* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Microscopes
Astronomical spectroscopy wikipedia , lookup
3D optical data storage wikipedia , lookup
Hyperspectral imaging wikipedia , lookup
Dispersion staining wikipedia , lookup
Ultraviolet–visible spectroscopy wikipedia , lookup
Diffraction topography wikipedia , lookup
Surface plasmon resonance microscopy wikipedia , lookup
Nonimaging optics wikipedia , lookup
Photon scanning microscopy wikipedia , lookup
Preclinical imaging wikipedia , lookup
Image intensifier wikipedia , lookup
Night vision device wikipedia , lookup
Image stabilization wikipedia , lookup
Optical aberration wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Ultrafast laser spectroscopy wikipedia , lookup
Fluorescence correlation spectroscopy wikipedia , lookup
Optical coherence tomography wikipedia , lookup
Chemical imaging wikipedia , lookup
Vibrational analysis with scanning probe microscopy wikipedia , lookup
Harold Hopkins (physicist) wikipedia , lookup
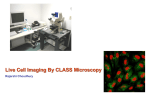
Microscopes Varieties and Applications Presented by Jeff Butler, Quorum Technologies Resolution Continuum and different resolving Techniques THE FIRST QUESTION WHAT DO YOU WANT TO SEE? Consider: Experimental design Controls Imaging Constraints • We rarely see things directly • So we often rely on indicators (fluorescent probes or refractive properties) • these may not reflect function Imaging Constraints The more we manipulate the sample, the less it reflects physiological processes Imaging Constraints Real Life Temporal Resolution Spatial Resolution ec tro n M ic ro Resolution Life sc op y O pt iG rid D ec on W volu id tio e n fie ld flu or Br es ig ce ht fie nc ld e Po in M tS ul ca ti Ph n n e ot r Li on ne Sc an O ne M r X Pi ST ED 4 El AF M Imaging Continuum Microscopy Techniques Overview ‘Classical’ Techniques • Electron Microscope: – Transmission Electron Microscope (< 1 nm) – Scanning Electron Microscope (~ 1-5 nm) • Confocals (~150 nm lateral, ~350 nm axial): – Point Scanning Confocal – MultiPhoton Confocal – Spinning Disk Confocal • Widefield Fluorescent Microscopy including: – Structured Illumination Microscopy (~180 nm lateral) – Deconvolution Microscopy (~180 nm lateral) • Transmitted Light Microscopy: – Brightfield, Phase, DIC, Darkfield (~200 nm) ‘New’ Techniques • Scanning Tunneling Microscope (0.1nm lateral, 0.01 nm axial - surface technique) • Atomic Force Microscope (0.1nm lateral, 0.01 nm axial - surface technique) • 4Pi or I3M (2 objectives) (< 100 nm) • STED (stimulated emission depletion) (< 100 nm) • OMX (structured illumination) (~ 50 nm?) • TIRF (100 nm axial) Resolution The ability to resolve two separate spots Resolution Resolution Airy Disks Rayleigh criterion • The peak to peak minimum distance that allows separation of two distinct signals • Rayleigh criterion: the radius of one Airy disk: measured from its point of maximum intensity to its first ring of minimum intensity. Resolution measurements Raleigh Equation: sin Θ = 1.220 λ D where: • Θ is the angular resolution • λ is the wavelength • D is the lens aperture diameter Raleigh Equation Derivations: 1. Δl = λ 2 n.a. 2. Δl = 0.61 λ n.a. 3. Δl = 1.22 (n.a.(obj) + n.a.(cond)) . . where: • Δ l is the minimal spatial resolution • λ is the wavelength • n.a. is the lens numerical aperture Resolution Spatial resolution is the first determinant in microscope choice Electron Microscopes High resolution Electron Microscope • Uses electrons to image the sample • Electromagnets act as lenses (though very weak) • Electron λ ~ 0.0055 nm • Thus resolutions of < 1 nm • WONDERFUL RESOLUTION BUT: Electron Microscope • • • • • • E.M. requires nonphysiological conditions including: very high vacuum Dehydration Fixation Very thin samples (50 - 100 nm)* exposure to electron opaque heavy metal salts (Ur, Pb, Os) Ab staining (immunogold) Optical Microscopy Good resolution Transmitted Light Microscopy The classical technique Transmitted light microscopy • Considered the simplest approach • All transmitted light microscopes in use are designed for Kohler illumination Kohler illumination Basic format for all transmitted light systems Ensures that: 1. 2. 3. The lamp is out of focus at the specimen The field diaphragm is in sharp focus at the specimen plane The n.a. of the illuminating beam can be adjusted without affecting the size of the illuminated field Contrast Techniques • Does not require any stain, but they enhance contrast: – Phase Contrast – Darkfield – DIC • All require an initial Kohler setup Transmitted Light Techniques Transmitted Light Advantages: Disadvantages: • Simple hardware requirements: • Minimal axial resolution • Difficult to identify specific cellular components (e.g. proteins) – Microscope • Excellent sensitivity and speed (with the right camera) Fluorescent Microscopy Imaging labeled molecules Fluorescent Microscopy • One of the most prevalent techniques • Allows (requires) a probe specific to molecule of interest • BUT: you are visualizing the probe, not the object of study – This is even true of GFP-labeled proteins the GFP moiety is often considerably smaller than the protein Fluorescence • Short wavelength absorbed • Longer wavelength emitted • But this occurs over over all possible excitation states Fluorescence Excitation • The complexity of multiple possible excitation and emission energies yields a spectrum for excitation and emissions Fluorescence There is a symmetrical distribution of excitation and emission wavelengths Fluorescence Microscope • Generally in an epifluorescence format • Require excitation filter, dichroic & emission filter Transmitted vs. epifluorscence Haze/Blur • Where does it come from? Convolution through optics Convolution through optics Why? Convolution through optics Why? Perfect Optics Convolution through optics Why? Perfect Optics DIFFRACTION Convolution through optics Why? Perfect Optics DIFFRACTION Real world Convolution through optics Why? Perfect Optics DIFFRACTION Real world Aberrations caused by the objectives What are some of the solutions? • Deconvolution • Structured Illumination (Grid Confocal) • Confocal Systems – Point Scanners (classical method) – Nipkow Spinning Disk (live-cell imaging) – MultiPhoton (deep tissue imaging) Fluorescent Microscopes Why not just use one solution: • Different microscopy techniques offer different advantages regarding: – Speed – Sensitivity – Resolution – Viability – Penetration Deconvolution Removal of haze/blur using math Point spread function Calculated PSF Measured PSF • The point spread function is a description of the convolution of light for a given objective or wavelength • Dependent on wavelength, n.a. and refractive index DEBLURRING ALGORITHMS • • • • Nearest-neighbor Multi-neighbor No-neighbor Unsharp mask Treats each 2-D plane of a 3-D image separately Nearest Neighbor Deblurring • Operates on plane ‘z’ by blurring its neighboring planes (z±1) using a digital blurring filter • Subtracts the blurred planes from z • Multi-neighbor methods extend this method From: Wallace et al, 2001. BioTechniques 31:1076-1097 IMAGE RESTORATION: INVERSE FILTERS Division of the Image FT by the PSF FT Algorithms: • Wiener deconvolution • Regularized least squares • Tikhonov-Miller regularization From: Wallace et al, 2001. BioTechniques 31:1076-1097 IMAGE RESTORATION: CONSTRAINED ITERATIVE • Nonlinear method that produces quantifiable data • Uses iterations to test different image estimates • Adds constraints or rules to each Iteration – – – – Distortion removal (smoothing) Non-negativity Boundary constraints (non-saturation) Noise removal From: Wallace et al, 2001. BioTechniques 31:1076-1097 IMAGE RESTORATION: CONSTRAINED ITERATIVE CONSTRAINED ITERATIVE ALGORITHMS: • Jansson-VanCittert (JVC) algorithm • Maximum Likelihood • Expectation maximization • Maximum Entropy • Blind Deconvolution* From: Wallace et al, 2001. BioTechniques 31:1076-1097 Constrained Iterative Estimate of deconvolved image Apply PSF* Compare to original image *blind vs. non-blind Deconvolution examples Deconvolution examples Deconvolution Advantages: Disadvantages: • Simple hardware requirements: • Less Axial resolution • Requires post processing – Microscope – Z-drive – Camera • Excellent sensitivity and speed (with the right camera) Structured Illumination Defining the focal plane Structured Illumination • The in focus sample plane is defined by a grid (grating) in a conjugate focal plane • The grid is only in clear focus when the sample is in focus Structured Illumination http://www.microscopyu.com/articles/confocal/confocalintrobasics.html Image A Image B Image C Image B Image A Grid off in Lower Focal Plane Grid on in Lower Focal Plane Structured Illumination • How does it work? • A combination of optical elements (the grid) and basic math Structured Illumination • When the Sample is in Focus the Grid is in Focus • The Sharper the Image the Sharper and Stronger the Grid • Weak or out of Focus information is removed in the Math The Math! Image A Image B Image C (A-B)2 (B-C)2 (A-C)2 Image OptiGrid 1 1 0 0 1 1 1.414 0.8 0.8 0.2 0 0.36 0.36 0.848 0.5 0.5 0.5 0 0 0 0 Widefield Structured Illumination QuickTime™ and a Cinepak decompressor are needed to see this picture. Structured Illumination Advantages: • Simple hardware requirements: – – – – Microscope Z-drive Camera Grid Paddle • No lasers needed • Excellent axial resolution • No post image processing needed Disadvantages: • Slow acquision: requires 3 captured frames for one complete image Confocal Removing out of focus light Convolution through optics Why? Perfect Optics DIFFRACTION Real world Aberrations caused by the objectives Confocal Out of focus emission light is removed by the pinhole Confocal Out of focus emission light is removed by the pinhole Confocal Out of focus emission light is removed by the pinhole Confocal • The laser aperture minimizes haze by reducing excitation outside of the point of interest Point Scanning Confocal http://www.microscopyu.com/articles/confocal/confocalintrobasics.html Point Scanning Confocal Laser Source PMT X Y Mirrors Dichroic mirror Pinhole Raster Scan http://www.microscopyu.com/articles/confocal/confocalintrobasics.html Point Scanning Confocal Point Scanner Advantages: Disadvantages: • Excellent Axial and Spatial resolution • Pinholes adjusted for each wavelength and objective • Not so fast, typically around 5 fps ( though this can be faster with resonant scanners - but these are less accurate) • Phototoxicity is a problem - not ideal for living cells Multiphoton Confocal • Uses multi-photon excitation - no pinholes needed • Requires powerful laser to generate multiphoton effect Multiphoton Confocal • Inherently confocal (no pinhole needed) • Less toxic to thick living samples (because it uses lower-energy longer wavelength excitation light) • More penetrating (because infrared light is scattered less going into the sample). Multiphoton Confocal Advantages: Disadvantages: • Less phototoxicity • Excellent tissue penetration • Ideal for living tissue • Requires highly specialized laser • Not optimal for all fluorophores • Slow for many physiological processes Spinning Disk Confocal Laser beam Collector disc Microlens CCD Camera Pinhole disc Dichroic Objective lens Sample Pinhole Spinning Disk Confocal QuickTime™ and a Sorenson Video 3 decompressor are needed to see this picture. Spinning Disk Confocal QuickTime™ and a Animation decompressor are needed to see this picture. Spinning Disk Confocal Advantages: Disadvantages: • Excellent speed (~30 fps) • Less phototoxicity: laser power divided between pinholes • Fixed pinhole QUESTIONS ???? Resources • Molecular Expressions website: http://micro.magnet.fsu.edu/ • Microscopy U website: www.microscopyu.com • Wallace et al. A Workingperson’s Guide to Deconvolution in Light Microscopy BioTechniques, 2001. 31:1076-1097 • James Pawley, Handbook of Biological Confocal Microscopy