* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Biology project Lz
Isotopic labeling wikipedia , lookup
Coordination complex wikipedia , lookup
Nuclear chemistry wikipedia , lookup
Chemistry: A Volatile History wikipedia , lookup
Biological aspects of fluorine wikipedia , lookup
History of chemistry wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Biochemistry wikipedia , lookup
List of phenyltropanes wikipedia , lookup
Aromaticity wikipedia , lookup
Triclocarban wikipedia , lookup
Liquid–liquid extraction wikipedia , lookup
Natural product wikipedia , lookup
Abiogenesis wikipedia , lookup
Microbial metabolism wikipedia , lookup
Drug discovery wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Homoaromaticity wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
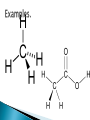
Luila Zaimi An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon (such as CO and CO2), and cyanides are considered inorganic.[1] The distinction between organic and inorganic carbon compounds, while "useful in organizing the vast subject of chemistry... is somewhat arbitrary."[2] Organic chemistry is the science concerned with all aspects of organic compounds. Organic synthesis is the methodology of their preparation. The word organic is historical, dating to the 1st century. For many centuries, Western alchemists believed in vitalism. This is the theory that certain compounds could be synthesized only from their classical elements—earth, water, air, and fire—by the action of a "life-force" (vis vitalis) that only organisms possessed. Vitalism taught that these "organic" compounds were fundamentally different from the "inorganic" compounds that could be obtained from the elements by chemical manipulation. Vitalism survived for a while even after the rise of modern atomic theory and the replacement of the Aristotelian elements by those we know today. It first came under question in 1824, when Friedrich Wöhler synthesized oxalic acid, a compound known to occur only in living organisms, from cyanogen.[citation needed] A more decisive experiment was Wöhler's 1828 synthesis of urea from the inorganic salts potassium cyanate and ammonium sulfate. Urea had long been considered an "organic" compound, as it was known to occur only in the urine of living organisms. Wöhler's experiments were followed by many others, where increasingly complex "organic" substances were produced from "inorganic" ones without the involvement of any living organism. Even though vitalism has been discredited, scientific nomenclature retains the distinction between organic and inorganiccompounds. The modern meaning of organic compound is any compound that contains a significant amount of carbon—even though many of the organic compounds known today have no connection to any substance found in living organisms. There is no single "official" definition of an organic compound. Some textbooks define an organic compound as one that contains one or more C-H bonds. Others include C-C bonds in the definition. Others state that if a molecule contains carbon―it is organic.[3] Even the broader definition of "carbon-containing molecules" requires the exclusion of carbon-containing alloys (includingsteel), a relatively small number of carbon-containing compounds such as metal carbonates and carbonyls, simple oxides of carbon and cyanides, as well as the allotropes of carbon and simple carbon halides and sulfides, which are usually considered inorganic. The "C-H" definition excludes compounds that are historically and practically considered organic. Neither urea nor oxalic acid is organic by this definition, yet they were two key compounds in the vitalism debate. The IUPAC Blue Book on organic nomenclature specifically mentions urea[4] and oxalic acid. Other compounds lacking C-H bonds that are also traditionally considered organic include benzenehexol, mesoxalic acid, and carbon tetrachloride. Mellitic acid, which contains no C-H bonds, is considered a possible organic substance in Martian soil. C-C bond is found in most organic compounds, except some small molecules like methane and methanol, which have only one carbon atom in their structure.[6] The "C-H bond-only" rule also leads to somewhat arbitrary divisions in sets of carbon-fluorine compounds, as, for example,Teflon is considered by this rule "inorganic" but Tefzel organic. Likewise, many Halons are considered inorganic, whereas the rest are considered organic. For these and other reasons, most sources believe C-H compounds are only a subset of "organic" compounds. In summary, most carbon-containing compounds are organic, and almost all organic compounds contain at least a C-H bond or a C-C bond. A compound does not need to contain C-H bonds to be considered organic (e.g., urea), but many organic compounds do. Natural compounds Synthetic compounds Biotechnology Natural compounds refer to those that are produced by plants or animals. Many of these are still extracted from natural sources because they would be more expensive to produce artificially. Examples include most sugars, some alkaloids andterpenoids, certain nutrients such as vitamin B12, and, in general, those natural products with large or stereoisometricallycomplicated molecules present in reasonable concentrations in living organisms. Further compounds of prime importance in biochemistry are antigens, carbohydrates, enzymes, hor mones, lipids and fatty acids, neurotransmitters, nucleic acids, proteins, peptides and amino acids, lectins, vitamins, and fats and oils. Synthetic compounds[ Compounds that are prepared by reaction of other compounds are referred to as "synthetic". They may be either compounds that already are found in plants or animals or those that do not occur naturally. Most polymers (a category that includes all plastics and rubbers), are organic synthetic or semisynthetic compounds. Biotechnology Several compounds are industrially manufactured utilizing the biochemistry of organisms such as bacteria and yeast. Two examples are ethanol and insulin. Regularly the DNA of the organism is altered to express desired compounds, often not ordinarily produced by that organism. Sometimes the biotechnologically engineered compounds were never present in nature in the first place.