* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 7-Heme Degradation
Survey
Document related concepts
Transcript
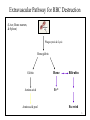
Heme Degradation Dr. Shumaila Asim Lecture # 7 1 FATE OF RED BLOOD CELLS Life span in blood stream is 60-120 days RBCs are phagocytosed and/or lysed Normally, lysis occurs extravascularly in the reticuloendothelial system subsequent to RBC phagocytosis Lysis can also occur intravascularly (in blood stream) 2 Extravascular Pathway for RBC Destruction (Liver, Bone marrow, & Spleen) Phagocytosis & Lysis Hemoglobin Globin Heme Amino acids Fe2+ Amino acid pool Bilirubin Excreted 3 Handling of Free (Intravascular) Hemoglobin Purposes: 1. Scavenge iron 2. Prevent major iron losses 3. Complex free heme (very toxic) • Haptoglobin: hemoglobin-haptoglobin complex is readily metabolized in the liver and spleen forming an iron-globin complex and bilirubin. Prevents loss of iron in urine. • Hemopexin: binds free heme. The heme-hemopexin complex is taken up by the liver and the iron is stored bound to ferritin. • Methemalbumin: complex of oxidized heme and albumin. 4 Bilirubin Metabolism Bilirubin formation Transport of bilirubin in plasma Hepatic bilirubin transport Hepatic uptake Conjugation Biliary excretion Excrete through intestine system 5 • Bilirubin is the terminal product of heme metabolism. Heme is present in hemoglobin and in other oxidative compounds such as hepatic mitochondrial and microsomal cytochromes (P450). • Thus plasma bilirubin is part erythropoietic and part non-erythropoietic. • Approximately, 85 % erythropoietic and 15% nonerythropoietic. 6 • The erythropoietic fraction originates from two sources: • The circulating normal aging red cells and • The immature defective red cells of the bone marrow. • The daily production of bilirubin is 250 to 350 mg. 7 • Shunt bilirubin is called that portion that does not originate from circulating red cells but originates from immature and defective red cells (7%) and from nonhemoglobin heme compounds, particularly from hepatic cytochromes and from myoglobin 8 • Bilirubin from erythropoietic heme is produced by monocytic macrophages, reticulo-endothelium, in every organ but especially in the spleen, liver and bone marrow in order of importance. • The bilirubin from non-erythropoietic hepatic heme is produced in the hepatocytes. 9 Bilirubin formation microsomal cytosol The iron-free porphyrin portion of heme is also degraded, mainly in the reticuloendothelial cells of the liver, spleen, and bone marrow. 10 The first step Heme oxygenase Heme oxygenase (HO) is an enzyme that catalyzes the degradation of heme. This produces biliverdin, iron, and carbon monoxide. 11 • The tetrapyrrolic ring of heme is broken by an oxygenase at the alpha bridge, the bond between the two carbons opposite to the gamma bridge which is between the two carbons carrying the two propionic acids. • The tetrapyrrolic molecule from a ring is transformed into a tetrapyrrolic chain without iron 12 Heme oxygenase There are three known isoforms of heme oxygenase. Heme oxygenase 1 (HO-1) is an inducible isoform in response to stress such as oxidative stress, hypoxia, heavy metals, cytokines, etc. Its activity is induced by its substrate heme and by various nonheme substances. Heme oxygenase 2 (HO-2) is a constitutive isoform which is expressed under homeostatic conditions. Both HO-1 and HO-2 are ubiquitously expressed and catalytically active. A third heme oxygenase (HO-3) is not catalytically active, but is thought to work in oxygen sensing. 13 In mammalian cells Heme oxygenase (HO1) has two basic functions: 1. it recycles iron supplies within the cell to maintain homeostasis. 2. biliverdin and biliruben (its reduced form), are powerful antioxidants believed to aid in the prevention of oxidative cell damage. 14 HOOC M V M CH2 CH2 CH O N H CH2 CH2 M CH2 N H M:-CH2 V:-CH=CH2 M V COOH N H O CH N H 15 The building of intermolecular hydrogen bonds by the NH and COOH groups is spatially hided. Bilirubin is lipophilic and therefore insoluble in aqueous solution.the solubility in water is less. 16 • HEME + Heme oxygenase = OXYHEME ( closed tetrapyrrolic ring with iron) • OXY- HEME + heme reductase = BILIVERDIN (open tetrapyrrolic ring without iron) • BILIVERDIN + biliverdin reductase = BILIRUBIN (unconjugated) 17 DEGRADATION OF HEME TO BILIRUBIN 75% is derived from RBCs P450 cytochrome In normal adults this results in a daily load of 250-300 mg of bilirubin Normal plasma concentrations are less then 1 mg/dL Hydrophobic – transported by albumin to the liver for further metabolism prior to its excretion “unconjugated” bilirubin 18