* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Running head: ATRIAL FIBRILLATION
Coronary artery disease wikipedia , lookup
Remote ischemic conditioning wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Heart failure wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Antihypertensive drug wikipedia , lookup
Rheumatic fever wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Myocardial infarction wikipedia , lookup
Cardiac surgery wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Electrocardiography wikipedia , lookup
Atrial septal defect wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
Heart arrhythmia wikipedia , lookup
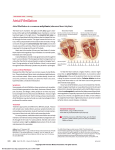
Atrial Fibrillation Running head: ATRIAL FIBRILLATION Atrial Fibrillation: Causes and Effects Lisa J. King Otterbein College 1 Atrial Fibrillation 2 During the last decade there has been a great shift of the responsibility for knowledgeable healthcare. More and more responsibility is being placed on the patients to gain knowledge about their health issues and their subsequent causes and treatment. The public knowledge of major cardiac events such as hypertension, heart attack and angina are becoming more commonly known to the average person. However, the most common heart rhythm abnormality has received very little publicity and its risks and overall health outcomes are still unknown to many people. Atrial fibrillation is responsible for 15-25% of all strokes in the United States. With the rise of the baby boomers it is important to consider that the risk of this arrhythmia increases with age. This is why it is important for us to understand the pathophysiology of atrial fibrillation as well as its causes and treatments. This article provides an overview of the basic pathophysiology associated with atrial fibrillation, diagnostic criteria, clinical manifestations and appropriate treatment. By understanding this condition patient care of these individuals can be better suited to provide the best possible outcomes. The tracing and diagnosis of atrial fibrillation can be traced back more than 100 years ago when Willem Einthoven first reported the invention of the electrocardiograph (Fye, 2006). This invention aided in the diagnosis of atrial fibrillation as well as the development of cardiology as a medical specialty (Fye, 2006). Over the last century, the prevalence of atrial fibrillation has dramatically increased. It has become one of the most common cardiology problems affecting about 5 million Americans (Gottleib, 2006). It has shown growing interest in the medical profession due to its potential negative health outcomes. Patients with atrial fibrillation are believed to be twice as likely to die as those in a normal sinus rhythm (Neal, 2007). This is thought to be because of its association with stroke and heart failure (Huntzinger, 2006). Who is affected? Atrial fibrillation increases with age and is thought to be higher in men than in women (Perrot, 2004). The aging process makes most disease process more prevalent simply because of the normal pathological changes in the body when one ages. For example the stiffening and possible scar tissue in the heart potentially alters the electrical pathway leading to or sustaining an abnormal heart rhythm (Gottlieb, 2006). The atrium can also become fibrotic leading to structural changes in the atrial myocytes which may lead to persistence of atrial fibrillation (Khaja & Flaker, 2005). The arrhythmia also is thought to be more common in those individuals whom have had a heart attack or recent heart surgery, heart failure, heart valve problems, or heart muscle inflammation (No author, 2006). These conditions too increase with age. Clinical Manifestations Some patients never know they are experiencing atrial fibrillation, while others find their symptoms are debilitating, limiting their activities and diminishing their quality of life (No author, 2006). The noticeable signs and symptoms are usually related to lack of rate control and not that of rhythm control (Rocca, 2007). These symptoms generally include: an irregular pulse, palpitations, fatigue, chest pain, dyspnea, dizziness/lightheadedness, nausea, diaphoresis, malaise, polyuria, decreased exercise tolerance and syncope (Scarabelli, 2005). In Atrial Fibrillation 3 patients that remain symptomatic their diagnosis occurs by chance encounters at a routine checkup. Pathophysiology In a normal functioning heart, the electrical impulses originate in the sinoartial node in the right atrium of the heart. The electrical impulse travels across to the left atrium where the heart is then stimulated to start beating (Managing atrial fibrillation, 2006). The electrical current then travels to the ventricles through the atrioventricular node causing the ventricles to contract (Managing atrial fibrillation, 2006). Atrial fibrillation is an abnormal arrhythmia caused by a disruption of the electrical pathway of the heart. The electrical impulses in atrial fibrillation are random impulses firing rapidly around the pulmonary veins, the right atrium, superior vena cava and coronary sinus (Neal, 2007). This uncoordinated and chaotic electrical activity is what is referred to as atrial fibrillation. This loss of normal electrical conduction causes the atria to contract improperly causing them to quiver or fibrillate instead of contract. The rapid quivering of the atrium causes an irregular and rapid ventricular response, which causes ventricular filling to decline and a reduction in the cardiac output often results (Bentz, 2006). This improper conduction of the atrium causes a pooling of blood inside the atria and atrial appendage. The stasis blood in the atria has a high potential to clot and could potentially lead to thrombus formation and thromboembolism (Managing atrial fibrillation, 2006). Atrial fibrillation accounts for almost 150,000 strokes per year (Managing atrial fibrillation, 2006). Atrial fibrillation can also lead to cardiomyopathy and heart failure (Rocca, 2007). Atrial fibrillation can induce electrophysiologic changes in the heart that promote further atrial fibrillation including electrical contractile and structural changes to the atria otherwise known as atrial remodeling (Veenhuyzen, 2007). In some instances atrial fibrillation recurs after conversion to a normal sinus rhythm. Over time this recurrence can lead to a permanent type of atrial fibrillation in which a conversion to normal sinus rhythm is considered impossible or inappropriate (Camm, 2006). This form of atrial fibrillation is believed to be caused by the atrial remodeling that occurs in the heart (Korantzopoulos, Kolettis, Kountouris, Siogag, & Goudevenos, 2005). Remodeling of the heart can be either electrical, contractile, or structural (Veenhuyzen, 2007). Increasing atrial dilatation and fibrosis as with structural remodeling causes an enlargement of the left atrium, increasing intaatrial pressure which slows atrial conduction velocity and allows a greater area for electrical reentry which contributes to maintenance of atrial fibrillation (Boos, 2005). Electrical remodeling indirectly affects ion channels and the potassium current which promotes increased atrial refractoriness thus maintaining an atrial fibrillation state (Boo, 2005). The inability of the atrium and ventricles to properly contract contributes to the contractile remodeling of the heart. Once remodeling has occurred treatment and or conversion to a normal sinus rhythm is much more difficult to achieve. Diagnosis The diagnosis of atrial fibrillation is determined based on whether the patient is symptomatic or not. Patients who have no symptoms of the arrhythmia are diagnosed by chance at a routine office visit by an irregular pulse during the physical exam. When a patient Atrial Fibrillation 4 presents with signs and symptoms of the arrhythmia an electrocardiogram is used to confirm the diagnosis. Atrial fibrillation appears as an irregular tachycardia with no recognizable P waves (Bentz, 2006). Other possible diagnostic tests that could be completed are a chest x-ray and an echocardiogram to check the heart valves, the size of the left atrium and other heart chambers as well as the thickness of the heart’s walls (Managing atrial fibrillation, 2006). At times of suspected atrial fibrillation a patient might be asked to wear a holter monitor to check to patient’s rate and rhythm over a longer period of time. This might be done if the patient is thought to be a paroxysmal atrial fibrillation (Adkins, 2007). Paroxysmal atrial fibrillation is where episodes of AF terminate spontaneously (Camm, 2006). Two other classifications of atrial fibrillation are persistent, which requires electrical or pharmacological cardioversion to normal sinus rhythm and permanent in which conversion is impossible or inappropriate (Camm, 2006). Treatment Much research has been done on the treatment of atrial fibrillation. Many different options are available and are chosen based on a variety of details. The patients symptoms, the type of atrial fibrillation as well as the risk factors of the patient are all considered when determining the appropriate course of action in the treatment of this arrhythmia. Once these factors are considered management of atrial fibrillation is directed by one of two approaches: either to restore and maintain normal sinus rhythm or to control the ventricular rate response to atrial fibrillation (Dorian, 2006). Recent clinical trials suggest that there is no difference in mortality or stroke rate between patients assigned to either rate or rhythm control (Bentz, 2006). Rate control strategies slow ventricular response to the fibrillating atrium while using anticoagulation to reduce stroke risk, but rhythm control strategies involve medical or electrical conversion to sinus rhythm to improve hemodynamics and reduce the risk of stroke (Cayley, 2005). Regardless of the treatment the goal remains the same; to reduce unpleasant symptoms and minimize the risk of stroke and heart muscle damage (Managing atrial fibrillation, 2006). Medications are the first line of treatment (Managing atrial fibrillation, 2006). Various other procedures are used when trying to control rhythm such as cardioversion, ablations, Maze surgery, and atrial or Bachmann’s bundle pacing. Rate control can be achieved by using atrioventricular nodal blocking agents such as beta-blockers and calcium channel blockers (Rocca, 2007). By controlling heart rate diastolic filling time, stroke volume and cardiac output increase (Bentz, 2006). In addition to rate control medications anticoagulants are added to the treatment regime to help prevent clotting and stoke (Managing atrial fibrillation, 2006). When choosing an anticoagulant the American College of Chest Physicians (ACCP) has presented a decision making tool to classify individuals at risk for clotting and possible stroke. The ACCP recommends the use of warfarin for atrial fibrillation patients at high risk for stroke and aspirin for those at low risk (Ebell, 2005). When using anti-coagulation it is important to consider the maintenance of the medications. The patients are usually required to have blood work weekly to maintain therapeutic INR levels. The goal is typically to keep anticoagulated patients INR between 2-3. It is also important to consider the many dietary modifications that are necessary with certain anticoagulants. Atrial Fibrillation 5 Another class of medications used in the treatment of atrial fibrillation is ACE inhibitors and ARBs. Interruption of the RAAS has been shown to effectively reduce the known cardiovascular outcomes such as CVD, HTN, stroke and heart failure therefore decreasing the risk of atrial fibrillation recurrence (Boos, 2005). Inhibition of the reninangiotensin system also has proven to significantly reduce the duration of known atrial fibrillation (Vennhuyzen, 2007). Anti-arrhythmic drugs are used to prevent multiple electrical wavelets by interrupting the electrical excitation or repolarization (Dilaveris, Giannopoulos, Synetos, & Stefanadis, 2005). The use of anti-arrhythmic medications is not used with all patients, due to the negative side effects of the medications. These medications are usually reserved for patients who are symptomatic, under age 65, presenting with atrial fibrillation for the first time, or who have atrial fibrillation secondary to a known cause (Neal, 2007). Prevention of the arrhythmia is of course always the best alternative. Research studies have been done on the usage of vitamin C and its reduction of atrial fibrillation recurrences (Wilson, 2007). Atrial fibrillation remains incompletely understood and many research studies are needed to pose “cures” for the disorder as apposed to the current treatment regime that already exists. Medication is not the only option for the treatment of atrial fibrillation. In some patients a conversion to a normal sinus rhythm is warranted so treatments such as cardioversions, ablations, Cox Maze surgery and atrial pacing are preferred. Cardioversions via drugs or electric shock is used to restore the heart to a normal sinus rhythm in patients with persistent atrial fibrillation (Huntzinger, 2006). Direct current cardioversion by use of electric shock is indicated for patients who have been in atrial fibrillation less than 48 hours and whom are hemodynamically unstable with serious signs and symptoms from the arrhythmia. With direct cardioversion low-voltage electrical shocks interrupt the irritable foci in the heart, letting the sinoartrial node resume its role as the pacemaker of the heart controlling rhythm and rate (Rocca, 2007). Cardioversion is less effective for patients with long histories of atrial fibrillation due to the atrial remodeling of the heart that occurs. Cardioversion is successful in 80-90% of patients but may be short lived so is typically followed by a long term antiarrhythmic therapy to sustain the normal sinus rhythm (Rocca, 2007). Another form of treatment for atrial fibrillation is an ablation. Ablation therapy is not recommended for older adults because it is more likely to cause complications, so it is typically reserved for younger patients (Rocca, 2007). Ablation or pulmonary vein isolation uses radio waves to ablate circles of tissue around the opening of the pulmonary veins and block inappropriate signals being given to the heart (Managing atrial fibrillation, 2006). Ablations can occur in other areas of the heart such as the AV nodal ablation which results in a complete heart block requiring that a permanent pacemaker be inserted (Chen-Scarabelli, 2004). Although atrial fibrillation has the potential to reoccur after an ablation an international trial found that 87% of subjects who underwent the procedure had no recurrent symptoms after one year (Managing atrial fibrillation, 2006). Atrial Fibrillation 6 Erratic electrical impulses can come from a variety of areas within areas of the heart. One of the areas that have been discovered to produce these impulses is a group of muscle fibers that link the sinus node to the left atrial appendage called Bachmann’s Bundle (Khaja & Flaker, 2005). Atrial pacing prevents bradycardia, synchronizes the atria, and reduces the dispersion of refractoriness thus reducing the incidence of atrial fibrillation (Khaja & Flaker, 2005). An additional benefit of atrial pacing is that it synchronizes the atria thus reducing conduction delay (Khaja & Flaker, 2006). Many different types of pacing are can be used in the treatment of atrial fibrillation based on the area of abnormal electrical conduction. Once that area is identified the appropriate placement of the pacer can be inserted thus interrupting the abnormal electrical current of the heart and restoring a normal sinus rhythm. A much less popular form of treatment is the Cox Maze surgery invented by Dr. James L. Cox in 1987. This procedure is always considered as a last resort because open heart surgery is required to perform the procedure. This surgery involves the surgeon making small incisions in the atria that interrupt and channel erratic electrical signals that cause atrial fibrillation. The left atrial appendage is also removed during this procedure (Managing atrial fibrillation, 2006). The surgeon uses radio waves, ultrasound, or cryosurgery to create a ring of scar tissue around the opening of the pulmonary vein (Managing atrial fibrillation, 2006). It is believed that some forms of atrial fibrillation may be caused by rapidly firing muscle cells in the pulmonary veins so disruption of this area interrupts the pattern of irregular electrical flow (Managing atrial fibrillation, 2006). The long term effects of this procedure have not yet had time to be evaluated. Conclusion Working primarily with cardiac patients it has become very evident of the growing number of problems associated with atrial fibrillation. Over the past 5 years the increase in the number of patients admitted to the hospital with a history of atrial fibrillation has rapidly increased. These patients usually have a long list of various other cardiac disorders. Each case of atrial fibrillation is treated differently based on risk factors, symptoms, and type of atrial fibrillation. It is important to find the appropriate treatment for the individual patient. Different types of atrial fibrillation may warrant different treatments. With the aging of the baby boomers generation the incidences of atrial fibrillation are going to continue increasing over the next several years. For appropriate treatment to occur it is important to understand the mechanisms in which atrial fibrillation effects the heart and the various treatments involved to correct it. Atrial Fibrillation 7 References Adkins, L. (2007, April). Atrial fibrillation. Nursing, 37(4), 43-44. Azamuddin, K. & Flaker, G. (2005, April 13). Bachmann’s Bundle: Does it play a role in Atrial Fibrillation? Pace, 28, 855-863. Bentz, B. (2006, December). Gaining control over a-fib. RN, 69(12), 35-38. Boos, C., & Lip, G. (2005, November). Targeting the rennin – angiotensin – aldosterone system in atrial fibrillation: from pathophysiology to clinical trials. Journal of Human Hypertension, 19(11), 855-859. Brembilla-Perrot, B., Burger, G., Beurrier, D., Houriex, P., Nippert, M., Miljoen, H., Andronache, M., Khaldi, E., Popovic, B., Terrier De La Chaise, A., & Louis, P. (2003, July 17). Influence of age on atrial fibrillation inducibility. Pace, 27, 287-292. Camm, J. (2006, September). Medical management of atrial fibrillation: State of the art. Journal of Cardiovascular Electrophysiology, 17, 2-6. Cayley Jr, W. (2005, December 1). Pharmacologic cardioversion for atrial fibrillation and flutter. American Family Physician, 72(11), 2217. Chen-Scarabelli, C. (2004, June 15). Supraventricular Arrhythmias: An electrophysiology primer. Cardiovascular Nursing, 20, 24-31. Dilaveris, P., Giannopoulos, G., Synetos, A., & Stefanadis, C. (2005, October). The role of rennin angiotensin system blockade in the treatment of atrial fibrillation. Current Drug Targets. Cardiovascular & Hematological Disorders, 5(5), 387-403. Dorian, P. (2006, September). The future of atrial fibrillation therapy. Journal of Cardiovascular Electrophysiology, 17, 11-16. Ebell, M. (2005, June 15). Choosing between warfarin (coumadin) and aspirin therapy for patients with atrial fibrillation. American Family Physician, 71(12), 2348. Fye, B. (2006, October 5). Tracing atrial fibrillation 100 years. New England Journal of Medicine, 355(14), 1412-1414. Gottlieb, S. (2006, November). Atrial fibrillation a little-known big problem. Diabetes Forecast, 59(11), 29. Atrial Fibrillation 8 Huntzinger, A. (2006, May 15). Joint guideline released for atrial fibrillation. Journal of the American College of Cardiology, 75(10), 1565-1568. Korantzopoulos, P., Kolettis, T., Kountouris, E., Siogas, K., & Goudevenos, J. (2005, August). Variation of inflammatory indexes after electrical cardioversion of persistent atrial fibrillation. Is there an association with early recurrence rates?. International Journal of Clinical Practice, 59(8), 881-885. Managing atrial fibrillation. (2006, August). Harvard Women’s Health Watch, 13(12), 4-6. Neal, J. (2007, June 8). Atrial fibrillation. Practice Nurse, 33(11), 4. Rocca, J. (2007, April). Responding to atrial fibrillation. Nursing, 37(4), 36-42. Venguyzen, G., Simpson, C., &Abdollah, H. (2004, September 28). Atrial fibrillation. CMAJ: Canadian Medical Association Journal = Journal De L’association Medical Canadienne, 171(7), 755-760. Wilson, J. (2007). A Day without orange Juice is like an Invitation to Atrial Fibrillation. Texas Heart Institute Journal, 34(3), 265-267. Atrial Fibrillation 9