* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Ecological community integration increases with added trophic
Survey
Document related concepts
Molecular ecology wikipedia , lookup
Occupancy–abundance relationship wikipedia , lookup
Restoration ecology wikipedia , lookup
Unified neutral theory of biodiversity wikipedia , lookup
Island restoration wikipedia , lookup
Latitudinal gradients in species diversity wikipedia , lookup
Habitat conservation wikipedia , lookup
Storage effect wikipedia , lookup
Biodiversity action plan wikipedia , lookup
Transcript
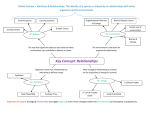
ecological complexity 5 (2008) 140–145 available at www.sciencedirect.com journal homepage: http://www.elsevier.com/locate/ecocom Ecological community integration increases with added trophic complexity Christopher K. Wright * U.S. Geological Survey Center for Earth Resources Observation and Science, 47914 252nd Street, Sioux Falls, SD 57198-0001, United States article info abstract Article history: The existence of functional biological organization at the level of multi-species commu- Received 12 February 2007 nities has long been contested in ecology and evolutionary biology. I found that adding a Received in revised form trophic level to simulated ecological communities enhanced their ability to compete at the 5 October 2007 community level, increasing the likelihood of one community forcing all or most species in a Accepted 25 October 2007 second community to extinction. Community-level identity emerged within systems of Published on line 20 February 2008 interacting ecological networks, while competitive ability at the community level was enhanced by intense within-community selection pressure. These results suggest a reas- Keywords: sessment of the nature of biological organization above the level of species, indicating that Ecological organization the drive toward biological integration, so prominent throughout the history of life, might Metacommunities extend to multi-species communities. # 2007 Elsevier B.V. All rights reserved. Levels of selection Lotka-Volterra Predator-prey Dynamical systems 1. Introduction A fundamental question in ecology is whether communities of co-existing species are organized by deterministic forces, like competition and consumption, into functional wholes possessing emergent properties (Clements, 1936; Odum, 1969; Pimm, 1991), or are simply random assortments of species with similar environmental requirements (Gleason, 1926; Whittaker, 1962; Hubbell, 2001). Parallel to this question of holism in ecology is the levels of selection debate in evolutionary biology (Reeve and Keller, 1999), where individual selectionists (Williams, 1966) and proponents of selfish gene theory (Dawkins, 1976) have rejected group selection and the idea of the superorganism (Wilson and Sober, 1989). Over a decade ago, Gilpin (1994) found that LotkaVolterra communities of competing species could form entities capable of competing en masse against other such entities. Although this finding has been confirmed (Toquenaga, 1997), its potential implications with respect to multispecies ecological organization have not been investigated further. Here I extend Gilpin’s theoretical result from competition-only systems to communities containing two trophic levels. One might imagine community-level competition occurring after some barrier between two separate communities is removed. Indeed, Gilpin’s study was motivated by Vermeij’s (1991) observation that large-scale biotic interchanges tend to be asymmetric (e.g., movement of species between North America and South America following formation of the Isthmus of Panama), with more species from one assemblage successfully invading newly accessible habitat of another assemblage than vice versa. * Tel.: +1 605 594 2553; fax: +1 605 594 6529. E-mail address: [email protected]. 1476-945X/$ – see front matter # 2007 Elsevier B.V. All rights reserved. doi:10.1016/j.ecocom.2007.10.004 ecological complexity 5 (2008) 140–145 2. Methods With some modification, I repeated Gilpin’s (1994) simulation experiment, and then added a second trophic level (for ease of exposition, two trophic-level systems are referred to as predator-prey communities, but can also be thought of as communities of primary producers and their consumers, or plants and herbivores). Two different types of five-species predator-prey communities were created. The first, what I call ‘naı̈ve communities’, contained four prey species and one predator species that were mutually compatible with one another de novo, i.e., no extinction events occurred when their population trajectories were simulated over time. The second type of five-species community was assembled via a sequence of extinction events. These communities began with eight prey species and two predator species, and only those systems that collapsed to four prey species and a single predator were kept. I call these ‘self-organized communities’. Pairwise community mixing was simulated within source pools of four different community types (one or two trophic levels by naı̈ve or self-organized community assembly). Competitive interactions in the lower trophic level were specified such that, on average, intraspecific competition was twice as strong as interspecific competition—assuming that individuals from the same species, and sharing the same niche, compete more intensely than different species occupying similar, but not identical niches. Among predators, direct intraspecific competition was set to zero, although indirect competition was modeled implicitly by prey depletion. However, direct interspecific competition between predators was allowed, representing behaviors where predators interfere with other species’ access to prey, e.g., through territoriality or intraguild predation. Negative effects of predators on their prey were, on average, an order-of-magnitude greater than reciprocal, positive effects of prey on predators, reflecting intrinsic inefficiencies in converting prey individuals into predator offspring. Following Gilpin (1994), I simulated the dynamics of single trophic level communities using a normalized version of the Lotka-Volterra equations: dx ¼ xðb þ AxÞ; dt (1) where population sizes are normalized by their carrying capacities. All elements of the b vector of intrinsic growth rates, and all diagonal elements (intraspecific competition coefficients) of the community matrix, A, were set to one. With these parameters fixed, different competition communities were created by randomly specifying interspecific competition coefficients on the off-diagonal elements of A from the uniform interval (1, 0). In five-species predator-prey communities, one prey species in x was replaced with a predator. Correspondingly, one element in b was replaced with a density-independent predator death rate of 0.1. Predator effects on prey species were randomly sampled from the uniform interval (1, 0), while positive effects of the four prey species on predators were randomly sampled from a smaller uniform interval (0, 0.1). Predator self-limitation, one diagonal element in A, was set to zero. When more than one predator species was present (during 141 assembly of self-organized communities, and at the onset of community mixing) interspecific competition coefficients were randomly sampled from the uniform interval (1, 0). In assembling naı̈ve and self-organized communities, I simulated population trajectories over 104 time steps using a 4th-order Runge-Kutta method. Initial conditions were randomly drawn from a uniform (0, 1) distribution. If a population’s size dropped below 105, it was set to zero. This threshold reflected the vulnerability of very small populations to stochastic events causing extinction. If communities contained five species after 104 time steps, I checked analytically whether they had an interior fixed point where equilibrium population sizes were all greater than zero. Communities that satisfied this criterion were added to source pools for community mixing. Randomly selected pairs of five-species communities were mixed using an augmented community matrix: A1;2 ¼ A1 C2 C1 : A2 (2) The two C matrices determined the effects of species in one community on species in the other community, and were randomly sampled from the same uniform distributions determining interactions in the original communities. Note that in Gilpin’s (11) study the C1 and C2 matrices were symmetric, i.e. C2 ¼ C01 . This guaranteed that any given pair of species from different communities had identical competitive effects on one another. Although obviously not a realistic simplification, Gilpin (1994) argued that balancing interactions between species from different communities was necessary to establish that asymmetric outcomes of community mixing resulted from within-community, not between-community, properties. I disagreed with this line of reasoning. My question of interest was the effect of building five-species communities in four different ways (one or two trophic levels by naı̈ve or self-organized community assembly) on the outcome of community competition. This was a within-community treatment effect that I varied, all the while keeping the mechanics of community mixing fixed. Source pools for community mixing contained 20 communities. Within each pool, 2000 mixing trials were conducted, with a new set of between-community interaction coefficients randomly selected at the onset of each trial. Initial conditions for the two communities were the same as their final values at the end of the assembly process. Community dynamics were simulated over 104 time steps and final community composition was classified as symmetric or asymmetric. In asymmetric outcomes, the post-mixing community contained four or five species from one community, and zero or one species from the other community. All other post-mixing combinations of species were classified as symmetric. Entire experiments – assembly of 20 communities followed by 2000 mixing trials – were repeated 20 times for each of the four community types (Fig. 1). 3. Results Community type had a substantial effect on the frequency of asymmetric outcomes (Fig. 2, F3,76 = 175.1, P = 4.8E34). In 142 ecological complexity 5 (2008) 140–145 Fig. 1 – Flowchart of steps in community mixing experiments repeated for each of the four community types: naı̈ve competition, self-organized competition, naı̈ve predator-prey, and self-organized predator-prey. particular: (i) asymmetric outcomes were more frequent among self-organized communities than in their naı̈ve counterparts (P < 0.01); (ii) adding a predator increased asymmetric outcomes in naı̈ve communities (P < 0.01), but not to the level of self-organized prey communities (P < 0.01); (iii) adding a predator increased asymmetric outcomes among self-organized communities (P < 0.01). By results (ii) and (iii), added trophic complexity generated an increase in ecological organization—in the sense that such assembled five-species communities had an elevated tendency to win or lose as wholes, or nearly so. Remarkably, well over one-third of mixing trials between self-organized, predator-prey communities resulted in one community dominating the other (Fig. 2). Under a null model assuming that each species has the same probability of Fig. 2 – Mean (WS.E.M.) number of asymmetric outcomes by community type (n = 20 community assembly and mixing experiments). All values are significantly different at the P < 0.01 level (Bonferroni-adjusted multiple comparison test). extinction (Pextinction = 0.43, the observed extinction rate over all mixing trials), the probability of these results was vanishingly small (expected value = 130 asymmetric outcomes in 2000 community mixing trials, x2 = 62,926, d.f. = 19, P < 1.0E307). Among asymmetric outcomes, the winning community retained all five of its species while forcing all species from the other community to extinction in an average of 283 22 (S.E.M.) mixing trials, significantly more instances (n = 20, t = 8.1, P = 8.4E09) than when naı̈ve predator-prey communities were mixed (mean number of complete victories by one community = 86 11). The process by which pools of naı̈ve and self-organized communities were created was essentially a form of artificial selection on the coefficients determining interactions between species, or the off-diagonal elements of the community matrix in Eq. (1). Among self-organized predator-prey communities, the distributions of prey competition coefficients, and the effects of predators on prey species, were both shifted toward weaker interactions relative to naı̈ve communities (Fig. 3). These shifts are consistent with other studies demonstrating the importance of weak interspecific interactions in promoting community persistence (May, 1972; McCann et al., 1998; Kokkoris et al., 1999). Notably, naı̈ve and self-organized predator-prey communities had very different attractors. Jacobian matrices evaluated at the interior fixed point of each self-organized predator-prey community each had one eigenvalue with a positive real component, i.e., each community’s fixed point was a saddle, with both stable and unstable manifolds. By contrast, all but seven naı̈ve predator-prey communities had an asymptotically stable interior fixed point. 4. Discussion Community integration, or the tendency of Lotka-Volterra communities to compete against one another as whole ecological complexity 5 (2008) 140–145 143 Fig. 3 – Distributions of prey competition coefficients and predator effects on prey. Histograms (a) and (c) were assembled from the 400 naı̈ve predator-prey communities created over the course of this study. Histograms (b) and (d) were compiled from 400 self-organized predator-prey communities. entities, is of obvious benefit to species from the winning community, and allows rare species to persist in the face of disruption from other communities. But integration also imposes costs—particularly on dominant species of the losing community. Community integration increases the ability of some communities to resist perturbation, while at the same time making other communities sensitive to wholesale invasion. Perhaps this dynamic played a role in the globally observed pattern of asymmetric biotic interchange (Vermeij, 1991). As human activities eliminate barriers separating communities (e.g., by construction of canals, long-distance transport of multiple species, etc.) or force separate communities to occupy common habitat (due to habitat destruction or global warming-induced shifts in species distributions), asymmetric community competition could potentially generate cascades of species extirpations. A recurring theme in the history of life is the combination of previously independent components as a basis for major evolutionary transitions, e.g., in the origins of chromosomes, eukaryotes, sexual reproduction, multicellular organisms, and social groups (Maynard Smith and Szathmáry, 1998). Vermeij (2006) recently commented, ‘‘Union, cooperation, and integration are so widely advantageous by enhancing power and competitive ability that selection favoring them should be strong and common regardless of the hierarchical level at which unions take place.’’ From this perspective, it would seem odd if the drive toward biological integration did not extend to multi-species communities. One argument that ecological communities are not functional unions is that they lack a degree of individuality and separateness from other entities of the same kind (Maynard Smith and Szathmáry, 1998). But synonymous with the property of community integration is a type of community identity. Given a propensity to not mix randomly, the theoretical communities in this study exhibit a degree of boundedness. This identity is by no means complete—in some asymmetric outcomes one species from the losing community remains or one species from the winning community goes extinct. Nonetheless, there is an underlying dynamical attractor that increases in strength as a trophic level is added or communities are self-organized prior to mixing. 144 ecological complexity 5 (2008) 140–145 The probability of a randomly constructed 10-species Lotka-Volterra system having a stable fixed point is low (May, 1972), and thus mixed communities are expected to collapse, with the probability of stability increasing with decreasing dimensionality. However, the mixed communities that I constructed were only semi-random, containing fivespecies subcommunities known to be bi-stable or asymptotically stable in 5D space. These subcommunities bias the collapse of higher dimensional systems toward combinations of species dominated by one community. This type of attractor – acting on concatenated dynamical systems – has not been described in the dynamical systems literature, to the best of my knowledge, and is the source of apparent community identity. Gilpin’s (1994) original contribution was to show that community identity emerged spontaneously from competitive interactions combining elements of both spatial segregation and mixing. I find that adding additional aspects of biological reality exaggerates this tendency. Although my predator-prey model remains a relatively simple caricature of underlying reality, perhaps this phenomenon is common to more complicated, realistic models and, more importantly, actually operates in nature. The ability of Lotka-Volterra communities to compete en masse presents a scenario in which, given some initial degree of spatial segregation, a population of communities, or a metacommunity (Wilson, 1992), could form—with individual subcommunities exhibiting differential fitness as conditions allow them to interact. Within this scenario we have the essential elements necessary for natural selection to operate at the community level (Wilson, 1992; Johnson and Boerlisjt, 2002). However, theories of multi-level natural selection assume a conflict between levels such that selection within communities favors species that disrupt community-level function (Wilson and Sober, 1989; Wilson, 1992; Reeve and Keller, 1999; Johnson and Boerlisjt, 2002). But, in building selforganized communities, I found that the collapse of 10-species systems resulted in five-species communities that were more integrated than their naı̈ve counterparts (Fig. 2). Intense within-community selection was disruptive of larger communities, but the end result was to enhance community-level function (albeit in smaller communities). Within-community selection, by increasing community integration, or, conversely, by engendering community identity, set the stage for between-community selection to occur. The Lotka-Volterra equations belong to the more general class of replicator equations (Schuster and Sigmund, 1983; Hofbauer and Sigmund, 1998), which have been applied in population genetics (Fisher, 1930), prebiotic chemistry (Eigen and Schuster, 1979), game theory (Taylor and Jonker, 1978), and economics (Standish, 2000). This wide-ranging homology suggests that the Lotka-Volterra equations are a canonical model for the study of complex systems. In terms of generality, the state variables ‘species’ might be replaced with ‘agents’ (e.g., genes, individuals, business firms, or nation-states) in the models studied. We then have a mechanism whereby agents acting solely in their own self-interest might form higher-level entities with both potential benefits and costs to individual constituents. The key insight is to treat agents not as if they are isolated within a single network, but as being embedded within a matrix of interacting networks, where the fate of agents is tied to the members of their local network as they interact with members of a more distant network. The increase in ecological organization that I attribute to added trophic complexity is obviously limited to a universe of five-species Lotka-Volterra communities under a prescribed set of conditions. Whether demonstration of community-level competition, in theory, is representative of a real ecological (or wider-ranging) phenomenon remains to be seen. It is striking, however, that the novel behavior of the Lotka-Volterra equations that Gilpin (1994) originally discovered, and I have expanded upon, has gone unnoticed in such a well-studied and venerable model. In our models, perhaps we look for that which we believe possible. The possibility that ecological communities might form integrated, novel evolutionary entities deserves serious re-examination. Acknowledgements This research was developed while the author was a National Research Council Postdoctoral Associate at the U.S. Geological Survey Center for Earth Resources Observation and Science. The author thanks Michael Gilpin, Guy Hoelzer, Robert May, and an anonymous reviewer for helpful comments on earlier drafts of the manuscript. references Clements, F.E., 1936. Nature and structure of the climax. J. Ecol. 24, 252–284. Dawkins, R., 1976. The Selfish Gene. Oxford University Press, Oxford. Eigen, M., Schuster, P., 1979. The Hypercycle—A Principle of Natural Self Organization. Springer, Heidelberg. Fisher, R.A., 1930. The Genetical Theory of Natural Selection. Clarendon Press, Oxford. Gilpin, M.E., 1994. Community-level competition: asymmetrical dominance. Proc. Natl. Acad. Sci. U.S.A. 91, 3252–3254. Gleason, H.A., 1926. The individualistic concept of the plant association. Torrey Bot. Club 53, 7–26. Hofbauer, J., Sigmund, K., 1998. Evolutionary Games and Replicator Dynamics. Cambridge University Press, Cambridge. Hubbell, S.P., 2001. The Unified Neutral Theory of Biodiversity and Biogeography. Princeton University Press, Princeton. Johnson, C.R., Boerlisjt, M.C., 2002. Selection at the level of the community: the importance of spatial structure. Trends Ecol. Evol. 17, 83–90. Kokkoris, G.D., Troumbis, A.Y., Lawton, J.H., 1999. Patterns of interaction strength in assembled theoretical competition communities. Ecol. Lett. 2, 70–74. May, R.M., 1972. Will a large complex system be stable? Nature 238, 413–414. Maynard Smith, J., Szathmáry, E., 1998. The Major Transitions in Evolution. Oxford University Press, Oxford. McCann, K., Hastings, A., Huxel, G.R., 1998. Weak trophic interactions and the balance of nature. Nature 395, 794–798. Odum, E.D., 1969. The strategy of ecosystem development. Science 164, 262–270. Pimm, S.L., 1991. The Balance of Nature? Ecological Issues in the Conservation of Species and Communities. University of Chicago Press, Chicago. ecological complexity 5 (2008) 140–145 Reeve, H.K., Keller, L., 1999. Levels of selection: burying the units-of-selection debate and unearthing the crucial new issues. In: Keller, L. (Ed.), Levels of Selection in Evolution. Princeton University Press, Princeton, pp. 3–14. Schuster, P., Sigmund, K., 1983. Replicator dynamics. J. Theor. Biol. 100, 533–538. Standish, R.K., Barnett, W.A., Chiarella, C., Keen, S., Marks, R., 2000. The role of innovation within economics. In: Schnabel, H. (Ed.), Commerce, Complexity, and Evolution. Cambridge University Press, Cambridge, pp. 61–79. Taylor, P., Jonker, L., 1978. Evolutionary stable strategies and game dynamics. Math. Biosci. 40, 145–156. Toquenaga, Y., 1997. Historicity of a simple competition model. J. Theor. Biol. 187, 175–181. 145 Vermeij, G.J., 1991. When biotas meet: understanding biotic interchange. Science 253, 1099–1104. Vermeij, G.J., 2006. Historical contingency and the purported uniqueness of evolutionary innovations. Proc. Natl. Acad. Sci. U.S.A. 103, 1804–1809. Whittaker, R.H., 1962. Classification of natural communities. Bot. Rev. 28, 1–239. Williams, G.C., 1966. Adaptation and Natural Selection. Princeton University Press, Princeton. Wilson, D.S., Sober, E., 1989. Reviving the superorganism. J. Theor. Biol. 136, 337–356. Wilson, D.S., 1992. Complex interactions in metacommunities, with implications for biodiversity and higher levels of selection. Ecology 73, 1984–2000.