* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download the Presentation - Alabama Pharmacy Association
Discovery and development of ACE inhibitors wikipedia , lookup
Neuropharmacology wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Dydrogesterone wikipedia , lookup
Theralizumab wikipedia , lookup
Discovery and development of direct Xa inhibitors wikipedia , lookup
Discovery and development of direct thrombin inhibitors wikipedia , lookup
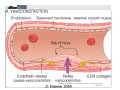
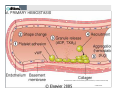
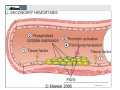
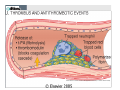
Newer Oral Anticoagulants and Available Reversal Agents Cara L. Leos, Pharm D, BCPS AQ‐ Cardiology Baptist Medical Center South Montgomery, AL Disclosure • Speakers bureau – Boehringer Ingelheim • As each of the newer agents is discussed, they will be presented in order of appearance on the US market, not in any order of personal preforance. • We will be discussing 1 investigational new drug Objectives • Describe novel anticoagulants and their characteristics • Compare differences between new anticoagulants • Assess bleeding risk of new anticoagulants • Explain different treatment options for bleeding patients • Discuss specific reversal agents Your Practice… How many of you practice in: A. Community Setting B. Health System Setting C. Other How many are: A. Pharmacists B. Technicians C. Interns Hemostasis • Circulatory hemostasis components – Vessel walls – Plasma proteins / clotting factors – Platelets – Microparticles / tissue factors • Activated by endothelial changes – Trauma or injury of some sort – Pathological (secondary to certain disease states / physical conditions) Hemostasis Big Picture • Reactionary to vascular changes • Rapid formation of platelet and fibrin plug at the site of injury • During formation, triggers it’s own dissolution (fibrinolysis) • When normal regulatory systems become overwhelmed: pathologic Thrombosis Pathogenesis Virchow triad • Endothelial injury • Stasis or turbulence of blood flow – Atrial fibrillation – Prosthetic heart valves – Immobility • Blood hyper‐coagulability – Factor V Leiden defect – Protein C or S deficiency – Antiphopholipid antibody syndrome 8 Downloaded from: Robbins & Cotran Pathologic Basis of Disease (on 17 March 2006 05:02 PM) © 2005 Elsevier Downloaded from: Robbins & Cotran Pathologic Basis of Disease (on 17 March 2006 05:02 PM) 9 © 2005 Elsevier 10 Downloaded from: Robbins & Cotran Pathologic Basis of Disease (on 17 March 2006 05:02 PM) © 2005 Elsevier Downloaded from: Robbins & Cotran Pathologic Basis of Disease (on 17 March 2006 05:02 PM) 11 But it’s not all about platelets… Coagulation Factors Procoagulants Anticoagulants • • • • • • • • • • • IIa (thrombin) Ia (fibrin) Xa Va VIIa IXa Protein C Protein S TFPI t‐PA Antithrombin (AT) Pop quiz… What factors does warfarin inhibit? A. II, V, VII, IX (2, 5, 7, 9), Proteins C & S B. II, V, X (2, 5, 10), Proteins C & S C. II, VII, IX, X (2, 7, 9, 10) Proteins C & S D. Dear Lord, I don’t remember. Please make it stop! And in the End… When good clots go bad… Thrombosis • Pathologic response to hemostasis • Inappropriate activation of the normal hemostatic processes – Formation of a thrombosis in an uninjured vessel – Occlusion of the vasculature by thrombis formation after relatively minor endothelial damage Thrombosis • Can result in emboli if dislodged – Pulmonary embolism – Deep vein thrombosis (DVT) – Myocardial infarction – Stroke • Dissolution – Fibrinolytic pathway – Surgery – Drug therapy 19 Is it a “NOAC” or a “DOAC”? DOAC’s on the Market In order of appearance… • Dabigatran (Pradaxa®) • Rivaroxaban (Xarelto®) • Apixaban (Eliquis®) • Edoxaban (Savaysa®) DOAC MOA Current U.S. Indications • Stroke prevention in afib – All four agents • Treatment of venous thrombo‐ embolism (VTE) – All four agents* • Prevention of VTE (surgical) – Apixaban, dabigatran, rivaroxaban • Secondary prevention of VTE – apixaban Direct Thrombin Inhibitor DABIGATRAN (PRADAXA®) Dabigatran: afib • 150mg PO BID – Dose found to be superior compared to warfarin for stroke risk reduction • 75mg PO BID if: – CrCl 30‐50ml/min PLUS dronedarone or ketoconazole – CrCl 15‐30ml/min (d/c drug if receiving P‐gp inhibitor) • Not to be used in CrCl < 15ml/min Dabigatran and FDA • 110mg dose: – Found to be non‐inferior to warfarin for stroke reduction, but less bleeding than the 150mg dose. – 110mg dose not FDA approved for afib • 75mg dose: – Not studied for endpoints in humans – Data extrapolated from kinetics models – FDA approved Dabigatran: VTE Treatment • 150mg PO BID after 5‐10days of parenteral anticoag (UFH/LMWH) – UFH/LMWH on own for 5‐10 days – Discontinue UFH/LMWH – Start dabigatran at next dose due • Safe for CrCl > 30ml/min except: – CrCl 30‐50ml/min PLUS P‐gp inhibitors (d/c dabigatran) Dabigatran: VTE Prevention Post‐op hip replacement surgery • 110mg PO X 1 taken 1‐4 hours after surgery & hemostasis achieved • 220mg PO qday for 28‐35 days – If 110mg dose not administered day of surgery, start 220mg regimen on POD 1 • Safe for CrCl > 30ml/min except: – CrCl 30‐50ml/min PLUS P‐gp inhibitors (d/c dabigatran) Which of the following is TRUE? A. Dabigatran 110mg dose proved superiority over warfarin in stroke reduction secondary to afib. B. Dabigatran can be used in patients on hemo‐ or peritoneal diaylsis C. Dabigatran is now approved post hip and knee replacement in the US D. Dabigatran can be started after the completion of 5‐10days UFH/LMWH therapy in acute VTE Dabigatran: Tidbits • Cannot be removed from stock bottle during dispensing – Need easy‐off? Pop the top! • Unit dose packaging only if to be used in pill boxes • Swallow whole! – Other administration can result in increased exposure to drug and increased ADE’s • Will increase an INR Factor Xa Inhibitor RIVAROXABAN (XARELTO®) Rivaroxaban: afib • 20mg PO qday with evening meal – Bioavailability ~ 60% fasting • 15mg PO qday with evening meal if: – CrCl 15‐50ml/min • Not to be used CrCl < 15ml/min Rivaroxaban: VTE Treatment • 15mg po BID (breakfast/supper) X 21 days, then 20mg po qday with evening meal – Provoked VTE: 3 months of therapy – Unprovoked: 3‐6 months of therapy • Can be continued at 20mg po qday for up to 1‐1.5 years if thought necessary – Secondary prevention Rivaroxaban: VTE Prevention Post‐op hip and knee replacement • 10mg po qday (no meal restrictions) • Started 6‐10hr post‐op & hemostasis established • Knee: 12‐14 days • Hip: 35 days • Avoid use in CrCl < 30ml/min Rivaroxaban: Tidbits • Has some data in extremes of weight – Less than 50 and up to 200kg • Can be crushed – Given in applesauce/pudding – Suspended in 50ml H2O & given via feeding tube – Still must have food/tube feeds for afib/VTE doses immediately after • Tablets are not scored • Will increase an INR Factor Xa Inhibitor APIXABAN (ELIQUIS®) Apixaban: afib • 5mg PO BID • 2.5mg PO BID if any 2 of the following: – Age 80+ – Bodyweight 60kg or less – SCr 1.5mg/dl + • ESRD on HD – 5mg po BID – 2.5mg PO BID if age 80+ or weight less than 60kg Apixaban: VTE Treatment • 10mg PO BID X 7 days, then 5mg PO BID • No renal dose adjustment, but patients with SCr 2.5+ or CrCl < 25 were not included in the trials. – Clinical decision regarding risk of clot vs risk of bleed. • Can be continued at 2.5mg PO BID after 6‐months of therapy to reduce secondary clot risk Apixaban: VTE Prevention Post‐op hip and knee replacement • 2.5mg PO BID • Started 12‐24hr post‐op • Knee: 12 days • Hip: 35 days • CrCl 15‐29ml/min: use with caution • CrCl < 15 and dialysis: not recommended Apixaban: Tidbits • FOCUS patients 80+!!! These are patients at highest risk for bleeding and are routinely low bodyweight or renally impaired!! • Can be crushed – Given in applesauce/pudding – Suspended in 60ml D5W for feeding tube administration – Not dependent on food for absorption • Will increase an INR Factor Xa Inhibitor EDOXABAN (SAVAYSA®) Edoxaban: afib • 60mg PO qday • 30mg PO qday if: – CrCl 15‐50ml/min • Do not use in: – CrCl < 15ml/min – CrCl > 95ml/min!! • AUC diminished in “normal” renal function resulting in potential diminished effectiveness!!! Increased risk of stroke Edoxaban: VTE Treatment • 60mg PO qday after 5‐10days of parenteral anticoag (UFH/LMWH) – UFH/LMWH on own for 5‐10 days – Discontinue UFH/LMWH – Start edoxaban at next dose due • 30mg PO qday after UFH/LMWH if: – Body weight 60kg or less – CrCl 15‐50ml/min – P‐gp inhibitors: verapamil, quinidine, azithro‐/clarithromycin or itra‐/ketoconazole Edoxaban: Tidbits • Only factor Xa inhibitor that can be used with phenytoin or phenobarbital • No info regarding feeding tube administration • Not food dependent • Renal function, renal function, renal function – watch your young afib pts! • Will increase an INR Which of the following is TRUE? A. Dabigatran needs to be swallowed whole. B. Rivaroxaban & apixaban are safe to use with phenytoin or phenobarbitol C. Edoxaban must be taken with food. D. Rivaroxaban is the only DOAC with dialysis dosing. Comparison of Oral Agents Agent Warfarin Dabigatran Rivaroxaban Apixaban Edoxaban Target IIv, VIIa, IXa, Xa IIa Xa Xa Xa Prodrug No Yes No No No Peak effect 4‐5 days 1.5 – 3hr 2‐4hr 1‐3hr 1‐2hr Half‐life 40hr 12‐17hr 5‐9hr 9‐14hr 9‐11hr Renal elim. None 80% 33% 25% 35‐50% Dialyzable No Yes No No No Interactions MANY P‐gp CYP 3A4, P‐gp CYP3A4, P‐gp P‐gp Monitoring Yes No No No No Antidote Vitamin K, FFP Idarucizumab In study In study In study Cove CL, Hyleck EM. J Am Heart Assoc 2013; 2:e000136 Drug in the pipeline • Betrixaban • Factor Xa inhibitor • Currently study: VTE prophylaxis in the medically ill – Replacement for LMWH? – Inpatient and post discharge while convalescing • Estimated market share: 30 million people Therapeutic Drug Monitoring These ain’t your momma’s warfarin… • ALL DOAC’s increase the INR – No relationship to degree of anticoagulation – Don’t be fooled! Vitamin K isn’t going to help • PTT: can be used to assess degree of anticoagulation in association with time of last dose These ain’t your momma’s warfarin… Dabigatran • aPTT (2.5 X reference range) may indicate overanticoagulation • Ecarin clotting test (ECT) – Academic medical centers • Thrombin time (TT) – Most reliable (direct thrombin inhibitor) – Likely send‐out lab These ain’t your momma’s warfarin… Factor Xa Inhibitors • Anti factor Xa level – Drug/reagent specific – ask your lab – Values have not been established for DOAC’s • What levels are therapeutic? • How do you adjust? These ain’t your momma’s warfarin… All anticoagulants: • CBC • Chemistries/renal function • AST/ALT Patients should be aware of: • Abnormal bruising/bleeding • Fatigue, dizziness, shortness of breath Reversal Agents Classics and Bestsellers • Vitamin K (PO or IV) • FFP (Fresh frozen plasma) • Prothrombin Complex Concentrate – Factors II, VII, IX, X, proteins C & S – Brand name Kcentra • None of which has been found effective in DOAC’s • Dabigatran: hemodialysis Idarucizumab (Praxbind®) • Approved for use October 2015 • Monoclonal (humanized) anti‐ body for dabigatran – Will not work on bivalirudin • 5g total infusion (2, 2.5g bottles) • Fast onset Use: Need for emergent surgery or life-threatening bleeds Pipeline: Andexanet alfa • Modified factor Xa molecule • Becomes a decoy for endogenous factor Xa – Anticoagulant binds to decoy preventing anticoagulation – Will also inhibit actions of enoxaparin and fondaparinux • Normally bind to ATIII and potentiate the inhibition of factor Xa Pipeline: Andexanet alfa • Phase III trials completed • Attempting accelerated approval from the FDA • Intended indication: – Emergent surgery – Life threatening bleed Reversal Agents and Role of Pharmacy • Good medication history as possible – Last refill, last dose – Other potentiating factors including OTC drugs/herbals and antiplatelets • Basic PK/PD knowledge • Laboratory interpretation • Protocol development Case • Independent pharmacy – medications for a local nursing home – Access to basic patient information and all medications provided to the residents. • You receive a telephone RX for Mr Smith, a 72yo resident, for vitamin K and the physician wants you to dose it for him. Mr Smith • Recently hospitalized for a PE • Now on apixaban 5mg PO BID • Routine labs resulted today – SCr 1.2 – CrCl 42ml/min – H/H 13.2/38 – PT/INR 35.31/3.21 • No recent falls or reports of bleeding Mr Smith Based on this information you recommend: A. Vitamin K 5mg IM X 1 B. Vitamin K 1mg IV X 1 C. Vitamin K 2.5mg PO X 1 D. No vitamin K – an elevated INR with apixaban is to be expected Case 2 Mrs Rodriguez arrives at the ED where you work. You receive a call from the ED doctor to dose Kcentra. • 53yo female on dabigatran 150mg po BID for afib. Last dose yesterday at bedtime • PT/INR 82.61/7.51 • H/H 12.3/36.9 • SCr 2.7; CrCl 42 ml/min Mrs Rodriguez • Presenting to the ED with congestion and sinus pressure unrelieved with OTC agents (X 3 days) • Denies abnormal bruising/bleeding • You quickly review the remaining information available – nothing else pertinent Mrs Rodriguez You recommend: A. Kcentra weight based dosing for INR > 6 B. Vitamin K and FFP because that is the recommended treatment in this case C. No action: patient does not meet criteria for dabigatran reversal D. Praxbind 2.5g IV X 2 Mrs Rodriguez What if it’s 3am and she was instead involved in a motor vehicle accident with her last dose of dabigatran 4 hours ago? She has massive internal injuries and is being taken to the O.R? Mrs Rodriguez You recommend: A. Kcentra weight based dosing for INR > 6 B. Vitamin K and FFP because that is the recommended treatment in this case C. No action: patient does not meet criteria for dabigatran reversal D. Praxbind 2.5g IV X 2 Recommended References Acforum.org • Free access • Guidelines – including recent clinical guidance publication • Protocols/procedures • Webinars monthly on anticoag topics • AC Forum Bootcamp information Recommended References Apps • Xarelto • Anticoag Evaluator (From ACC) • MAQI2 Anticoagulation Toolkit • Afib Educator Newer Oral Anticoagulants and Available Reversal Agents Cara L. Leos, Pharm D, BCPS AQ‐Cardiology Baptist Medical Center South Montgomery, AL [email protected]