H = E + PV H = Enthalpy E = internal energy PV = small component accounting for pressure and volume Enthalpy is a state function, and depends on temperature, state , and composition of the substance, so we must include states in chemical equations used with enthalpy and enthalpy changes. Enthalpy is an extensive property it is proportional to the quantity of reactants. We can easily measure the change in enthalpy of a chemical reaction. This is called: ∆Hrxn, or enthalpy (heat) of reaction and is equal to heat absorbed or released in a chemical rxn (assuming constant pressure) ∆ Hrxn = Hproducts - Hreactants Endothermic reactions have a positive ∆ H, meaning the H products > H reactants Exothermic reactions have a negative ∆ H,meaning the H reactants > H products We will be dealing with reactions at constant pressure so Heat, q = ∆ Hrxn Enthalpy Diagrams: Show the enthalpy change of a reaction. The y axis is increasing enthalpy, ∆H The reactants are written on a line then an arrow points to a line with the products. Standard Enthalpy Change, ∆Ho are enthalpy changes that occur under standard conditions of 1 atm, 25o C AND assuming the reactants and products are in their standard states. (not to be confused with STP, 1 atm, 0OC of gases) the standard state of a substance is its natural state in pure form at 1 atm if it has 2 natural states (such as graphite and diamond) the most stable form will be considered the standard state (graphite) ∆Ho values are used to compare values because the heat absorbed or released varies with temp, pressure and state. When writing thermochemical equations to show ∆ Ho, you pull energy out of the equation and write it to the right using a + or – sign to designate endothermic or exothermic reactions (recall + ∆Ho = endothermic and - ∆ Ho = exothermic) so writing the same equations as before in this way…. C3H8 + 5O2 3CO2 + 4H2O C + 2H2O CO2 + 2H2 ∆ Ho = - 2043 kJ ∆Ho = + 113 kJ Stoichiometry Calculations involving Enthalpy (heat) We can solve for ∆ Ho by using a conversion factor relating moles of any substance in a balanced equation to the ∆ Ho for that chemical reaction. Ex: 2H2O2 (l) 2H2O(l) + O2 (g) ∆Ho = -190 kJ 1. Conversion factors you can make from the relationships in this equation are: 2 mole H2O2 and -190 kJ 2 mole H2O -190 kJ and 1 mole O2 -190 kJ 2. these conversion factors can be used to covert from moles kJ or kJ mole 3. use other stoichiometry relationships to make other conversions….such as moles A from the coefficients in the balanced eq moles B __1 mole molar mass,g 1 mole 6.02 x 1023parts 1 mole 22.4 Lgas at STP STEPS FOR SOLVING ∆Ho ENTHALPY PROBLEMS You will usually be given a quantity of a substance and will be asked for the change in enthalpy. 1. Start with a balanced chemical equation with ∆Ho. 2. Write given quantity as a fraction over 1. 3. Convert given quantity to moles by multiplying by 1 mole molar mass, g (omit this step if you are given moles) 4. Then convert from moles of chemical to ∆Ho (heat) x ∆Ho moles substance (from coefficients in eq) Example: How much heat will be released if 1.0g hydrogen peroxide decomposes in a Bombedier beetle to produce a steam spray? 2H2O2(l) 2H2O(l) + O2(g) ∆Ho = -190 kJ Balanced chemical equation Write given over one and convert to moles 1gH2O2 x 1 mole H2O2 1 34g H2O2 Then multiply by moles H2O2 : kJ from equation 1gH2O2 x 1 mole H2O2 x -190 kJ 1 34g H2O2 2 mole H2O2 = -2.79 kJ Ex2: How much heat is transferred when 9.22g glucose in your body reacts with O2? C6H12O6(s) + 6O2(g) 6CO2(g) + 6H2O(l) Ho = -2803kJ Ex 3: How much heat is transferred when 147g NO2 gas is dissolved in 100g H2O? 3NO2(g) + H2O(l) 2HNO3(aq) + NO(g) Ho = -138 kJ You must do 2 conversions, because we do not know which compound is limiting the amount of product, the 147 g NO2 or the 100g H2O. HW –# 22 – 26 and Enthalpy Stoichiometry Additional problems sheet Hess’s Law: If a series of reactions are added together, the enthalpy changes for the overall (net) reaction will be the sum of the enthalpy changes for the individual steps. Ex. The formation of smog occurs in two steps: Step 1: N2(g) + O2(g) 2NO(g) ∆Ho = +181 kJ Step 2: 2NO(g) + O2(g) 2NO2(g) ∆Ho = -113 kJ The overall (net) reaction is N2(g) + 2O2(g) 2NO2 (g) ∆Ho = 181kJ + (-113kJ) = +68 kJ (Notice that the overall reaction is endothermic although one of the steps is exothermic) This illustration is an enthalpy diagram showing the two step reactions and the overall reaction for the combustion of methane. Hess’s Law allows us to calculate energy changes that are difficult to measure directly , by measuring the ∆H of the individual step reactions. Steps for applying Hess’s Law to solve enthalpy problems: When you are given individual (step) reactions and ∆Ho for each and asked to solve for the ∆Ho of the overall or net reactions, which is also usually given, you must: 1. Compare the step reactions to the net reaction you are asked to solve for. Arrange the equations so you can add them together to give you the overall reaction. 2. Rules for arranging equations in order to get the net reaction: If you multiply or divide the coefficients of a reaction, you must do the same to the ∆Ho value. If you reverse an equation, you must change the sign on the ∆Ho. (Logically, if a reaction absorbs heat in one direction, it will give off heat in the other direction.) When adding equations together to get the net (overall) reaction, you cross out equal quantities of the same substances that appear on both sides of the equation. Add the ∆Ho values of the re-arranged step reactions to get the ∆Ho of the net reaction. 3. Check that your final equation matches the net reaction you were asked to solve for. Sometimes you must simplify the coefficients by dividing them AND the ∆Ho value. Ex: The combustion of sulfur can produce SO2 as well as SO3 depending on the supply of oxygen. From the following reactions and Ho, calculate the standard enthalpy changes for the combustion of sulfur to produce SO2. Net rxn: S(s) + O2(g) SO2(g) Step rxns: 2SO2(g) + O2(g) 2SO3(g) ∆Ho = -196 kJ 2S(s) + 3O2(g) 2SO3(g) ∆Ho = -790 kJ Solution: We need SO2 as a product – it is a reactant in the given step equation,, so we must reverse that equation AND change the sign on the ∆Ho. 2SO3(g) 2SO2(g) + O2(g) ∆Ho = +196 kJ Write the step reactions and their ∆Ho in columns so they can be added together: 2SO3(g) 2SO2(g) + O2(g) ∆Ho = +196 kJ 2S(s) + 3O2(g) 2SO3(g) ∆Ho = -790 kJ 2S(s) + 2O2(g) 2SO2(g) ∆Ho = -594 kJ We must divide the coefficients and the ∆H of our resultant equation by 2 to match the given net reaction. S(s) + O2(g) SO2(g) ∆Ho = -594/2 = -297 kJ Ex. 2: From H2S + 3/2 O2 H2O + SO2 ∆Ho = -563 kJ CS2 + 3O2 CO2 + 2SO2 ∆Ho = -1075 kJ Calculate ∆Ho for CS2 + 2H2O CO2 + 2H2S Solution: We need to reverse the first reaction to get H2S as a product – remember to change the sign of ∆Ho. H2O + SO2 H2S + 3/2 O2 ∆Ho = +563 kJ Must multiply coefficients and ∆Ho of the rxn 1 so that quantities of O2 and SO2 are equal and can cancel out. (1) 2H2O + 2SO2 2H2S + 3O2 ∆Ho = +1126 kJ (2) CS2 + 3O2 CO2 + 2SO2 ∆Ho = -1075 kJ CS2 + 2H2O CO2 + 2H2S ∆Ho = 51 kJ HW #27 – 31 and Hess’ Law Practice Problems Enthalpy of formation, ∆Hfo , enthalpy changes associated with the formation of one mole of a compound from its constituent elements in their standard states. Aka “heat of formation” Under standard conditions of 1 atm, 25oC Equations are always written to show 1 mole of the compound, so fraction coefficients , like ½ are commonly used. ∆Hfo units are kJ/mole Ex: ½ N2(g) + O2(g) NO2(g) ∆Hfo = 34 kJ/mol The ∆Hfo for an element in its standard state is 0. Using ∆Hfo values to calculate Enthalpies of Reactions 1. Look up ∆Hfo values for each reactant and product. 2. Write a step reaction showing the formation of 1 mole of each reactant and product that is a compound. The reactants in these formation reactions must be elements (including diatomic gases). You may use fraction coefficients when needed to form just one mole of the compound. 3. Use Hess’s Law to combine step reactions and calculate the ∆Ho for the overall reaction Example: What is the ∆Ho for the combustion of propane? C3H8(g) + 5O2 (g) 3CO2 (g) + 4H2O(l) 1. Write chemical equations to show formation of one mole of each reactant and product and look up ∆Hfo values in a table. 3C(s) + 4H2 (g) C3H8 (g) C(s) + O2 (g) CO2 (g) H2 (g) + ½ O2 (g) H2O (l) ∆Hfo = -103.85 kJ/mol ∆Hfo = -393.5 kJ/mol ∆Hfo = -285.8 kJ/mol 2. Use Hess’s Law to rearrange these equations to get the reactants on the left and the products on the right. Remember to change signs on the ∆Hfo values if you reverse a reaction. Multiply coefficients and ∆Hfo values if needed to enable you to cross out substances and add sides to get the net reaction. C3H8 (g) 3C(s) + 4H2 (g) 3C(s) +3 O2 (g) 3 CO2 (g) 4H2 (g) + 2 O2 (g) 4H2O (l) ∆Hfo = 103.85 kJ/mol ∆Hfo = 3 * -393.5 kJ/mol ∆Hfo = 4* -285.8 kJ/mol C3H8(g) + 5O2 (g) 3CO2 (g) + 4H2O(l)∆Hfo = -2220 kJ/mol Practice problem: Using step reactions and Hess’s law, find the ∆H° for the following reaction: H2S (g) + 4F2 (g) 2HF(g) + SF6(g) Another way…. The ∆Ho of a reaction is equal to the sum of the ∆Hfo of the products minus the ∆Hfo of the reactants. ∆Horxn = ∑ n( ∆Hfo products) - ∑ n( ∆Hfo reactants) ∑ = sum of values for all products (or reactants) n = coefficient of specific compound from balanced eq Elements are NOT included in this equation, since their ∆Hof values are zero. Example: Using standard enthalpies of formation, calculate the ∆Ho for the net reaction below: 4NH3 (g) + 7O2 (g) 4NO2 (g) + 6H2O (l) NH3 (g) O2 (g) NO2 (g) H2O (l) ∆Hof = -46.2 kJ/mol ∆Hof = 0 (element) ∆Hof = 33.8 kj/mol ∆Hof = -285.8 kJ/mol coeff = 4 coeff = 4 coeff = 6 ∆Horxn = ∑ n( ∆Hfo products) - ∑ n( ∆Hfo reactants) = [(4 * 33.8) + (6 * -285.8)] – (4 * -46.2) = - 1396 kJ When you use this equation, you do not switch signs on any ∆Hof values. Problem: Calculate the ∆Ho for the combustion of 1 mol benzene , C6H6: C6H6 (g) + 15/2 O2 (g) 6 CO2 (g) + 3H2O (l) Calculate the ∆Ho for the combustion of 1 mol of sulfuric acid SO3(g) + H2O(l) H2SO4(aq) FOOD & FUEL Fuel value: the energy released when 1 g of a substance is combusted. The fuel values of food are measured in a calorimeter. Most energy for our bodies metabolism comes from the combustion of carbohydrates and fats. Carbohydrates are broken down easily and provide rapid source of energy for our cells Average food value for Carbohydrate = 17 kg/mol Fats = 38 kJ/mol Protein = 17 kJ/mol Our bodies store extra calories as fat because it stores more energy per gram (its lighter) and it is not soluble in water. Proteins are used primarily for building materials, not fuel, although they provide the same energy/mol as carbohydrates. Fuels : the complete combustion of fuels converts all Carbon to CO2 and all hydrogen to H2O. The greater the proportion of C and H in a fuel, the greater the fuel value. The US has only 4.5% of the world’s population, yet accounts for 20% of the world’s energy consumption. Fossil Fuels – petroleum (oil), coal, natural gas - All formed from the remains of plants and animals deposited millions of years ago. - Natural gas is a mixture made up of mainly methane, CH4, with some ethane, C2H6 , some propane, C3H8, and some butane, C4H10. - Petroleum is a liquid combination of large and small hydrocarbons with some S, N, and O - Coal is solid hydrocarbons with some S, O, and N. We have coal reserves to last about 100 years, but coal use has concerns including o SO2 pollution and CO2 pollution (contributing to global warming) o Hazards associated with mining