* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Superconductors: Better levitation through
Electromagnetism wikipedia , lookup
Aharonov–Bohm effect wikipedia , lookup
Electromagnet wikipedia , lookup
State of matter wikipedia , lookup
Electrical resistance and conductance wikipedia , lookup
Electrical resistivity and conductivity wikipedia , lookup
Condensed matter physics wikipedia , lookup
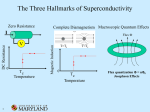
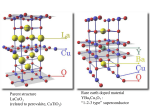
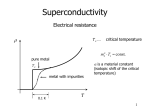
Superconductors Better Levitation through Chemistry Arthur B. Ellis University of Wisconsin-Madison, Madison. WI 53706 Nineteen eighty-seven will be remembered as an extraordinary year for the physical sciences. I t was a year in which new superconductive materials created unprecedented excitement in the scientific community. And it was a year in which the public became spellbound by new vistas in technology. The title of this article reflects the notion that chemistry can have a positive impact upon our lives-"better living through chemistry". And it includes the phenomenon that best captures the almost magical quality of superconductivity-levitation. First, I will outline the history of superconductivity, then describe the physical and chemical principles upon which i t rests, and, finally, speculate on some of the applications tbat may he forthcoming ( I ) . Hlstory Superconductivity owes its discovery to a Dutch physicist, Heike Kamerlingh Onnes. In the early 1900's, Kamerlingh Onnes accomplished the then remarkable feat of liquefying helium. The ability to drop temperatures to a few degrees Kelvin (helium boils at 4.2 K a t atmosoheric oressure) suddrnly o;,ent.d a iarger svindow for studies of thermal effects. Kamerlineh Onnrs exvloited his new t d hs measurina the electrical iesistance df metals a t these very low temperatures. In 1911, while measuring the resistance of mercury, he found an astonishing result: at about 4 K, the resistivity abruptly dropped below his ability to measure it. Nor was mercury unique in displaying what appeared to be zeroresistance to a flow of electrical current. Several other metallic elements exhibited the same effect a t temperatures of a few degrees Kelvin. This remarkable electrical property was treated as a diagnostic for a new state of matter, the superconducting state. 87 UI Fr RI Ac I IN Unqs Unp' IM Unh' 107 Un5' IOB About 20 years later, in 1933, another surprising characteristic of superconductivity was discovered by Meissner and Ochsenfeld. They found that a superconducting material will not permit a magnetic field to penetrate its hulk, a property that has come to be called the Meissner effect. These two diagnostics of superconductivity, resistanceless current flow and perfect diamagnetism, were appreciated even 50 years ago as having tremendous technological implications. But two maior obstacles to imolementina- anv - new technology existed. First, extremely cold temperatures were reauired to achieve the suoerconducting- state. Although . liq. uid helium could serve as a coolant, its scarcity and processing costs made it expensive; moreover, sophisticated equipment was required to handle it. The second problem was tbat the superconducting state of these elemental metals was easily destroyed by the application of modest external magnetic fields or electrical transport currents, making their use in electromagnetic applications impractical. These problems prompted an intensive search for new materials that would become superconducting a t higher temperatures and retain their superconductivity in the presence of large magnetic fields and electrical currents. In particular, scientists who studied this phenomenon dreamed of achieving superconductivity a t or above 77 K (-196 ' C ) , in which case liquid nitrogen (boiling point, 77 K), which is cheap and easily handled, could be used as a coolant. Until recently, alloys of niobium, particularly Nb-Ti, had been responsible for the greatest advances in superconductor technology. Their ability to remain in the superconducting state while supporting large electrical currents led to limited but extremely important applications such as the construction of powerful magnets. In 1973, superconductivity was observed at 23 K with NbaGe. Another breakthrough 10) U d Figure 1. The peridic table with elemems that exhibit superconductivity at ambient pressure indicated in boldface autilne; all d these elements requlre temperatures of <10 K to main the superconducting state. Adapted from ref. 2. 838 Journal of Chemical Education involved the observation of superconductivity with certain organic salts, whose structur& feature one-dimensional stacks of organic moieties that can be modified by chemical substitution. These salts have required liquid-helium-range' temperatures to become superconducting. In spite of these advances, there was a feeling of despair among some scientists that perhaps superconductivity a t 77 K was simply not meant to be. This gloomy picture reflected the fact that, while more than 20 metallic elements exhibit superconductivity (Fig. I),they do so only a t very low temperatures near that of liquid helium (2). Furthermore, most bf the simple alloys or compounds based on combining two or three elements also required disappointingly low temneratures for sunerconductivitv. I t was against this setting that Georg Hednorz and K. Alex Mi~llcr.two scientist3 working at the IBM facility in Zurich, reported a startling result in early 1986: an oxide of lanthanum, barium, and copper lost its resistance a t about 30 K (3). That a metallic oxide, generally regarded as a mineable ore (or worse, as a form of dirt), should not only exhibit superconductivity but do so a t such a high temperature struck many in the scientific community as incredible. Suddenly, a whole new familvof materials. ceramics. was r i ~ for e investi----~ -~~ gation, and the pace of researih accelerated markedly. In early 1987, groups a t the University of Houston and University of Alabama, headed by physicists Paul Chu and M. K. Wu, Jr., respectively, announced (4) the discovery of a related oxide that was superconducting above 77 K! Superconductivitv at the temperature of liquid nitrogen (in fact, above 90 K)became a ;eality, to the delight of many researchers who never expected to see it. In fact, we cannot rule out the possibility that room temperature superconductivity is in the offing. At this writing, several research groups have seen tantalizing evidence for such a possibility. Let usnow turn to the physics and chemistry that underlie these momentous advances. ~ .................... .................... .................... ..................... .................. .................... .................... vacancy interstitial impurity Physical Properties of Superconductors Loss of Resistance A material's oassaee into the suoerconductine state is characterized b; an &rupt change k its resistivzy as i t is cooled. In ordinarv conductine - materials the flowing- electrons that represent current always encounter some resistance, which can he likened to friction. Ohm's law relates the current, I, to the voltage, V, (the difference in electrical potential between points in a circuit that causes electrons to flow) and resistance, R, thus: V = ZR. The source of the resistance is the scattering of electrons from the atoms that make up the conducting material. Scattering occurs if the atoms are vibrating andlor if lattice defects are nresent. Defects. which are illustrated in Fieure 2, occur when a normally periodic arrangement of atoms, a crvstal lattice. is interrunted hv imnuritv atoms. hv dis~lacemerit of atoms to posiiions that they "would not nokwlly occupy (interstitials), and by the absence of atoms (vacan~ i e ~ ) Figure 3 graphs the resistivity of a superconducting material (the resistivity is proportional to resistance) as a function of temperature. As the sample is cooled, there is initially a smooth decline in resistivity with temperature; with less thermal energy available, the atoms vibrate less, causing less scattering. In a normal metal, this decline ceases a t very low temperatures when scattering becomes limited by fixed defects: a limitine or residual resistivitv exists. What characterizes the superconducting state, however, is that there is a sudden drop to zero resistivity a t what is called the critical temperature, T,.Below T,, a direct current can flow indefinitely in the material, so far as anyone has been able to determine. This is a form of perpetual motion that is one of the amazing-. ~ r o.~ e r t iof e sthe su~erconductinestate. The transition from a normaimetal to a s~perconductor can be regarded as a phase change. But unlike the phase change associated with, say, conversion of a liquid to a gas, the enthalpy and entropy of the phase change are zero (if no magnetic field is present); there is a change in the specific heat of the material, however. - The Melssner Effect and Levitation Besides resistanceless current flow, the other characteristic of a superconducting material is that it is perfectly diamagnetic. The expulsion of magnetic field lines from the interior of a material as it passes from the normal to the superconducting state, the Meissner effect, is illustrated in Figure 4. Figure 2. Types of lattice defects Temperature Figure 3. Resistivity as a function of temperature for a superconductor Figure 4. The Meissner effect. A superconductar (shaded sphere) expels magnetic field lines from its interior. Volume 64 Number 10 October 1987 837 In order to appreciate how the Meissner effect operates to produce levitation, we need t o review some of the principles of electromagnetism. As recently as the beginning of the 19th century, electricity and magnetism were believed to be two separate phenomena. In 1820, however, the Danish physicist Oersted observed a connection between them. As illustrated in Figure 5, Oersted found that when he passed an electric current through a wire, a deflection was observed in the magnetic pointer of a compass; in other words, the current had induced a magnetic field. Superconducting magnets are based on this principle: a magnetic field induced by passing current through continuous superconducting coils of wire will persist indefinitely, since there is no resistance to the flow of current. Following Oersted's obsenrations, two other scientists, Michael Faraday and Joseph Henry, investigated the related question of whether a magnetic field could induce a current in an electrical conductor. They found that a magnet could induce a current if the magnet was constantly in motion (or more fundamentally, if the magnetic flux linking the coil is continuously changing), as shown in Figure 6. Our present large-scale electricity needs are now met by generators that work on this ~ r i n c i ~ l e . The coupling of electrical and magnetic phenomena is eleeantlv demonstrated in a levitation experiment. We begin hycoolihg a pellet so as to make it su~rconducting.If an appropriate light-weight, strong magnet is now placed ahove the pellet, it will hover, as if by magic, over the pellet. The magnet retains this position so long as the pellet remains superconducting; once the pellet warms to its normal state, the magnet will no longer remain suspended in air. Why does levitation occur? Electromagnetism tells us that as the magnet first approaches the pellet, it will induce a current in the surface of the superconductor. Because the superconductor has no resistance, this current remains even after the magnet stoos movine. I t is a su~ercurrent.The supercurrent in turn induces a magnetic field. The induced magnetic field has just the right strength and geometry to cancel completely the effects of the magnetic field, arising from the maenet. . . . in the bulk of the suoerconductor. The interior d the supercondurtor is thuv perfectly diamagnetic. as demanded bv the Meiisner effect. Ourside of the superconductor, however, the field caused by the magnet a n d t h e field induced by the superconductor repel one another, just as two north or two south poles of conventional magnets would. The result is that the magnet is suspended in limbo, its position dictated by the equilibrium between the downward gravitational force and the upward magnetic repulsive f0rce.h its ability to respond electromagnetically to movements of the magnet, the superconductor can be regarded as a kind of magnetic mirror. Levitation is easily demonstrated with the new superconductors havine T, ahove 77 K. A pellet of the material is cooled with &id nitrogen, u s i n i a Styrofoam stand (an inverted coffee CUD,for example). When placed above the pellet, a standard Iefrigeratormagnet, if small enough, can become airborne. However, the lift is more dramatic with strong, rare-earth-based magnetic compounds like SmCoS and FelaNdlB. .. - We have cleveloped a technique, using an overhead projector, placed on its back, and mirror, for enabling a large audience to view alevitation experiment (5). This is truly a demonstration accomplished with magnets and mirrors! Compass The Mechanism of Superconductivity Superconductivity baffled theorists for many years after its discovery. I t was not until the late 1950's, some 40-odd years after Kamerlingh Onnes' experiments, that a satisfactory theory was found. Called the BCS theory after its authors. Bardeen. Cooper, and Schrieffer, i t satisfactorily accounts for virtually h l of the properties of the traditional, low-T, superconductors (2).The cornerstone of the theory is a not& that a t first seems counterintuitive: electrons are attracted to each other to form what are called Cooper pairs. That two electrons, both negatively charged, would feel an attraction seems to violate what we know about simple electrostatic interactions. The key to the attraction, though, is that i t is mediated by the lattice. Positive ions, whose positions are fixed in the lattice, result in any metal because the valence electrons of atoms composing the metal have been removed from their individual atoms and move about freely. Figure 7 shows that, as these electrons responsible for conductivity travel past the positive ions, the ions are drawn toward the path of the electron by electrostatic attraction. n Dry C e l l Figwe 5. Oersted's experiment. The magnetic needle of a compass placed beneath a conductor carrying current will be deflected by the Induced magnetic field Figure 6. Moving a magnet through a coiled wire induces a current (mare a voltage is induced. which drives the cunent). detected with a a~c~rately, galvanometer. 838 Journal of Chemical Education - a Flgure 7. The coupling (vertical lines) of two electrons (large blocks) Into Cooper pairs is mediated by lattice atoms (dots),whose positions are affected by electronic motion. And because the ions are much more massive than the electron and move more sluggishly, this "wake" of displaced nositive charee lone enough to attract a second elec. . nersists . trun. f$.rmingtht, Couper pair.'l'hr pair is thus bound by the mutual artniction of earh elrctron fi,r the ~ositiveions in the lattice. Important characteristics of the Cooper-pair electrons are that their spins are paired and the combined momentum of the pair is not affected by electron scattering. Consequently, scattering does not provide the energy transfer between the electrons and lattice that produces resistance in a normal conductor. In the superconducting state most of the conduction electrons are bound in Cooper pairs. A crude way to think of these pairs is as diatomic molecules. And like such molecules, they can be dissociated if sufficient energy is present to disrupt the bonding. The value of T,is a reflection of this energy: as a superconductor is warmed, the number of Cooper pairs drops as T, is apnroached. Tempernture is not rhr only experimental parameter that call affect the formntion of Cooper pairs. We have seen that. when magnetic fields are applied to a superconductor, currents will he induced in the surface of the superconductor. If these fields and corresponding currents are sufficiently large, they can impart so much energy to the superconductor that the Cooper pairs will be dissociated and the superconductivity destroyed. Many potential applications of superconductors are thus ~recludedbv the critical field H, (and corresponding critical current) a t which this conversion from suoerconductine to normal behavior occurs; recall that small magnetic field; and electrical currents destroyed superconductivity in the early superconductors. Figure 8 shows how T,varies with H, for a typical superconductor. The points on the curve represent the critical temperature at u,hich the phase change fntm normal cunductor t o s u p r r c ~ n ductor takes d a r e in the presence of different magnetic field strengths. AS-themagnet& field increases, lower values of T, result. The BCS theory makes no restriction on how large T,can be. But at present we do not know the extent to which the theory can be applied to the new superconductors. Chemical Properties of a Hlgh-T, Superconductor Svnthesis and Oxidation States The superconducting oxide that has created such a maelstrom of research activitv has the deceptively simple formula, YBa&~307-~(x 5 0.1). I t is often referred-to as the "1-2-3" compound because of the Y.Ba:Cu stoichiometry. Making a pellet of the compound is straightforward, although several steps are involved ( 5 , 6 ) .In a typical procedure, the starting materials, Y203, BaC03, and CuO, are ground together and heated to about 950 OC. After cooling, nellets can be messed and then sintered at 950 "C. Sinterina involves heating just below the melting point; this process ~ r o m o t e bondine s between the erains comnosina the pellet. ;hereby increasing the densityUand strength o r t h e pellet: v 6 Superconductor Normal conductor Temperature Figure 8. Relationship of H, and T, for a typical supercanductor. Once the pellet has been sintered, it is heated in 0 2 a t 500600 "C and slowly cooled to room temperature. The oxygen content of YBa2Cua07-, has been estimated from standard chemical methods, which indicate that the material is a nonstoichiometric compound: instead of having an integral number of oxygen atoms, the best supercondncting materials appear to have values of x of about a tenth or less. As will he discussed in more detail below, nonstoichiometric compounds are common in the solid state and reflect chemistrv associated with lattice defects. Formai oxidation states can be assigned to the elements of the 1-2-3 compound. Based upon the normal oxidation states of -2 for the oxide oxygen atom, +3 for the yttrium atom, and +2 for each barium atom, the resulting average oxidation state for each conoer . . atom is 713 (for x = 0). We can intrrpret this nonintegral oxidation state for Cu to mean thnt. on aieraee. two-thinlsof the C'u is wesent in Cu2' sites in the lattice &d one-third of the Cu isin Cw3+sites. It is natural to ask why the 1-2-3 oxide had not been prepared before, given the tremendous amount of research that has been conducted with oxides. The answer lies in the large number of elements that form oxides and the difficulty in predicting which of these combinations will yield new phases. Most of the elements in the periodic table form solid oxides, so that the number of ways we can combine three oxides is staggeringly large. Furthermore, we cannot always predict which combinations will lead to solid solutions. For example, AI2O3and Cr203 are miscible in any proportion to yield compounds of the type (A12-,Cr,)03, where x can take anv value from 0 to 2: in contrast. LiCl and KC1 are mutuallv in'oluble (7). whether compounds form solid solutions or simnlv retain their identities in a ~hvsicalmixture is often determined by X-ray and neutron diffraction: the interaction of X-rays and neutrons with a periodic crystal lattice yields a diffraction pattern that serves as a fingerprint for identifying the formation of a new phase. Both X-ray and neutron diffraction patterns of the 1-2-3 oxide were used to demonstrate that it was a new material. In trying to understand what makes the 1-2-3 oxide so special, researchers have tried to tune the material chemically by substituting for Y, Ba, and Cu. A variety of rare earth elements have been substituted for Y without greatly disturbing the superconducting characteristics of the material. However, substitution for Ba or Cu adversely affects superconductivity (8). ~ ~ ~ Structure Because the structure of a solid is intimately related to its physical properties, X-ray and neutron diffraction have assumed a prominent role in the characterization of the 1-2-3 oxide. The structure of this superconductor belongs to the perovskite family. Perovskites characteristically have aratio of two metal atoms for each three oxygen atoms. Representative comnounds with the nerovskite structure are CaTiOp (ca2+;~ i ~ ; )NaNbos , ( ~ a + l N b ~ and + ) , ~ a ~ 1 (0~3a ~AI"~); +; in these simnle the metal oxidation states must . ~erovskites . sum I,, tfiin order to yield nnelectricnlly neutral compound. Shown in Fieurc 1 is the solid-state structure for the mineral perovskite, CaTi03. Note that the larger Ca2+cation is a t the center of the cubic unit cell, the smaller Ti4+ions are located a t each corner of the cube, and the 02-ions bisect the edges of the cube. If the 1-2-3 compound had an idealized perovskite structure, it would possess nine oxygen atoms in its formula (metal-to-oxygen atom ratio of 23) and would have the tripledecker structure sketched in Figure 10a. The resulting idealized unit cell, consisting of three stacked cubic unit cells, is now tetragonal rather than cubic, having a square base but rectangular sides. Note that the top and bottom compartments of the tetragonal unit cell contain Ba2+ions and that the middle compartment contains a Y"+ ion. The Cu ions Volume 64 Number 10 October 1967 839 occupy the corners of the cubes composing the unit cell, while the oxide ions again hisect the cube edges. Why is the ideal structure not adopted by the 1-2-3 oxide compound? The answer presumably lies in the excessively high oxidation state required of Cu. A formula of YBa2Cu309 leads to an average copper oxidation state of 1113, which implies contrihutions from both Cu3+ and Cu4+ oxidation states. The fact that tetravalent Cu compounds are extremely rare strongly suggests that the average Cu oxidation state must be +3 or less. One avenue for achieving a lower oxidation state is to expel oxygen atoms from the lattice: as we saw above, the observed stoichiometry of the 1-2-3 oxide with annroximatelv seven oxveeu atoms vields an average copper oxidation statk of 713, whLch impliescontrihutions from bbth Cu2+and Cu3+ions (but not from Cu4+ions). How the structure is tuned by varying the oxygen content is illustrated in Figure lob. Although it is difficult (due to crystal disorder problems) to locate all of the oxygen atoms in the structure, the general geometrical pattern appears to be that four of the 12 02-ions surrounding the Y3+ion have been lost as well as four other 02-ions, two from the top face of the unit cell and two from the bottom. The oxygen vacancies are defects in the lattice that are governed by chemical equilibria. I t is helpful, in fact, to regard the solid as a solvent wherein vacancies, impurities, and interstitial atoms act as solutes that are subiect to the same kinds of mass action expressions that are used t o describe aaueous acid-base chemistry. In this case, the concentration df oxygen vacancies is coupled to the oxidation states of the Cu ions composing the lattice: as the oxygen content declines, so too does the average Cu oxidation state. Specifically, since the solid must remain electrically neutral, each oxygen titanium calcium Figure 9. The perovskite structure of CaTiO, possesses a cubic unit cell (the fundamental building block of a threedimansianal lattice). The conelation of the unit cell with the chemical formula is established by determining lhe enent to which each atom in lhe unit ceil contributes to this particular unit cell: The single Ca" ion Is fully contained in and belongs exclusively tothis ceil; each of the eight Ti4+ ions is shared by eight unit cells (8 X 118 = 1 Ti4+ ion); and each 01 the twelve 02-ions is shared by four unit cells (12 X 114 = 3 0'- ions). The ions Sires are not drawn to scale. b a -~ Fiaure 10. la1 YBa,CulOq, . . Idealized unit cell of the hvoolhetical .. . . . which is essumed to oe oared an a perovsrlte-lire wb-sw~cture.ib) Idealwed structure of the 1-2-3 Oxioe compamd. YBarC~,O,., ablaned hom X-ray d'nraction m a y e s . The ion szes are no1 drawn to scale. Aoapled from ref 8. 840 Journal of Chemical Education 02-ion that is removed reauires comnensatorv removal of two units of positive charge; this can he accomplished if, for example, two Cu3+ centers are converted to C U ~centers. + An equation describing the equilibrium that couples oxygen vacancies, V, with Cu oxidation states is, Are there any clues to the superconductivity of the 1-2-3 oxide from its structure? Speculation has focused on the hondine and coordination reouirements of Cu. The oxveen vacauci& in the 1-2-3 structu>e create sheets and chaihs of Cu atoms, linked through the remaining oxygen atoms. One consequence of such a bonding arrangement is that the physical properties of the material, including superconductivity, will show anisotropy, that is, they are dependent on the lattice direction along which they are measured. Much more information is needed, however, before the remarkable properties of this particular 1-2-3 oxide can be explained. Applications Will the hieh-T- oxide sunerconductors transform the technological Lndsiape of ou; world? Applications, a t least in nrincinle. . . increase with critical temnerature: liauid-nitrogen-hased superconductivity pcrmits geographically broader anulicntiuns thahdoes technolorn .. -- based on liquid helium; and the discovery of a room-temperature superconductor has the potential to bring superconductive devices into every household. Whether these applications come to fruition will depend on whether some formidable problems in the realm of materials science can be overcome. If we think hack to the notion that the high-T, materials are really a form of dirt, the difficulty in fabricating them into wires, ribbons, and films is readily appreciated. One of the major technical challenges associated with the ceramics is to find ways to mold them into useful shapes. If this can be done, applications await in such diverse areas as the transmission of electrical power, transportation, recreation, magnet-intensive technoloeies, - . and education. The ability of a superconductor to support a resistanceless direct current can be exploited in the lossless transmission of electrical power. At present, a substantial fraction of electricity is lost as heat through the resistance associated with traditional conductors. Whether conventional transmission of electricitv will he affected by the ceramic is difficult to assess. ~ h e i eis some loss of energy when alternating current, the form of current provided by utilities, is being transferred. A laree-scale shift to sunerconductine technoloevwill -. also hingeon whether wires can be prepared from the ceramics that retain their superconductivity at 77 K while supporting large current densities. Other potential applications of the new superconductors appear in the field of electronics. For example, miniaturization and increased s ~ e e dof computer chius are limited bv the generation of heat and the charging time of capacitors arising from the resistance of the interconnectinn metal tilrns.'i'he use of the new ceramics may rrwlt in more denseIs packed rhips that could transmit information more rapid& by orders i f magnitude. The use of superconductors for transpottation has already been established using liquid helium as a coolant. A prototype levitated train system has been constructed in Japan by equipping the train with superconducting magnets and placing magnets on the track. Since the Meissner effect permits levitation of a magnet with a superconductor or of a superconductor with a magnet, a variety of engineering strategies for transportation are possible: Magnetic vehicles riding above a superconducting freeway are shown in Figure 11. Once the vehicle is propelled, be i t by gasoline, a magnet, or blast of air, little energy should be required for sustained motion; on the other hand, means for starting, stopping, and changing direction will need to be developed. It should be - - Figure 12. Moving can be e breeze with levitation Figure 11. Levitation gives new meaning to "free"-ways. noted, too, that although the idea of three-dimensional personal transportation is appealing, a new set of traffic-control problems will await these magic-carpet rides. Recreation is an area that is ideally suited for superconductiveflights of fantasy. I t is easy to imaginelevitating toys and facilities. For example, a su~erconductingrink would literally to walk on air. A enable a magnetically shbd superconducting yki slope could create new forms of slalom and downhill competition. Furniture whose height can he adjusted by superconducting c4ectromagnet.i would provide new perspectives on interim and exterior decorating. And, as illustrated in Figure 12, moving cumbersome personal helongings could become cheaper and easier. Superconducting magnets are crucial components of seveta1 technologies. Magnetic resonance imaging (MRI) is playing an increasingly prominent role in medicine. The intense magnetic fields that underlie this technique provide a unique diagnostic tool for physiological studies. Similarly, the particle accelerators that serve the high-energy physics community are dependent on high-field superconducting magnets. The recent controversy surrounding the Superconducting Super Collider (SSC) also illustrates the political ramifications of new technologies. At issue is whether the multibillion-dollar project should be constructed now, using existing liquid-helium-based superconductor technology, or whether construction should be postponed to embrace the developing liquid-nitrogen-hased technology. Proponents of waiting argue that a substantial savings in cooling costs would result, assuming that the oxides can be fabricated into useable forms. Advocates of immediate construction believe that the cooling costs are a relatively small portion of the SSC costs and argue that developing the oxides to the point where they can be used in the SSC may take too long and offer little benefit. While the aforementioned applications are a t varying stages of development, the educational landscape is already being reshaped by the new superconductors. The 1-2-3 oxide is readily prepared, permitting demonstrations of levitation throughout the country. As I hope this article has illustrated, levitation can be used as a starting point to discuss physics, chemistrv. materials science. and even nolitics. Most oeoole are absokely entranced b; a levitate'd magnet. ~ n once d thev have said. "Gee whiz".. thev " ask whv it works.. soeculate . on what i t can be used for, and say, "Gee whiz", again. What more can you ask for in science education? Acknowledgment I am grateful to my research group and to J. E. Nordman, D. C. Larhalestier, J. C. Wright, L. F. Dahl, and J. Stewart for helpful comments. I thank Cheryl Agulnick for drawing the levitation cartoons. The University of Wisconsin Graduate School is acknowledged for generous support of our studies of superconductors. Llterature Cited 1. General references for this ertido include the following, Is) R e - l n n e s , A. C.: Rhoderick. E. H. lntrnduelion to Suparconducliuify; Pergamon: Oxford, 1876. lb) Grd, R. 2. 2. 4. 6. 6. 7. 8. New Ymk. 1973. IcI Beehgasrd, K.: Safeand Simpl~E1ectricalErprrimanfs;Douer: Jerom~,D.Sei.Am.1982,247.52.Id) Little, W.A.Sci.Am. 1965,212,21. le)Kunzler, J.E.:Tanenhaum. M. Sch. Am. 1962,206,60. If) 9uchhold.T.A. Sci. Am. 1960,202, 74. lg) Matthiss,B.T.Sci.Am. 1957,197, 92. Asheroff, N. W.; Mermin, N. D. S d i d State Physics; Saunden: Philsdelphii, 1976; Chapter 34. 9 e d n o n . J . G.: Moiler. K.A.Z. Phys. 6. 1986.64, 189. (a) Wu.M. K.. Jr.:Ashhum,J. R.;Torng,C.J.:Hor,P. H.;Meng,R.L.:Gao,L.;Husng, Z J . ; Wmp,Y.Q.:Chu,C. W. Phyr-Re". Lett. 1981.58.908. (h) Caus, R. J.;Batlogg, R. 9 . : M U C P ~ Yn. , w.: sunshine, s.: sicgrist. T.; ~ ~ ~J. P.; ~ i 6.: oouer, Rietman. E.A.;Zshursk.S.; E ~ p i n m a . G . P . P h y sRro.Lafi. . 1987,47,1676. Juergens. F. H.; Ellis. A. B.; Dieckmann, G. H.: and Perkins, T . R. I. J. Chem. Edue. 1987,61,&51. Grant, P. M. N u Sci. 1987, (July 301.36. 1.4 W e e , A. R. Solid Stof. Chemistry and Its Applienfionr; Wiley: Now York. 1984: Chapter 10. (h) Evans, R. C. An Introduction Lo Crystal Chemistry: Cambridge University: Cambridge, 1979 Chapters 8 and 9. Dagani, R. Chem. En#. News 1987.65lMay 11),7. Volume 64 Number 10 October 1987 841 k ~ .