* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Protein synthesis inhibitors Tetracyclines
Pharmacognosy wikipedia , lookup
Discovery and development of cephalosporins wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Drug design wikipedia , lookup
Drug discovery wikipedia , lookup
Drug interaction wikipedia , lookup
Prescription costs wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Theralizumab wikipedia , lookup
Dydrogesterone wikipedia , lookup
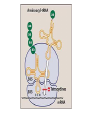
Protein synthesis Inhibitors Dr. Naza M. Ali Lec-8 3 -1-2017 Tetracyclines • They are a group of closely related compounds consist of four fused rings with a system of conjugated double bonds. • Substitutions on these rings are responsible for variation in the drugs pharmacokinetics, which cause small differences in their clinical efficacy. Tetracyclines Mechanism of action Entry of tetracycline into susceptible organisms is mediated both by 1. Passive diffusion 2. An energy-dependent transport protein mechanism unique to the bacterial inner cytoplasmic membrane. • The drug binds reversibly to the 30S subunit of the bacterial ribosome, • blocking access of the amino acyl-tRNA to the mRNA-ribosome complex at the acceptor site. Resistance Widespread resistance to tetracyclines limits their clinical use The most commonly naturally occurring resistance 1. An inability of the organism to accumulate the drug. 2. Enzymatic inactivation of the drug and production of bacterial proteins that prevent tetracyclines from binding to the ribosome. Classification of Tetracyclines according to T 1/2 Examples Short- acting Half-life in hrs 6-8 Tetracycline Oxytetracycline Chlotetracycline Intermediate acting Demeclocycline Long-acting 12 18 Doxycycline Minocycline Pharmacokinetics Absorption • Tetracyclines are adequately absorbed after oral ingestion. • Administration with dairy products or other substances that contain divalent and trivalent cations (Mg or Al antacids or iron supplements) decreases absorption, for tetracycline due to the formation of nonabsorbable chelates . Distribution: • The tetracyclines concentrate well in the bile, liver, kidney, gingival fluid, and skin. • They bind to tissues undergoing calcification (teeth , bones). • Only minocycline and doxycycline achieve therapeutic levels in CSF. • Minocycline also achieves high levels in saliva and tears, rendering it useful in eradicating the meningococcal carrier state. • All tetracyclines cross the placental barrier and concentrate in fetal bones and dentition. Elimination • Tetracycline is primarily eliminated unchanged in urine • Minocycline undergoes hepatic metabolism and is eliminated to a lesser extent via the kidney. • In renally compromised patients, doxycycline is preferred, as it is primarily eliminated via the bile into the feces. Clinical uses 1. Primary uses • Mycoplasma pneumoniae ( cause of community acquired pneumonia in young adults and in people who live in close connes, such as in military camps). • Chlamydia trachomatis ( sexually transmitted disease , It causes urethritis, pelvic infammatory disease). • Rocky mountain spotted fever Rickettsiae ( is characterized by fever, chills, and aches in bones and joints). • Cholera (is caused by Vibrio cholerae) 2. Secondary uses • are alternative in treatment of syphilis. • prophylaxis against infection in chronic bronchitis • in treatment of acne 3. Selective uses • GIT ulcer cause by H.pylori Tetracycline • Meningococal carrier state Minocycline • Lyme disease Doxycycline • Prevention of malaria Doxycycline • Treatment of amebiasis Doxycycline • In managment of patients with ADH-secreting tumors Demeclocycline Adverse effects 1. Gastric discomfort 2. Effects on calcified tissues This may cause discoloration and hypoplasia of teeth and a temporary stunting of growth. The use of tetracyclines is limited in pediatrics. 3. Hepatotoxicity 4. Phototoxicity: Severe sunburn in patient who exposed to sun or ultraviolet rays (tetracycline & demeclocycline) 5. Vestibular dysfunction: Dizziness, vertigo, and tinnitus with minocycline, which concentrates in the ear and affects function. Doxycycline may also cause vestibular dysfunction. 6. Pseudotumor cerebri: Benign, intracranial hypertension ( headache & blurred vision) Glycylcylines Tigecycline is the first available member of a new class of antimicrobial agents called glycylcyclines. is structurally similar to the tetracyclines has a broad-spectrum activity against multidrugresistant gram-positive, some gram-negative , anaerobic organisms. is indicated for treatment of complicated skin and soft tissue infections & complicated intra-abdominal infections. Chloramphenicol • A broad-spectrum antibiotic, • Is restricted to life-threatening infections for which no alternatives exist. Mechanism of action • Chloramphenicol binds reversibly to the bacterial 50S ribosomal subunit and inhibits protein synthesis at the peptidyl transferase reaction • Due to some similarity of mammalian mitochondrial ribosomes to those of bacteria, protein and ATP synthesis in these organelles may be inhibited at high circulating chloramphenicol levels, producing bone marrow toxicity. • ( The oral formulation of chloramphenicol was removed from the US market due to this toxicity.) Resistance 1. Occurs through the formation of acetyl transferase enzyme that inactivates chloramphenicol. 2. With an inability of the antibiotic to penetrate the organism so change in permeability may be the basis of multidrug resistance. Mechanism of action of chloramphenicol. aa = amino acid. Antimicrobial spectrum • Is either bactericidal or bacteriostatic • A broad-spectrum antibiotic, is active not only against bacteria but also against other microorganisms, rickettsiae. • Chloramphenicol has excellent activity against anaerobes. • Not active against Pseudomonas aeruginosa, chlamydiae. Clinical uses • Because of its toxicity, has very few uses as a systemic drug. • It is a backup drug for severe infections caused by Salmonella & for the treatment of pneumococcal & meningococcal meningitis in beta-lactam sensitive persons Pharmacokinetics • Chloramphenicol may be either IV or orally • It is completely absorbed via the oral route because of its lipophilic nature, and is widely distributed throughout the body. • It readily enters the normal CSF. • Chloramphenicol primarily undergoes hepatic metabolism to an inactive glucuronide, which is secreted by renal tubule & eliminated in urine. • Dose reductions are necessary in patients with liver dysfunction or cirrhosis. • Chloramphenicol is secreted into breast milk. Toxicity 1. GIT disturbance (candidiasis) 2. Anemia: • Hemolytic anemia occurs in patient with low G6-phosphate dehydrogenase • Reversible anemia is dose related • A plastic anemia is independent of dose and may occur after the therapy ceased. 3. Gray baby syndrome: in neonates if the dosage regimen of drug is not properly adjusted. 3. Gray baby syndrome: • • • • Neonates have a low capacity to glucuronylate the antibiotic, they have under developed renal function, a decreased ability to excrete the drug, which accumulates to levels that interfere with the function of mitochondrial ribosomes. This leads to poor feeding, depressed breathing, cardiovascular collapse, cyanosis & death. Adults who have received very high doses of the drug can also exhibit this toxicity. Drug interactions: • Chloramphenicol inhibits some of the hepatic mixed-function oxidases and, thus, blocks the metabolism of drugs • such as warfarin and phenytoin,so elevating their concentrations and potentiating their effects.