* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Clinical Trials
Polysubstance dependence wikipedia , lookup
Psychopharmacology wikipedia , lookup
Compounding wikipedia , lookup
Orphan drug wikipedia , lookup
Clinical trial wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Neuropharmacology wikipedia , lookup
Drug interaction wikipedia , lookup
Drug design wikipedia , lookup
Pharmacognosy wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Drug discovery wikipedia , lookup
Prescription costs wikipedia , lookup
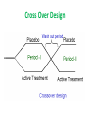
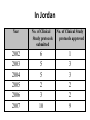
Basic & Clinical Evaluation of New Drugs Mayyada Wazaify, PhD Brain Massage! The development and testing process required to bring a drug to market DRUG DISCOVERY • Most new drugs or drug products are discovered or developed through one or more of six approaches : 1. Identification or elucidation of a new drug target . 2. Rational drug design of a new drug based on an understanding of biologic mechanisms, drug receptor structure, and drug structure 3. Chemical modification of a known molecule DRUG DISCOVERY 4. Screening for biologic activity of large numbers of natural products, banks of previously discovered chemical entities, and large libraries of peptides, nucleic acids, and other organic molecules 5. Biotechnology and cloning using genes to produce peptides and proteins. 6. Combinations of known drugs to obtain additive or synergistic effects or a repositioning of a known drug for a new therapeutic use. Evaluation in Humans • The need for careful design and execution is based on three major confounding factors inherent in the study of any therapeutic measure-pharmacologic or nonpharmacologic-in humans Evaluation in Humans • Confounding Factors in Clinical Trials 1.Variable natural history of most diseases (eg, spontaneous neoplasm remission) – to avoid errors, a crossover design is used 2. Presence of other diseases & risk factors (eg, life style) – to avoid errors, crossover design and valid methods of randomization are used 3. Subject & observer bias (placebo response) - to avoid, single-blind and double-blind design are used Cross Over Design Randomization The Food & Drug Administration (FDA) • The FDA is the administrative body that oversees the drug evaluation process in the USA and grants approval for marketing of new drug products. Jordan Food and Drug Administration • • • • • JFDA Established in 2004 Clinical Trials Unit (CTU) The Clinical Research Law established in 2001 Seven Contract Research Organizations (CROs) 19 IRB, 12 of which are active PRECLINICAL SAFETY & TOXICITY TESTING to correctly define the limiting toxicities of drugs and the therapeutic index comparing benefits and risks of a new drug the most essential part of the new drug development process PRECLINICAL SAFETY & TOXICITY TESTING • The goals of preclinical toxicity studies include: 1. identifying potential human toxicities; 2. designing tests to further define the toxic mechanisms; and 3. predicting the specific and the most relevant toxicities to be monitored in clinical trials • "no-effect" dose: the maximum dose at which a specified toxic effect is not seen • The minimum lethal dose: the smallest dose that is observed to kill any experimental animal • The median lethal dose (LD50)-the dose that kills approximately 50% of the animals.. • These doses are used to calculate the initial dose to be tried in humans, usually taken as one hundredth to one tenth of the no-effect dose in animals. Clinical Trials • Once a drug is judged ready to be studied in humans, a Notice of Claimed Investigational Exemption for a New Drug (IND) must be filed with the FDA • IND contains: (1) information on the composition and source of the drug, (2) chemical and manufacturing information, (3) all data from animal studies, (4) proposed clinical plans and protocols, (5) the names and credentials of physicians who will conduct the clinical trials, and (6) a compilation of the key data relevant to study the drug in man made available to investigators and their institutional review boards. Clinical Trials • Testing in humans is begun after sufficient acute & subacute animal toxicity studies have been completed • Chronic safety testing in animals is done concurrently with clinical trials • Usually 4-6 years of clinical testing • Ethical principles: Declaration of Helsinki, 1966 • Approval of: sponsoring organization, FDA (in Jordan: JFDA), interdisciplinary institutional review board at facility Clinical Trials • Phase I: - observes the effect of drug as a function of dosage - small number of healthy volunteers(25-50) - In some cases (cancer, AIDS, ie drugs with expected toxicity), patients with disease are used rather than normal volunteers - Nonblind (open) - Detect safety & pharmacokinetics - Done in research centers by clinical pharmacologists Clinical Trials • Phase II: - Drug studied in patients with the target disease to determine efficacy - Number of patients is 100-200 - Usually single-blind design with a placebo & positive control - Detects broader range of toxicities - Done in special clinical centers (eg, university clinics) Clinical Trials Phase III - Larger number of patients (e.g. Thousands) Further study of safety & efficacy Double-blind & crossover techniques Investigators are specialists in disease being treated If results meet expectations: application is made for permission to market the agent (NDA-new drug application) - FDA review of NDA may take up to 3 years Clinical Trials • For serious diseases, the FDA may permit extensive but controlled marketing of a new drug before phase 3 studies are completed; • For life threatening disease, it may permit controlled marketing even before phase 2 studies have been completed; • Once approval to market the drug has been obtained, phase 4 begins… Clinical Trials • Phase IV (post-marketing) - Constitutes monitoring the safety of the new drug under actual conditions of use in large numbers of patients - Some rare toxicities are revealed (low incidence) Orphan drugs: • drugs for rare diseases. Difficult to research, develop and market. FDA provides special assistance & grants for research of such drugs. • 120 orphan drugs are approved for 82 rare diseases since 1983. • Generic drug: a drug product that is produced by any pharmaceutical company after the patent of the originator drug is expired. In Jordan Year 2002 2003 2004 2005 2006 2007 No. of Clinical Study protocols submitted 6 5 5 2 3 10 No. of Clinical Study protocols approved 1 3 3 2 2 9