* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Reversible and irreversible Processes
Insulated glazing wikipedia , lookup
Solar air conditioning wikipedia , lookup
Intercooler wikipedia , lookup
Dynamic insulation wikipedia , lookup
Heat exchanger wikipedia , lookup
Thermal conductivity wikipedia , lookup
Building insulation materials wikipedia , lookup
Heat equation wikipedia , lookup
Copper in heat exchangers wikipedia , lookup
Cogeneration wikipedia , lookup
R-value (insulation) wikipedia , lookup
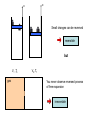
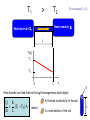
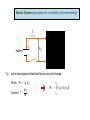
Reversible and irreversible Processes intuitive approach to reversible and irreversible processes later introduce entropy and the 2nd law foundation of thermodynamics Reversible process: can be defined as one whose “direction” can be reversed by an infinitesimal small change in some property of the system. “Gedankenexperiment” to picture a reversible process: 1 Make a video recording of a process Observable process 2 reversible Run the recording backwards Process impossible to observe irreversible Examples: Process is possible reversible Backward recording reversible Backward recording x x Small changes can be reversed reversible but V1 ,Ts gas V2 ,Tf You never observe reversed process of free expansion irreversible Reversibility is an idealization (in strictest sense, almost all real processes are irreversible) Reversibility requires equilibrium processes but Not every equilibrium process is reversible Almost perfect insulation gas in equilibrium at any time Example Tg > T0 Qout Although system in equilibrium, no small change of the system will reverse the heat flow Reversibility is an idealization Dry friction between 2 objects You never observe the reversed process: object starts to move without assistance x Friction between piston and cylinder irreversibility Heat Conductivity in Solids (an example for irreversibility) Remember: Heat is an energy transferred from one system to another because of temperature difference T1 > T2 System 2 System 1 Heat Q flows from 1 to 2 T1 > T2 *(in the textbook T >T ) 2 1 Heat reservoir 2 Heat reservoir 1 L T(x) T1 T2 0 L x A Heat transfer per time interval through homogeneous solid object: Q K (T1 T2 )A t L K: thermal conductivity of the rod where L A: cross-section of the rod Electric Systems (examples for reversibility and irreversibility) I #1 + battery VE - VE : work done against electrical forces per unit charge Work: W q VE dq Current I dt tf W VE ( t ) I( t ) dt t0 Irreversible case #1 #2 Resistor network with total resistance R VE R I W R I 2 t In the steady state: Internal energy of black box unchanged 1. law: U Q W 0 U 0 Q W R I 2 t 0 Heat leaving the system Application as heater #1 reversible case Capacitor network with total Capacity C #2 q2 - Charging an uncharged capacitor W 2C q2 - Discharge of the capacitor W done by the capacitor 2C No heat transferred (Q=0)