* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Cell Physiology
Survey
Document related concepts
Transcript
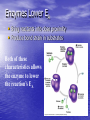
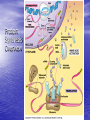
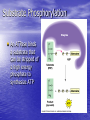
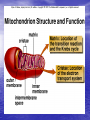
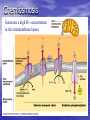
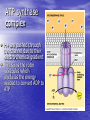
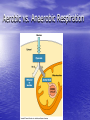
Cell Physiology: Metabolism Biology 201 Organism S&F Dr. Tony Serino Metabolism • Refers to all of the reactions that occur in the cell • Each reactions requires a specific enzyme • Energy may be released or consumed in these reactions Production Efficiency by Individual Only 1/6 (~15%) of the leaf’s energy is used to build the caterpillar’s body. The energy in the feces is left in the environment. and is used by decomposers. The part used as cellular respiration is to maintain life or is lost as heat. Only this fraction can be passed on in the food chain. Trophic Efficiency ~90% loss of energy per trophic level Metabolic Rate –total rate of energy use in an organism • Basal MR –MR of a fasting endotherm at rest • Standard MR –MR of a fasting ectotherm at rest at a specific temperature Energy Budgets Use of energy by organisms differ based on body size and survival strategies • Total energy consumed by an animal BMR/g BMR Metabolic Rate vs. Animal Size increases with body size, but when rate per gram is plotted against body mass a reciprocal function is seen • animal the higher the MR per gram of body mass, yet the overall energy consumption is greater in the larger animal Each class (reptiles, birds, mammals, etc) of organism follows this pattern, but on separate curves (BMR/g) • The smaller the •Unicellular organisms, endotherms, and ectotherms, all have a metabolic rate related to body mass at a slope of about 0.75. RQ Energy Flow in Reactions Metabolic Reactions (R P) • Most reactions are reversible • All reactions try to proceed to a dynamic equilibrium. Therefore, one way to favor a reaction is to manipulate the amount of reactants or products present. A + B C + D Metabolic Pathways • A series of reactions in the body. • Most are linked with other sets, so that the products of one reaction become the reactants of the next. • Two Kinds: – Degradative (Catabolism) – Biosynthetic (Anabolism) Pathway Map of Cell Metabolism Note: Kreb Cycle Enzymes • • • • • • • • Catalyze reactions Reactants = substrates (S) S bind to active site on E S bound non-covalently 3D structure give E specificity # of bonds formed gives affinity May use co-factors (co-enzymes) May bind other chemicals that act as modulators (change 3D shape of active site) Energy flow in a reaction • Every • reactions must overcome an energy barrier to begin. Energy of Activation (EA) Energy Flow with Enzyme Present Enzymes increase reaction rates by lowering the EA Enzymes Lower EA • Bring reactants into close proximity • Produce bond strain in substrates Both of these characteristics allows the enzyme to lower the reaction’s EA Control of Enzyme Function Proteins remain functional in a narrow range of pH and temp. Radical changes in these values can cause proteins to denature; that is, change its 3D shape Enzyme Control • Enzyme activity can be modified by changes in both enzyme and substrate concentrations • Excess substrate eventually hits a maximum or saturation point Enzyme Control • Other substances may bind • to the enzyme and modify its behavior; either as an activator or inhibitor If the substance competes with the substrate for the active site; it is a competitive inhibitor Enzyme Modulation: non-competitive inhibition and activation • Binding of a molecule to a site other than the active site may • result in an enzyme conformational change that either turns the enzyme “on or off” If the modulator is bound by non-covalent forces; it is allosteric modulation (the most common type); if bound covalently, it is covalent modulation (which is more difficult to change) Central Dogma Protein Synthesis Overview ATP cycle Utilization of ATP ATP Synthesis • Two ways to produce ATP – Substrate Phosphorylation – Oxidative Phosphorylation Substrate Phosphorylation • An ATPase binds a substrate that can be stripped of a high energy phosphate to synthesize ATP Oxidative Phosphorylation • High energy electrons are • • scavenged from the breakdown of food molecules and used to power an electron transport chain which allows the cell to synthesize ATP Uses a series of Redox reactions to power pumps Note: the PO4- is an ion of the environment and contains no extra energy Co-enzymes: NADH & FADH2 Oxidized Reduced NAD+ NADH FAD+ FADH2 The co-enzymes pick up high energy electrons and transport them to where they are needed, such as, the electron transport chain. Glycolysis Kreb Cycle Electron Transport Chain Glycolysis: Overview 2 PGAL Transition Reaction: Acetyl-CoA For one molecule of glucose, 2 pyruvates will be processed. Kreb Cycle Kreb Cycle • For one molecule of • • glucose, 2 acetyl-CoAs will be processed, so the Kreb cycle will make 2 complete turns All of the carbon atoms of the sugar have now been converted to CO2 After the co-enzymes are processed, the total amount of ATP produced per turn of the wheel will be 12 ATP Transition reaction Electron Transport Chain (Respiratory Chain) • NADH unloads its electrons • • at the start of the chain; yielding the maximum energy release per electron pair FADH2 unloads further down the line, thereby diminishing its energy return Oxygen is the final electron acceptor, it combines with hydrogen to form water Chemiosmosis Generates a high H+ concentration in the intermembranal space ATP synthase complex • H+ are pushed through • the channel due to their electro-chemical gradient This spins the rotor molecules which produces the energy needed to convert ADP to ATP Cellular Respiration Overview Aerobic vs. Anaerobic Respiration Anaerobic Respiration Food Processing Protein Metabolism • Proteins Amino Acids • Amino Acids – Deamination –removes NH forming a keto-acid – Transamination –transfers NH to other keto-acid • Keto-acids can be fed into Kreb Cycle • Amino group may form ammonia which can be converted to urea and excreted by kidney In mammals Fat Metabolism Triglyceride 3 fatty acids + glycerol ( a sugar) Fat Metabolism Fatty acids broken down • 2 C’s at one time = Beta-oxidation of fat 8 C fatty acid would yield 62 ATP molecules ((12 * 4)+15; -1 initial ATP used; -5 because last two carbons do not generate extra coenzymes) (17x-6) = # of ATP produced x = # of C pairs in the FA