* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download heat engine
Dynamic insulation wikipedia , lookup
Radiator (engine cooling) wikipedia , lookup
Passive solar building design wikipedia , lookup
Thermal conductivity wikipedia , lookup
Thermoregulation wikipedia , lookup
Solar water heating wikipedia , lookup
Building insulation materials wikipedia , lookup
Heat exchanger wikipedia , lookup
Heat equation wikipedia , lookup
Copper in heat exchangers wikipedia , lookup
Solar air conditioning wikipedia , lookup
R-value (insulation) wikipedia , lookup
Intercooler wikipedia , lookup
Cogeneration wikipedia , lookup
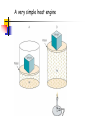
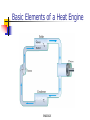
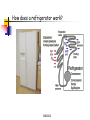
Conservation of Energy First Law of Thermodynamics ENGR302I True or False? Energy is conserved, only if no friction is present. Energy can be converted from one form to another. Work can be entirely converted to heat and vice versa. An ideal heat engine that works with no friction is 100% efficient. Construction of a time machine, although possible, must wait a very long time. ENGR302I Thermal Energy What is heat? Heat and Temperature Energy Conversion Conservation of Energy (1st Law) nd Law) Availability of Energy (2 Efficiency Applications ENGR302I Nature of Heat Historical Perspective Aristotle (Phlogiston) Lavoisier (caloric) William Thompson (motion) Heat and Temperature ENGR302I Temperature Conversion Temperature Conversion Formula Following are the temperature conversion equations for converting degrees centigrade (C), fahrenheit (F), and kelvin (K). F = 1.8 C + 32 C = (F – 32) / 1.8 K = C + 273 (1) (2) (3) ENGR302I Modes of Heat Transfer Conduction Convection Transfer of heat from molecule to molecule Transfer of heat by bulk motion Natural vs. Forced Convection Radiation Transfer of heat by electromagnetic waves. Unlike conduction and convection, radiative heat transfer requires no medium. ENGR302I Electromagnetic Spectrum ENGR302I First Law of Thermodynamics Historical Perspective Richard Meyer Kelvin Joule Energy Conversion First law efficiency ENGR302I Energy Conversion Devices EXAMPLES OF DIFFERENT KINDS OF ENERGY CONVERSIONS FROM/TO MECHANICAL THERMAL CHEMICAL MECHANICAL Bicycle Friction Bomb THERMAL Heat Engines Heat exchanger Pyrolysis CHEMICAL Rockets Food ELECTRICAL Electric Motor LIGHT Galvanometer ELECTRICAL LIGHT Wind generator Sparks Thermo-couple Luminescence Metabolism Fuel cell/ Battery Candle Resistor Electrolysis Transformer Light bulb Solar collector Photosynthesis Solar cell ENGR302I Fluorescence Availability of energy Second Law of Thermodynamics ENGR302I Second Law of Thermodynamics Natural Direction and Arrow of Time Entropy Consequence of the Second law Heat cannot, by itself flow from a low to a high temperature Work can be completely converted to heat. But heat cannot be completely converted to work. ENGR302I Why work is more valuable than heat? True or false: Which one is better? An electric heater or a gas heater? True or false: Which one is better? A gas hot water heater or a solar water heater? ENGR302I A very simple heat engine Heat Engines A heat engine is any device that uses heat to perform work. No real engine can have an efficiency greater than that of a Carnot engine when both engines work between the same two temperatures. ENGR302I Claims, Lies and bigger lies An engine manufacturer makes the following claims: The heat input of the engine is 9 kW at 375 K. The heat output is 4 kW at 225 K. Do you believe these claims? hactual = 1 - Qc/Qh = 1- 4/9 = 0.56 Hideal = 1 - Tc/Th = 1 - 225/375 = 0.4 ENGR302I Living Organisms A living organism is an example of a heat engine which transforms light energy into chemical energy ENGR302I Hurricane A hurricane is an elegant example of a natural Carnot heat engine. It draws heat from the ocean, releases some of it to the atmosphere via radiative cooling, and does work during this process. ENGR302I True or false: Heat can never flow from a cold reservoir to a hot one. ENGR302I True or false: A perfect engine produces no thermal pollution. ENGR302I Basic Elements of a Heat Engine ENGR302I Thermal Devices Internal Combustion Engines Gasoline, diesel, and gas turbine Refrigerator, Air Conditioners, and Heat Pumps Thermal Powerplants ENGR302I Thermal Power Plants ENGR302I Power Plant Operation (II) According to the second law of thermodynamics, the higher the boiler pressure (temperature), and the lower the condenser temperature, the higher is the efficiency of the power plant. Wnet Wturbine Wpump η Qin Q boiler ENGR302I How does it work? Power Plants Gasoline Engines Diesels Jet Engines Refrigerators and A/C Heat Pumps ENGR302I How does a refrigerator work? ENGR302I If you leave your refrigerator door open, the temperature in the room will be... Higher Lower The same ENGR302I How does a heat pump work? ENGR302I True or False? Energy is conserved, only if no friction is present. Energy can be converted from one form to another. Work can be entirely converted to work and vice versa. An ideal heat engine that works with no friction is 100% efficient. Construction of a time machine, although possible, must wait a very long time. ENGR302I