* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 17
Asymmetric induction wikipedia , lookup
George S. Hammond wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Petasis reaction wikipedia , lookup
Hydroformylation wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Aromaticity wikipedia , lookup
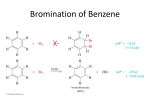
Organic Chemistry Chemistry, 7th Edition L. G. Wade, Jr. Chapter p 17 Reactions of Aromatic Compounds ©2010, Prentice Hall Electrophilic p Aromatic Substitution Alth Although h benzene’s b ’ pii electrons l t are iin a stable t bl aromatic ti system, they are available to attack a strong electrophile to give a carbocation. This resonance-stabilized carbocation is called a sigma complex because the electrophile is joined to the benzene ring by a new sigma bond. Aromaticity is regained by loss of a proton. Chapter 17 2 Mechanism of Electrophilic p Aromatic Substitution Chapter 17 3 B Bromination i ti off Benzene B Chapter 17 4 Mechanism for the Bromination of Benzene: Step 1 Br Br FeBr3 Br + Br FeBr3 (stronger electrophile than Br2) Before the electrophilic p aromatic substitution can take place, the electrophile must be activated. A strong Lewis acid catalyst, such as FeBr3, should be used. Chapter 17 5 Mechanism for the Bromination of Benzene: Steps 2 and 3 Step 2: Electrophilic attack and formation of the sigma complex. H H H H Br H H H Br Br FeBr3 H H H + FeBr4- H H Step 3: Loss of a proton to give the products. H H H Br H FeBr4H Br + FeBr3 + HBr H H H H H H Chapter 17 6 Energy Diagram for Bromination Chapter 17 7 Chl i ti and Chlorination d IIodination di ti Chlorination is similar to bromination. AlCl3 is most often used as catalyst, but FeCl3 will also work. Iodination requires an acidic oxidizing agent like nitric acid agent, acid, to produce iodide cation. H+ + HNO3 + ½ I2 Chapter 17 I+ + NO2 + H2O 8 Solved Problem 1 Predict the major product(s) of bromination of p-chloroacetanilide. Solution The amide group (–NHCOCH3) is a strong activating and directing group because the nitrogen atom with its nonbonding pair of electrons is bonded to the aromatic ring. The amide group is a stronger director than the chlorine atom atom, and substitution occurs mostly at the positions ortho to the amide. Like an alkoxyl group, the amide is a particularly strong activating group, and the reaction gives some of the dibrominated product. Chapter 17 9 Nit ti off B Nitration Benzene NO2 HNO3 H2SO4 + H 2O Sulfuric acid acts as a catalyst, allowing the reaction to be faster and at lower temperatures. HNO3 and H2SO4 react together to form the electrophile of the reaction: nitronium ion (NO2+). Chapter 17 10 Mechanism for the Nitration of Benzene Chapter 17 11 R d ti off th Reduction the Nit Nitro G Group NO2 NH2 Zn, Sn, Zn Sn or Fe aq. HCl T Treatment t t with ith zinc, i tin, ti or iron i iin dil dilute t acid id will reduce the nitro to an amino group. This is the best method for adding an amino group to the ring. Chapter 17 12 S lf Sulfonation ti off Benzene B + SO3 H2SO4 SO3H Sulfur trioxide (SO3) is the electrophile in the reaction. A 7% mixture of SO3 and H2SO4 is commonly referred to as “fuming sulfuric acid”. The —SO3H groups is called a sulfonic acid. Chapter 17 13 M h i Mechanism off S Sulfonation lf ti Benzene attacks sulfur trioxide, forming a sigma complex. complex Loss of a proton on the tetrahedral carbon and reprotonation of oxygen gives benzenesulfonic acid acid. Chapter 17 14 D Desulfonation lf ti R Reaction ti SO3H + H , heat H + H2 O + H2SO4 S Sulfonation lf ti is i reversible. ibl The sulfonic acid group may be removed from an aromatic ring by heating in dilute sulfuric acid. Chapter 17 15 M h i Mechanism off D Desulfonation lf ti In the desulfonation reaction, a proton adds to the ring (the electrophile) and loss of sulfur trioxide gives back benzene. Chapter 17 16 Nit ti off T Nitration Toluene l T Toluene l reacts t 25 ti times ffaster t than th benzene. b The methyl group is an activator. The product mix contains mostly ortho and para substituted molecules. p Chapter 17 17 O th and Ortho d Para P Substitution S b tit ti Ortho and para attacks are preferred because their resonance structures include one tertiary carbocation. carbocation Chapter 17 18 E Energy Di Diagram Chapter 17 19 M t S Meta Substitution b tit ti When substitution occurs at the meta position, the positive charge p g is not delocalized onto the tertiary y carbon, and the methyl groups has a smaller effect on the stability of the sigma complex. Chapter 17 20 Alk l G Alkyl Group Stabilization St bili ti CH2CH3 CH2CH3 CH2CH3 CH2CH3 Br Br2 FeBr3 + + Br o-bromo (38%) m-bromo (< 1%) Br p-bromo (62%) Alkyl groups are activating substituents and ortho, para-directors. This effect is called the inductive effect because alkyl groups can donate electron density to the ring through the sigma bond, bond making them more active. active Chapter 17 21 Substituents with Nonbonding g Electrons Resonance stabilization is provided by a pi bond between th —OCH the OCH3 substituent b tit t and d th the ring. i Chapter 17 22 M t Att Meta Attackk on Anisole A i l Resonance forms show that the methoxy group cannott stabilize t bili th the sigma i complex l iin the meta substitution. Chapter 17 23 B Bromination i ti off Anisole A i l A methoxy group is so strongly activating that anisole is quickly tribrominated without a catalyst. Chapter 17 24 Th A The Amino i G Group Aniline reacts with bromine water (without a catalyst) y ) to yyield the tribromoaniline. Sodium bicarbonate is added to neutralize the HBr that is also formed. Chapter 17 25 S Summary off Activators A ti t Chapter 17 26 A ti t Activators and dD Deactivators ti t If the substituent on the ring is electron donating, the para p positions will be activated. ortho and p If the group is electron withdrawing, the ortho and para positions will be deactivated. Chapter 17 27 Nit ti off Nit Nitration Nitrobenzene b Electrophilic substitution reactions for nitrobenzene are 100,000 , times slower than for benzene. The product mix contains mostly the meta isomer, only small amounts of the ortho and para isomers. Chapter 17 28 Ortho Substitution on Nitrobenzene The nitro group is a strongly deactivating group when considering its resonance forms. The nitrogen always has a formal positive charge charge. Ortho or para addition will create an especially unstable intermediate. Chapter 17 29 Meta Substitution on Nitrobenzene Meta substitution will not put the positive charge on the same carbon that bears the nitro group. Chapter 17 30 Energy Diagram Chapter 17 31 Deactivators and MetaDirectors Most electron withdrawing groups are deactivators and meta-directors. The atom attached to the aromatic ring has a positive or partial positive charge. Electron El t density d it is i withdrawn ithd iinductively d ti l along the sigma bond, so the ring has less electron l t d density it th than b benzene and d th thus, it will ill be slower to react. Chapter 17 32 O th Attack Ortho Att k off Acetophenone A t h In ortho and para substitution of acetophenone, acetophenone one of the carbon atoms bearing the positive charge is the carbon attached to the partial positive carbonyl carbon. carbon Since like charges repel, this close proximity of the two positive charges is especially unstable. Chapter 17 33 M t Attack Meta Att k on Acetophenone A t h The meta attack on acetophenone avoids bearing the positive charge on the carbon attached to the partial positive carbonyl. Chapter 17 34 Other Deactivators Chapter 17 35 Nit ti off Chlorobenzene Nitration Chl b Wh When chlorobenzene hl b iis nitrated it t d th the main i substitution b tit ti products are ortho and para. The meta substitution product is only obtained in 1% yield yield. Chapter 17 36 H l Halogens A Are D Deactivators ti t X IInductive d ti Effect: Eff t Halogens H l are d deactivating ti ti because they are electronegative and can withdraw ithd electron l t d density it ffrom th the ring i along l the sigma bond. Chapter 17 37 Halogens g Are Ortho,, ParaDirectors Resonance Effect: The lone pairs on the halogen can be used to stabilize the sigma complex by resonance resonance. Chapter 17 38 E Energy Di Diagram Chapter 17 39 S Summary off Directing Di ti Eff Effects t Chapter 17 40 Eff t off Multiple Effect M lti l Substituents S b tit t The directing effect of the two (or more) groups may reinforce each other. Chapter 17 41 Effect of Multiple p Substituents (Continued) The position in between two groups in Positions 1 and 3 is hindered for substitution, and it is less reactive. Chapter 17 42 Effect of Multiple p Substituents (Continued) OCH3 OCH3 OCH3 Br Br2 FeBr3 O2N O2N O2N Br major j products p obtained If directing effects oppose each other, the most powerful activating group has the dominant influence. Chapter 17 43 F i d l C ft Alkylation Friedel–Crafts Alk l ti Synthesis of alkyl benzenes from alkyl halides and a Lewis acid, usually AlCl3. Reactions of alkyl halide with Lewis acid produces a carbocation, which is the electrophile electrophile. Chapter 17 44 Mechanism of the Friedel–Crafts Reaction Step 1 Step 2 Step 3 Chapter 17 45 P t Protonation ti off Alkenes Alk An alkene can be protonated by HF. This weak acid is preferred because the fluoride ion is a weak nucleophile and will not attack the carbocation carbocation. Chapter 17 46 Al h l and Alcohols dL Lewis i A Acids id Alcohols can be treated with BF3 to form the carbocation. Chapter 17 47 Limitations of Friedel–Crafts Reaction fails if benzene has a substituent that is more deactivating than halogens. Rearrangements are possible. The alkylbenzene product is more reactive than benzene, so polyalkylation occurs. Chapter 17 48 R Rearrangements t Chapter 17 49 Solved Problem 2 Devise a synthesis of p-nitro-t-butylbenzene from benzene. Solution To make p-nitro-t-butylbenzene, we would first use a Friedel–Crafts reaction to make tb t lb butylbenzene. Nit Nitration ti gives i th the correctt product. d t If we were tto make k nitrobenzene it b fifirst, t the th Friedel–Crafts reaction to add the t-butyl group would fail. Chapter 17 50 F i d l C ft Acylation Friedel–Crafts A l ti Acyl chloride is used in place of alkyl chloride. The product is a phenyl ketone that is less reactive than benzene. Chapter 17 51 M h i Mechanism off A Acylation l ti Step 1: Formation of the acylium ion. Step 2: Electrophilic attack to form the sigma complex. Chapter 17 52 Cl Clemmensen Reduction R d ti Th The Clemmensen Cl reduction d ti is i a way tto convert acylbenzenes to alkylbenzenes by t t treatment t with ith aqueous HCl and d amalgamated zinc. Chapter 17 53 Nucleophilic p Aromatic Substitution A nucleophile replaces a leaving group on the aromatic ring. This is an addition–elimination reaction reaction. Electron-withdrawing substituents activate the ring for nucleophilic substitution substitution. Chapter 17 54 Mechanism of Nucleophilic p Aromatic Substitution Step 1: Attack by hydroxide gives a resonance-stabilized complex. Step 2: Loss of chloride gives the product. Step 3: Excess base deprotonates the product. Chapter 17 55 A ti t d Positions Activated P iti Nitro groups ortho and para to the halogen stabilize the intermediate (and the transition state leading to it). Electron-withdrawing groups are essential for the reaction to occur. Chapter 17 56 Benzyne y Reaction: EliminationAddition Reactant is halobenzene with no electronwithdrawing groups on the ring ring. Use a very strong base like NaNH2. Chapter 17 57 B Benzyne Mechanism M h i Sodium amide abstract a proton. The benzyne intermediate forms when the bromide is expelled and the electrons on the sp2 orbital adjacent p with the empty p y sp p2 orbital of the carbon to it overlap that lost the bromide. Benzynes are very reactive species due to the high strain of the triple bond bond. Chapter 17 58 Nucleophilic p Substitution on the Benzyne Intermediate Chapter 17 59 Chl i ti off B Chlorination Benzene Addition to the benzene ring may occur with excess of chlorine under heat and pressure. The Th first fi t Cl2 addition dditi iis difficult, but the next t two moles l add dd rapidly. idl Chapter 17 An insecticide 60 C t l ti H Catalytic Hydrogenation d ti CH 3 CH 3 3 H 2, 1000 psi Ru, 100°C CH 3 CH 3 El Elevated t dh heatt and d pressure iis required. i d Possible catalysts: Pt, Pd, Ni, Ru, Rh. Reduction cannot be stopped at an intermediate stage. g Chapter 17 61 Bi h R Birch Reduction d ti H H H H Na or Li NH3 (l), ROH H H H H H H H H H H Thi This reaction ti reduces d the th aromatic ti ring i tto a nonconjugated 1,4-cyclohexadiene. The reducing agent is sodium or lithium in a mixture of liquid ammonia and alcohol. Chapter 17 62 Mechanism of the Birch Reduction Chapter 17 63 Li it ti Limitations off th the Bi Birch hR Reduction d ti Chapter 17 64 Sid Ch i Oxidation Side-Chain O id ti CH2CH3 KMnO4, NaOH H2O, 100oC CO2H (or Na2Cr2O7, H2SO4 , heat) Alkylbenzenes are oxidized to benzoic acid by heating in basic KMnO4 or heating in Na2Cr2O7/H2SO4. The benzylic carbon will be oxidized to the carboxylic acid. Chapter 17 65 Sid Ch i H Side-Chain Halogenation l ti Br CH2CH3 Br2 or NBS hν CHCH3 Th The benzylic b li position iti iis th the mostt reactive. ti Br2 reacts only at the benzylic position. Cl2 is not as selective as bromination, so results in mixtures. Chapter 17 66 Mechanism of Side-Chain Halogenation Chapter 17 67 SN1 R Reactions ti Benzylic carbocations are resonancestabilized, easily formed. Benzyl halides undergo SN1 reactions reactions. CH 2Br C H 3 CH 2 O H, heat Chapter 17 C H 2 O CH 2C H 3 68 SN2 R Reactions ti Benzylic halides are 100 ti times more reactive than primary i h halides lid via i SN2. The transition state is stabilized by a ring. Chapter 17 69 O id ti off Phenols Oxidation Ph l OH O Cl Cl Na2Cr2O7 H2SO4 O 2-chloro-1,4-benzoquinone Phenol will react with oxidizing agents to produce quinones. Quinones Q i are conjugated j t d1 1,4-diketones. 4 dik t This can also happen (slowly) in the presence of air. Chapter 17 70