* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Function of E. coli RNA Polymerase Factor 70 in
Survey
Document related concepts
Transcript
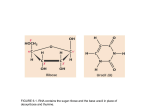
Cell, Vol. 86, 485–493, August 9, 1996, Copyright 1996 by Cell Press Function of E. coli RNA Polymerase s Factor s70 in Promoter-Proximal Pausing Brian Z. Ring, William S. Yarnell, and Jeffrey W. Roberts Section of Biochemistry, Molecular and Cell Biology Cornell University Ithaca, New York 14853 Summary The s factor s70 of E. coli RNA polymerase acts not only in initiation, but also at an early stage of elongation to induce a transcription pause, and simultaneously to allow the phage l gene Q transcription antiterminator to act. We identify the signal in DNA that induces early pausing to be a version of the s70 210 promoter consensus, and we show that s70 is both necessary for pausing and present in the paused transcription complex. Regions 2 and 3 of s70 suffice to induce pausing. Since pausing is induced by the nontemplate DNA strand of the open transcription bubble, we conclude that RNA polymerase containing s70 carries out basespecific recognition of the nontemplate strand as single stranded DNA. We suggest that s70 remains bound to core RNA polymerase when the 210 promoter contacts are broken, and then moves to the pause-inducing sequence. Introduction s factors of bacterial RNA polymerase mediate recognition of promoters, and possibly the opening of duplex DNA, to allow initiation of RNA synthesis (Burgess et al., 1969; Helmann and Chamberlin, 1988; Gross et al., 1992). Essential elements of promoters reside upstream of the initiation site; for the predominant E. coli s factor s70, for example, these are sequences centered at 235 and at 210 (Figure 1) (McClure, 1985). A few nucleotides of the transcript, usually no more than 10, are made while the holoenzyme remains fixed to these recognition sequences (McClure, 1985). At some juncture, the recognition sequences contacted at 235 and 210 by s in holoenzyme are released, and the enzyme transitions to a distinct elongation conformation (Carpousis and Gralla, 1985; Straney and Crothers, 1987; Krummel and Chamberlin, 1989). It is believed that s 70 separates entirely from core enzyme at some time during or after this transition; it is at least true that s 70 usually can be released in vitro after 5–10 nucleotides of RNA are made (Hansen and McClure, 1980; Krummel and Chamberlin, 1989), and the remaining core enzyme then is competent for elongation and termination of transcription. We have found that s70 acts in an unexpected way after RNA synthesis initiates at the bacteriophage l late gene promoter: it mediates a transcription pause that is related to the transcription antitermination activity of the phage l gene Q protein. RNA polymerase pauses both in vitro and in vivo for the order of a minute after transcribing 16 and 17 nucleotides of the l late gene transcript (Grayhack et al., 1985; Kainz and Roberts, 1992; Yarnell and Roberts, 1992). This pause is encoded primarily in nontemplate strand bases of the transcription bubble, particularly at 12 and 16 (Ring and Roberts, 1994). Only the naturally paused complex has the correct conformation or components to be modified in vitro by the gene Q antiterminator, as assayed either by footprinting of Q protein bound in the complex or by transcription antitermination downstream; a complex made of mutant DNA (e.g., mutant at 12 or 16), in which RNA synthesis is stopped by lack of a nucleotide substrate at the site of the wild-type pause, is competent for neither modification (Yarnell and Roberts, 1992). The transcribing complex paused at 116/17 has many essential properties of an elongation complex. According to both MPE and exonuclease footprinting, the RNA polymerase-s70 contact has been released from the 235 segment, and, almost certainly, from the 210 segment as well, although some pause-specific interactions do occur near the 210 segment (Yarnell and Roberts, 1992). The length of the exonuclease footprint, about 30–35 nucleotides, is typical of elongation complexes. The paused complex is stable to incubation and to filtration, in contrast to complexes that contain abortive RNAs. RNA polymerase initiated at the late gene promoter of a related lambdoid phage, phage 82, contains RNA paused at 125, even farther from the promoter (Yang et al., 1989; Guo, 1990). These characteristics would give rise to the expectation that s 70 has been released at the 116/17 pause. In contrast, we have found that s70 is present in the paused complex—both at 116/17 for l and at 125 for phage 82—and that s70 is necessary for the pause to occur. Furthermore, major elements of the sequence in the early transcribed segment that induces the pause are similar or identical to the s70 210 consensus. Thus it appears that s 70 releases from the promoter and then rebinds to the early transcribed segment, thereby inducing the pause. Since the pause is induced primarily by the nontemplate DNA strand, an important implication is that s 70 also makes base-specific contacts to nontemplate strand DNA of the 210 sequence in open promoter complexes (Roberts and Roberts, 1996). We describe here the evidence for s70 involvement in pausing, implications for s70 function and its release after the elongation complex forms, and possible relations to the activity of the l gene Q antiterminator protein. Results Two major sequence determinants of pausing at 116/ 117 of the l late gene promoter are an A at 12 and a T at 16 of the nontemplate DNA strand (Figure 1), in each of which mutation reduces pausing on the order of 10-fold (Yang, 1988; Kainz and Roberts, 1992; Roberts, 1992; Yarnell and Roberts, 1992). There is a similar sequence displaced 8 nucleotides farther downstream in the phage 82 late gene promoter region, for which mutations of A and T at 110 and 114 strongly reduce pausing at 125 (Guo, 1990). We have shown for l that changing Cell 486 Figure 1. DNA Structures of Open and Paused Promoter Complexes of the Phage l Late Gene Promoter pR9 Melted regions of the open promoter were determined by Kainz and Roberts (1992). Matches to consensus of promoter elements in the 235 and 210 regions are shown. This “extended 210” sequence contains the most important bases for promoter function (Kumar et al., 1993); its match to the pauseinducing sequence is also shown. the base in these positions in the nontemplate strand of DNA strongly affects pausing, whereas changing the template strand base has relatively little effect (Ring and Roberts, 1994). A comparison of the disposition of these bases in the paused complex and the bases of the conserved 210 consensus in the open complex (Figure 1) shows a striking similarity: the A at 12 and T at 16 are congruent with the most highly conserved A and T of the 210 consensus (underlined here), TATAAT. This relation holds among five examples of pause-inducing sequences in lambdoid phages (Figure 2) and involves presumptive matches to other strongly conserved elements of the “extended 210” consensus: T-TG-TATAAT (Keilty and Rosenberg, 1987; Kumar et al., 1993), which includes an additional 59 segment. The best match to this consensus, 7/9 bases including the highly conserved A and T, occurs in DNA that induces the pause most distant from the start site, that for the phage 82 late gene transcript at 125. There also exist important elements of these pauseinducing sequences that are not part of the 210 consensus, including particularly a G/C rich segment after the final T (Ring and Roberts, 1994); we discuss these sequences further below. Figure 2. Match of Lambdoid Bacteriophage Late Gene Promoter Pause-Inducing Sequences to Important Elements of the Extended 210 Consensus Pause sites and DNA sequences for l, 21, and phage 82 were described (Grayhack et al., 1985; Goliger and Roberts, 1989; Guo, 1990; Guo et al., 1990); the DNA sequence for phage 80 was determined by L. Matthews, D. Kadosh, and J. W. R. (unpublished data), and its features were surmised by analogy with l. The initiation site is designated by the arrow, and the pause site is underlined. A Consensus 210 Sequence Induces Efficient Pausing If the pause-inducing sequence includes a reiteration (although possibly a variant) of the 210 consensus sequence, then substitution of the full consensus for an otherwise weak pause-inducing signal should increase pausing. To test this, we used a derivative of the wildtype l sequence in which pausing is greatly weakened by displacement of the pause-inducing sequences downstream by means of a 10 bp insertion at 11 (template X10#1); we consider below why the pausing is weaker in this DNA. Figure 3 shows that the pause is displaced downstream in transcription from [X10#1] to 127/28, an amount consistent with the insert length, and is very weak relative to wild type. The template X10#1CON has 5 nucleotides of the X10#1 sequence changed to bring the pausing sequences to full extended 210 consensus; in contrast to X10#1, it supports efficient pausing at the displaced position (see also Figure 6). s70 Is Required for Pausing The apparent equivalence of the pause-inducing and s70 210 consensus sequences naturally suggests, but does not demand, that s70 must be present for pausing to occur at 116/17. A contrary possibility, for example, is that a site in core enzyme at least partly forms the base-specific contacts mediated during promoter recognition by s70 , and this site can bind the nontemplate DNA strand to induce pausing even in the absence of s70 . To test this possibility, we used the discovery that core enzyme initiates accurately, if bidirectionally, from the edge of a 12 base heteroduplex bubble extending from 210 to the start site (Aiyar et al., 1994). Holoenzyme also initiates accurately from the bubble (Aiyar et al., 1994), allowing initiation to occur with or without s70 , so that the effect of s 70 on pausing can be tested. We constructed two bubble heteroduplex templates in which an arbitrary but completely variant sequence replaced DNA of either strand from 212 to 11. Figure 4 shows that transcription of both templates by either core or s70 holoenzyme yields a distinct runoff, z100 s Factor Induces a Transcription Pause 487 Figure 3. Effect of the Extended 210 Sequence on Pausing at 10 bp Displacement The autoradiogram shows a gel resolution of transcripts of l1 pR9 DNA, a l pR9 variant with 10 bp of extraneous DNA introduced just before the site of the first transcribed base (X10#1), and a further variant in which the 10 bp insert DNA is modified to match the extended 210 consensus in the region that induces pausing (X10#1 con). In addition to changes made to match the consensus T-TGTATAAT, there is a change to pyrimidine at 210 (Kumar et al., 1993) and an irrelevant change at 15. Matches to the extended 210 sequence are shown by bullets. RNAs below the 116/17 pause RNAs are abortive initiation products, which vary in pattern and intensity with different initial transcribed sequences. nucleotides in length. Although Aiyar et al. (1994) reported bidirectional transcription from bubbles, we find accumulation mostly of the 100 nucleotide rightward transcript with or without s 70, from either bubble; conceivably, upstream sequences recognized by the a subunit of RNA polymerase (Ross et al., 1993) orient the synthesis. The important result is that both core and holoenzyme make runoff transcripts, whereas only holoenzyme yields transcripts at a significant level at the site of pausing. On the wild-type template strand bubble holoenzyme makes two strong transcripts at 116/117, exactly coinciding with the natural paused RNAs, whereas core enzyme gives only a faint background of short products. The template strand mutant bubble produces a pair of transcripts about a nucleotide shorter, presumably corresponding to its shorter runoff transcript. (Whereas runoff synthesis with wild type template strand bubble exactly matches that of homoduplex DNA, runoff from the mutant template strand bubble is slightly shorter, possibly because the enzyme seeks the first convenient template site for its preferred purine initiation base, at 12 of the wild-type sequence.) Thus core initiates accurately from the bubble, but fails to recognize the pause-inducing 210 consensus in the early transcribed segment: s70 is required for the pauseinducing sequence to be recognized. Transcription of bubbles does not exactly replicate that of natural DNA. First, it was difficult to determine if the prominent transcripts at 116/117 are paused rather than arrested permanently, because the inactivity of the initiation inhibitor rifampcin in blocking synthesis from bubbles prevented single round synthesis (Ring, 1995). But pausing and arrest are closely related in any event, since a few sequence changes in the early transcribed segment, particularly just after the final T, can convert a 210 consensus-induced pause at 125 of phage 82 to an arrested complex (Yarnell and Roberts, unpublished data). Second, there is a background of short transcripts extending to about 118 that is not made by holoenzyme on natural DNA. Since the 116/117 RNAs made from bubble templates are within this background, it could be argued that they arise in some way unrelated to pausing. We thus performed two further analyses. First, we used a bubble template in which a consensus pauseinducing sequence is displaced downstream 6 nucleotides relative to the wild-type sequence; both core and holoenzyme again yield runoff, and in this case the pause is 6–7 nucleotides farther downstream at 123, away from the background, and again is dependent upon s 70 (Figure 4B). Second, we used a mutation at 16 (A to G of the nontemplate strand) which prevents pausing in natural DNA; 16G also prevents appearance of the 116/117 RNAs from the bubble template in the presence of s70 (Figure 4B). Thus it is clear that s70dependent RNAs made from bubble template do also require the pause-inducing sequence that acts on natural DNA templates. s70 Fragments Containing Region 2 Induce Pausing Since s 70 is not required for initiation of RNA synthesis on bubble templates, fragments of the 613 amino acid s70 protein that are inactive for initiation can be assayed for recognition of the pause-inducing sequence. We tested three s70 fragments that lack major portions of the polypeptide but contain region 2 (amino acids 384–453), which is required for recognition of the 210 promoter segment (Kenney et al., 1989; Siegele et al., 1989; Zuber et al., 1989; Waldburger et al., 1990). GST[360–528] is a constructed fragment that contains both region 2 and region 3, thought to be involved in binding of s 70 to core enzyme, fused to glutathione-S-transferase; it binds DNA containing the 210 consensus in vitro, but no other activities have been reported (Dombroski et al., 1992). Fragments [104–448] and [114–448] are tryptic portions of s70 that contain most of region 2, but are known only to be active in core binding (Severinova et al., submitted). Cell 488 Figure 4. The 116/17 Pause Transcript Depends upon the Presence of s 70, and upon the Pause-Inducing Sequence, in Transcription from Bubble Templates Templates were PCR products made and transcribed as described. The substituent sequence was ATCTCGACTGAGT in place of the wildtype TAAATTTGACTCA in positions 212 to 11 of the nontemplate strand mutant bubble, or ACTCAGTCGAGAT in place of the wild-type TGAGTCAAATTTA in the template strand mutant bubble. (A) Transcripts of both bubbles by RNA polymerase holoenzyme (1s70) and core (2s70), compared with pause transcripts of wild-type DNA. The prominent runoff derives from rightward initiation at the bubble; a larger leftward transcript (not shown) is present at <20% of this level. (B) Transcripts of nontemplate strand altered 13 bp bubbles, as above, but with the (double-stranded) pausing sequence displaced downstream by a 6 bp insertion, and converted to consensus 210 sequence (in these bubbles TTATGCTATAAT—with consensus elements underlined— replaces nucleotides 11 to 16 of the wild-type sequence); and a bubble with a 16 T to G change in the nontemplate strand of the early transcribed segment. This single base heteroduplex at 16 of the nontemplate strand is expected to express the nonpausing mutant phenotype (Ring and Roberts, 1994). (C) Effect of s 70 fragments on pausing at 116/117 in the wild-type configuration, and at 123 in the extended 210 sequence with 6 bp insert. Numbers at top designate amino acids present in s70 fragments; the 360–528 fragment also contains GST fused to its N-terminus (Dombroski et al., 1992). None of the fragments contain region 4, which binds the 235 consensus (Gross et al., 1992), and thus none should be active in normal initiation from duplex templates. All three fragments induce pausing from the bubble template (Figure 4C). High concentrations of the tryptic fragments are required, possibly because they bind core less well than fragments containing region 3. Thus region 360–448 of s70 contains elements that induce pausing, although adjacent regions may be required for efficient initiation function. s70 Is Present in Paused Complexes The requirement for s70 to induce pausing at the l 116/ 17 site and its variants suggested that s70 might be detectable physically in these complexes in vitro, if its association is strong enough. Analyses of isolated complexes made from other promoters, and containing RNAs as long as 115, show that s 70 has been released, whereas s70 may be present if RNAs are shorter (<z10 nucleotides); in these cases footprinting shows that the 235 region is still occupied when s 70 is present and vacated when s 70 is released (Krummel and Chamberlin, 1989); recall that the 235 region is vacated in the l paused complex. We tested complexes from the l and phage 82 promoters, made from both wild-type DNA and from mutants that strongly reduce pausing. For convenience we used the l 115 complex, which is made easily by nucleotide deprivation; it has the essential properties of the 116/17 complexes (Yarnell and Roberts, 1992). The 82 complex is stopped by nucleotide deprivation at 125, exactly the site of the natural pause. Complexes were made from DNAs fixed to magnetic beads; excess proteins were washed from the beads and remaining proteins analyzed by SDS gel and silver staining. Figure 5 shows that s 70 survives washing in substantial amount from the wild type, but not from the nonpausing variants of these promoters. To reduce potential background due to released s70 or RNA polymerase adhering nonspecifically to beads, we released the l complexes from beads by restriction enzyme digestion; although there is a new background of polypeptides from the restriction enzyme preparation, it is clear that the s70 content of the released mutant complexes is even less than that of unreleased mutant complexes. Possibly some s70 remains in the complexes because the mutations do not completely destroy the binding sequence. We conclude that s 70 is fixed in the paused complex through the pause-inducing sequence. Pausing Decreases as the Pause-Inducing Sequence Is Moved from the Promoter To determine if s70-induced pausing requires a particular relation to the promoter—e.g., distance or angular orientation around the DNA—we moved sequences inducing s Factor Induces a Transcription Pause 489 Figure 5. s 70 Is Present in Complexes Stopped at the Pausing Site of l and 82 Promoter Segments, But Reduced in Amount about 5-fold when the Pause-Inducing Sequence Is Altered Figure 6. Effect of Displacing the Pause-Inducing Sequence of the l pR9 Promoter Downstream, and Replacing Displaced Sequences with the Extended 210 Consensus Complexes were made and analyzed as described; a photograph of a silver-stained SDS gel is shown. The s 70 content relative to RNA polymerase holoenzyme standard is shown. The l sequences have been described (Yarnell and Roberts, 1992). The phage 82 sequence is a variant with transcript cytidines removed up to 125, so that complexes stopped at the pause site can be constructed. Mutants of this variant are a deletion at 111, which removes the highly conserved T at 114 of the natural transcript (see Figure 2), and a 110G change of this site; both are defective in pausing (W. S. Y. and J. W. R., unpublished data). Insertions of 2 to 11 bp in insertion series #1 contain successive segments from the 59 end of the sequence ATTGGTAGTGA inserted before 11 of the wild-type sequence; the 1 bp insertion of series #1 has T inserted between 11 and 12. The variant 8 bp insertion is AATTACTC placed before 11, and two variant 10 bp insertions are GGAATTACTC (#2) and AAGGTTACTC (#3), both placed before 11. The 16 extended 210 consensus insertion contains TTATGCTA TAAT replacing the wild-type sequence from 11 to 16; the 110 extended 210 consensus insertion (template X10#1con) is described in Figure 3; the 120 extended 210 consensus insertion contains ATTGGTAGTGAATTATATGCTATAAT, substituted for the wild-type pR9 sequence from 11 to 16. Templates were PCR copies made with the primers described for synthesis of heteroduplex bubble templates. The “fraction RNA polymerase paused per minute” is the sum for all sampled times (0.59, 19, 29, 59, 109) of the mole fraction of transcripts at the pause site, divided by the total time of the reaction; it is an arbitrary measure that gives weight to both the fraction captured at the pause and the half-time of the pause. A substantially similar view is obtained by plotting the fraction that decays between 0.5 min and 5 min, or the fraction initially captured at 0.5 min. Error bars indicate variance over several experiments. the l 116/17 pause downstream, by inserting between 21 and 11 arbitrary segments that contain no elements of the extended 210 sequence. All bases of the original pause-inducing segment that match the extended 210 sequence (i.e. 12, 15, and 16) are included in the segment displaced downstream. We characterized these templates by measuring pausing in vitro and plotted for each a measure of pausing efficiency, as described in Figure 6 (legend); measuring pause half-life instead of occupancy gave a similar result. The major effect is that pausing drops off abruptly as the segment is displaced: the one base insertion reduces pausing somewhat, and pausing on insertions of 2 through 11 nucleotides fluctuates around 10%–20% of the wild-type level. Several different 8 and 10 base pair insertions give similar levels of pausing. We considered that pausing might recur with a periodicity related to the repeat of the DNA helix, but there is no trace of this. Previous results correlated the ability of Ql to antiterminate with the strength of the natural pause; this correlation holds for the insertions as well, since Ql acts about half as well on the 11 insertion as on wild type, slightly on the 12 insertion, and not at all on the longer insertions (B. Z. R. and J. W. R., unpublished data). We conclude that the natural l sequence induces efficient pausing only in close vicinity to the promoter. As described above and shown again in Figure 6, changing the l sequence to full consensus (T-TGTATATT) does provide efficient pausing at the 110 displacement, and even more efficient pausing at 16 displacement; the consensus insert also supports pausing at 120 displacement, although the latter is little or no stronger than the remaining pause displayed by insertions of 3–10 nucleotides of the natural l sequence. We conclude that failure of the natural sequence to induce efficient pausing at extensions of 16 and 110 is not due to the novel position per se, but instead to the incomplete match of the natural sequence to the 210 consensus: this region is accessible to s 70, but s70 can bind only if the site is strong. Even with the consensus sequence, pausing is strongly diminished by a displacement of 20 nucleotides from the natural pause site. Elements besides the 210 Consensus Contribute to Pausing The slight remaining pause made from DNAs in which the l sequence is displaced 3 or more base pairs from its natural site appears to reflect a component of the pause-inducing signal different from the 210 consensus sequence. To determine if elements of the consensus affect pausing at 10 or 20 bp displacement, we changed the highly conserved T originally at 16 of the natural sequence (and thus at 116 of the displaced sequence) to G; although this change reduces pausing at the natural position at least 10-fold (Ring and Roberts, 1994; Cell 490 Ring, 1995), it has little or no effect on the weak but distinct pause at the 10 or 20 bp displaced position (B. Z. R. and J. W. R., unpublished data). Therefore, the remaining pause appears not to be induced by s 70 recognition of the 210 consensus. Loci distinct from the 210 consensus also affect pausing at the natural position in l and phage 82, including sequences at the pause site itself, other positions within the bubble, and, particularly, a G/C rich segment just downstream of the final T of the 210 consensus (Goliger and Roberts, 1989; Guo, 1990; Ring and Roberts, 1994). Some or all of these elements may encode the remnant pause seen when the 210 consensus sequence is mutated. However, it is nonetheless possible that the presence of s70 is required even if the 210 consensus does not act, because we observed no (i.e. much less than the remnant level) pausing in synthesis initiated by core enzyme on bubble templates (see Figure 4B). Further work should resolve this. Discussion We have shown that the s 70 initiation subunit of E. coli RNA polymerase also can act on RNA polymerase during an early step of elongation, through binding a second copy of the 210 consensus at sites as much as 20 base pairs downstream of the 210 sequence that acts in promoter recognition and opening. This interaction has at least two consequences: there is a pause in transcription in vivo and in vitro, and RNA polymerase becomes sensitive to modification by the l gene Q antiterminator protein. A model that describes the structure of this paused complex and its origin after RNA synthesis initiates at the open promoter complex is shown in Figure 7. We note first that s70 carries out base-specific recognition of the nontemplate strand as single-stranded DNA, both in the open promoter complex and the paused complex (Ring and Roberts, 1994; Roberts and Roberts, 1996). This strand selectivity has been shown for positions 212, 211, 28, and 27 in the open promoter complex and/or the comparable sites in the paused complex. Es70 first binds the promoter specifically in a closed, duplex complex (Figure 7, row 1), which then is reconfigured into the melted open complex (Figure 7, row 2) (McClure, 1985); the high affinity of Es70 for bases of nontemplate strand DNA in single-stranded form may be important in its activity to melt the promoter region. Region 2 of s 70 carries specificity for 210 recognition, whereas region 4 of s70 is thought to bind the 235 segment (Gross et al., 1992); both interactions occur in the context of the holoenzyme Es70 , so that the core could contribute to the binding. RNAs of 10 or more nucleotides can be polymerized while Es70 remains fixed in the open complex, and these frequently dissociate from the complex (“abortive initiation”), forcing RNA synthesis to begin again (Johnston and McClure, 1976). Elongation succeeds when the 235 and 210 associations are broken and the RNA chain continues beyond the initial region. However, elongation is interrupted if s70 can associate with the pause-inducing sequence (Figure 7, row 3). Presumably, pausing reflects some of the same Figure 7. Model of s 70 Function in Promoter Opening and Pausing, and the Interaction l Q Protein with the Paused Complex Details are described in the text. (A), (B), and (C) represent formation of closed promoter complex, open promoter complex, and paused complex; (D) represents the interaction of l Q protein with the paused complex. Region 2 of s70 is placed to bind the upstream end of the extended 210 consensus as duplex DNA, in agreement with KMnO4 mapping (Kainz and Roberts, 1992). A pronounced stop in upstream exonuclease digestion characteristic of complex containing the wild-type pause sequence (Yarnell and Roberts, 1992) could reflect such a structure. forces between s70, DNA, and core enzyme that restrain the enzyme in the open promoter complex. We have no information where region 4 of s70 resides during the pause, although it no longer occupies 235 segment DNA and, as we have shown, is not necessary to induce pausing. (There is a near reiteration of the 235 sequence beginning at 227 in l [Figure 1], but changing this sequence does not affect pausing.) s70 might reach the pause-inducing sequence either by an internal pathway, whereby it transits (“hops”) from the 210 promoter sequence, or externally, through rebinding of released s70 to core at the pause site. There are several arguments that the former is more likely, although none are definitive. First, we attempted to increase pausing by adding a 35-fold excess of s 70 during in vitro transcription of either wild-type l or the consensus extended 210 sequence displaced to 120 relative to wild-type l (see Figure 6); there was no increase in pausing (Ring and Roberts, 1994). Second, the affinity of s 70 for elongating complexes has been measured directly and found to be orders of magnitude lower than for core RNA polymerase (Gill et al., 1991). Third, it appears plausible that s70 binding to core might persist after s 70 s Factor Induces a Transcription Pause 491 releases promoter contacts, so that a newly presented DNA site of high affinity (especially the melted nontemplate strand) would again fix s 70 in the complex. We envision the following: in the l open complex (Figure 7, row 2), extension of the transcription bubble a few bases downstream during the beginning of RNA synthesis exposes single-stranded nontemplate DNA containing the binding sequence, to which s70 jumps as it is released from the promoter 210 sequence (Figure 7, row 3). Even for a pause at around 125, for which the s70 binding segment is in the region 110 through 115, the initial part of this sequence might be unwound before s70 is released from the promoter 210 sequence. Whatever the interval in time and space, however, the hopping model proposes that s 70 remains bound to core between sites, and that s 70 can carry out a significant interaction with core after initiation and after the promoter 210 sequence bonds are broken. The discovery that s 70 can interact with core after initiation but independently of the promoter contacts begs the question when s70 actually is released in vivo when there is no pause. It is clear that s70 can be released in vitro after 10 nucleotides of RNA are made. However, most such measurements involve complexes stopped for at least minutes, whereas elongation through hundreds of base pairs requires only seconds. One measurement found that s70 release is time and not position dependent, occurring with a half-time of 5 s (Shimamoto et al., 1986). We suggest that the ability of the consensus extended 210 sequence to invoke pausing at a 10 base pair displacement, where the natural l sequence does not invoke pausing, implies that s70 is present but unable to grasp the weaker binding sequence in the displaced position. The fact that even the consensus sequence fails at greater extensions could mean that s70 is not there, or that other influences prevent the interaction; in fact, it is worth considering that s70 might not be released in some conditions in the cell, and that it might have other roles in transcription elongation. A number of eukaryotic genes also have associated promoter-proximal pause sites, including Drosophila heat shock genes (Rougvie and Lis, 1990), the human c-myc gene (Krumm et al., 1995), and possibly the HIV-1 LTR promoter (Cullen, 1990). The heat shock and c-myc associated pauses are affected by mutations removing various promoter elements (Krumm et al., 1995; Lee et al., 1992), suggesting that promoter bound factors are important for pausing by polII. We directly demonstrate here that a promoter element binding factor of E. coli RNA polymerase, s70 , can induce promoter-proximal pausing. It is interesting that similar mechanisms may govern a common regulatory step in both prokaryotes and eukaryotes. There are important elements of the pause-inducing sequence besides the 210 promoter consensus. Mutation of any one of the three G/C base pairs following the 210 consensus in l reduces pausing severalfold (Yang, 1988); like the 210 consensus bases, these sites act primarily through the nontemplate strand (Ring and Roberts, 1994). Although it is not part of the derived 210 consensus, this region is the site of the “discriminator” (de Boer et al., 1979; Travers, 1980), thought to be important in the stringent regulation of promoter function by the nucleotide ppGpp; G/C richness is associated with inhibition of promoter function by ppGpp, whereas A/T richness is associated with stimulation (Cashel and Rudd, 1987; Riggs et al., 1986). Conceivably the interaction by which this promoter segment influences pausing also is involved in mediating the stringent response. A single mutation such as 16G that prevents pausing and s70 incorporation into the complex also blocks l gene Q antiterminator binding and function, even if RNA polymerase is stalled at the nucleotide where pausing would occur (Yarnell and Roberts, 1992). This finding suggests strongly that s70 is directly required for Q function. We thus show (Figure 7, row 4) a new view of the paused complex, with Q and RNA polymerase placed on the linear DNA sequence according to DNA protection analysis. When Q binds, it alters the DNA footprint in the early transcribed region where s 70 region 2 binds (Yarnell and Roberts, 1992). This change could reflect a direct s70 –Q interaction or an indirect effect of s 70 in providing a favorable conformation of RNA polymerase for the interaction. Whichever is true, it is clear that the influence of s 70 in this incipient elongation complex is essential to allow its modification by Q to the antiterminating form. Experimental Procedures Proteins and DNA RNA Polymerase holoenzyme was purified as described (Hager et al., 1990). Core RNA polymerase was prepared from holoenzyme by phosphocellulose chromatography as described (Burgess, 1969). s 70 was purified as described (Dombroski et al., 1992) as a fusion to glutathione-S-transferase at amino acid 8, the plasmid source of which was a gift from C. Gross. Holoenzyme made by reconstitution of core RNA polymerase with a 10-fold excess of GST-s70 at 08C for 10 min behaved identically to purified holoenzyme. The 110–448 and 114–448 fragments of s70 were gifts of E. Severinova, K. Severinov, and S. Darst. Other enzymes were obtained from New England BioLabs. For experiments except that of Figure 5, the plasmid source for the l late promoter and 16G mutant was pXY306 and its mutant derivative (Yang, 1988; Kainz and Roberts, 1992). All insertion/deletion mutations of the l late promoter region were produced by polymerase chain reaction (PCR) overlap mutagenesis. In brief, an oligonucleotide containing the mutant sequence was combined with a downstream oligonucleotide in a PCR templated by pXY306, or an appropriate derivative, to produce a right-half PCR fragment. An oppositely oriented oligonucleotide matching 15 bp of the end of the mutagenic oligonucleotide was combined with an upstream oligonucleotide in a PCR templated by pXY306 or a derivative to produce a left-half PCR product. Hybrid was produced by combining the right and left half fragments in equimolar amounts and exposing them to five primer extension cycles in the presence of dXTP’s, with critical ramp speeds and annealing temperature as follows: 948C to 358C at a ramp of 10 s/8C, and then from 358C to 728C at 2 s/8C. A restriction fragment of the resulting hybrid was cloned into the parent plasmid of pXY306 and DNA prepared as described; or the hybrid was used as template for further PCR and the product used as transcription template after purification with glass milk (National Scientific). For the experiment of Figure 5, l DNAs were BamHI–EcoRI fragments of pBY300 and pBY301 (Yarnell and Roberts, 1992) that contain l DNA from 2282 to 183 relative to the pR9 start site. Phage 82 DNAs were HinDIII–SalI fragments of pBY400 (wild-type), pBY402 (111 deletion), and pBY405 (110G), which contain phage 82 DNA from 297 to 1141 relative to the phage late gene promoter. Phage Cell 492 82 “WT” DNA of Figure 5 is from pBY40u, a derivative of true wildtype made by PCR mutagenesis that allows stopped complexes to be formed at the 125 pause site in a single step, through introduction of encoded cytidine at 12 and 126 and removal of encoded cytidine at 15, 116, and 117. Initiation at the 82 late promoter on pBY40u produces transcribing complexes that are partially arrested at 125, are released by GreB, and are fully modified by Q protein. “111 deletion” and “110G” templates are derivatives of pBY40u and do not produce arrested complexes. Heteroduplex Promoter Bubble Templates Heteroduplex templates were made by denaturing and reannealing mixed PCR products of mutant and wild-type DNAs, in which the template strand of the mutant template and the nontemplate strand of the wild-type template were biotinylated by synthesis with a biotinylated primer; heteroduplexes then were separated from homoduplexes and from each other by differential bandshift with streptavidin (C. Roberts and J. W. R., unpublished data). One heteroduplex (nontemplate strand mutant and template strand wild type) does not bind streptavidin and is not shifted; the homoduplex fragments are biotinylated on one strand and shift singly; and the other heteroduplex is biotinylated on both strands and shifts doubly. The mutation used in heteroduplex bubble templates was 212ATCTCGACTG AGT11 in the template strand, replacing the wild-type sequence of 212TAAATTTGACTCA11. The upstream primer for PCR was AAGG ATGCCAGCAAGC, which begins in l DNA at bp 44,344, 243 bp upstream of the pR9 start site in pXY306; the downstream primer was CACCACAGAAAGGTCG, which ends 100 bp downstream of the pR9 start site in pXY306 DNA. DNAs to be reannealed were combined in equimolar amounts, supplemented with 5 mM EDTA, heated 10 min at 958C, annealed 10 min at 658C, and chilled. Streptavidin was then added to a concentration of 0.5 mg/ml and the shifted and unshifted DNAs separated on a 1% agarose gel. DNA was removed in agarose and purified with glass milk. In Vitro Transcription Transcripts were made by preincubating 1 nM DNA template and 15 nM holoenzyme, or core enzyme, or core enzyme and s or s fragment, for 15 min at 378C in 20 mM Tris–Cl (pH 8.0), 0.1 mM EDTA, 1.0 mM DTT, 50 mM KCl, 200 mM ATP, CTP, and GTP, 50 mM UTP, and 0.5 mCi/ml [a-32P]UTP in a volume of 25 ml. RNA synthesis was initiated by addition of 2.5 ml of 40 mM MgCl 2 and 100 mg/ml rifampicin. RNA was processed and analyzed by denaturing gel electrophoresis and autoradiography as described (Grayhack et al., 1985). Dried gels were also analyzed on a Betascope or phosphorimager. s70 Content of Stopped Transcription Complexes DNA templates purified from plasmid DNA were labeled upstream of the promoter with 32P (top strand) and biotin (bottom strand), using T4 polynucleotide kinase and Klenow fragment, respectively. Stopped transcription complexes were formed on DNA templates attached to streptavidin-coated paramagnetic beads (Promega) by incubation of stoichiometric amounts of RNA polymerase and DNA in transcription buffer (TB; 20 mM Tris–HCl [pH 7.9], 0.1 mM EDTA, 1.0 mM dithiothreitol, 50 mM KCl, 2% glycerol, 0.04 mg/ml bovine serum albumin, and 4 mM MgCl2) for 12 min at 378C followed by initiation of transcription with 25 mM ATP, 25 mM GTP, 25 mM UTP, 0.4 mCi/ml [a-32P]UTP, and 50 mM initiating nucleotide (ApApC or ApC) for 8 min. Residual open promoter complexes and free RNA polymerase were removed by 08C incubation with 350 mM KCl and 0.2 mg/ml poly(dA-dT) for 5 min followed by five rinses with buffer (TB; 0.1 mg/ml BSA, 0.02 mg/ml poly[dA-dT]) and resuspension in TB. Aliquots were removed for analysis of protein subunits (2.5 pmol complexes) and nascent RNA (0.2 pmol complexes; >90% of RNAs were the desired stopped product) as well as for estimation of contaminating open promoter complexes by Exonuclease III digestion (0.3 pmol complexes; open complexes represented <2% of total complexes). Additional l DNA complexes (2.5 pmol) were released and separated from beads by 10 min digestion with 40 U MseI, which cuts upstream of the promoter, for analysis of protein subunits. Samples for protein subunit analysis were separated on SDS– polyacrylamide gel (7%), silver stained, photographed on a Fotodyne imager, and quanititated using Fuji MacBas software. RNA and DNA were analyzed by sequencing gels and phosphorimager. Acknowledgments We thank J. Lis and members of the laboratory for reading the manuscript; J. Helmann for advice and for use of bubble templates; C. Gross for a gift of plasmids; E. Severinova, K. Severinov, and S. Darst for providing s70 fragments and suggesting they be tested in the bubble pausing assay; and the National Institutes of Health for support (grant 21941). Received May 16, 1996; revised June 25, 1996. References Aiyar, S.E., Helmann, J.D., and deHaseth, P.L. (1994). A mismatch bubble in double-stranded DNA suffices to direct precise transcription initiation by Escherichia coli RNA polymerase. J. Biol. Chem. 269, 13179–13184. Burgess, R.R. (1969). A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J. Biol. Chem. 244, 6160–6167. Burgess, R.R., Travers, A.A., Dunn, J.J., and Bautz, E.K.F. (1969). Factor stimulating transcription by RNA polymerase. Nature 221, 43–46. Carpousis, A., and Gralla, J. (1985). Interaction of RNA polymerase with the lacUV5 promoter DNA during mRNA initiation and elongation: footprinting, methylation, and rifampicin-sensitivity changes accompanying transcription initiation. J. Mol. Biol. 183, 165–177. Cashel, M., and Rudd, K.E. (1987). The stringent response. In Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology, F. Neidhardt, J. Ingraham, B. Low, B. Magasanik, M. Schaechter, and H. Umbarger, eds. (Washington, D.C.: Am. Soc. Microbiol.), pp. 1410–1438. Cullen, B.R. (1990). The HIV-1 Tat protein: an RNA sequence-specific processivity factor? Cell 63, 655–657. de Boer, H.A., Gilbert, S.F., and Nomura, M. (1979). DNA sequences of promoter regions for rRNA operons rrnE and rrnA in E. coli. Cell 17, 201–209. Dombroski, A.J., Walter, W.A., Record, M.T.J., Siegele, D.A., and Gross, C.A. (1992). Polypeptides containing highly conserved regions of transcription initiation factor Sigma-70 exhibit specificity of binding to promoter DNA. Cell 70, 501–512. Gill, S.C., Weitzel, S.E., and von Hippel, P.H. (1991). Escherichia coli sigma 70 and NusA proteins. I. Binding interactions with core RNA polymerase in solution and within the transcription complex. J. Mol. Biol. 220, 307–324. Goliger, J.A., and Roberts, J.W. (1989). Sequences required for antitermination by phage 82 Q protein. J. Mol. Biol. 210, 461–471. Grayhack, E.J., Yang, X., Lau, L.F., and Roberts, J.W. (1985). Phage lambda gene Q antiterminator recognizes RNA polymerase near the promoter and accelerates it through a pause site. Cell 42, 259–270. Gross, C., Lonetto, M., and Losick, R. (1992). Bacterial sigma factors. In Transcriptional Regulation, S. McKnight and K. Yamamoto, eds. (Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press), pp. 129–176. Guo, H.-C. (1990). Mutational analysis of transcription antitermination mediated by lambdoid phage gene Q products. Ph.D. thesis, Cornell University, Ithaca, New York. Guo, H.-C., Kainz, M., and Roberts, J.W. (1990). Characterization of the late gene regulatory region of phage 21. J. Bacteriol. 173, 1554–1560. Hager, D.A., Jin, D.J., and Burgess, R.R. (1990). Use of mono Q high-resolution ion-exchange chromatography to obtain highly pure and active Escherichia coli RNA polymerase. Biochemistry 29, 7890– 7894. Hansen, H.M., and McClure, W.R. (1980). Role of the s subunit of s Factor Induces a Transcription Pause 493 Escherichia coli RNA polymerase in initiation. II. Release of s from ternary complexes. J. Biol. Chem. 255, 9564–9570. Yang, X. (1988). Transcription antitermination mediated by lambdoid phage Q proteins. Ph.D. thesis, Cornell University, Ithaca, New York. Helmann, J.D., and Chamberlin, M.J. (1988). Structure and function of bacterial sigma factors. Annu. Rev. Biochem. 57, 839–872. Yang, X., Goliger, J.A., and Roberts, J.W. (1989). Specificity and mechanism of antitermination by Q proteins of bacteriophages l and 82. J. Mol. Biol. 210, 453–460. Johnston, D.E., and McClure, W.R. (1976). Abortive initiation of in vitro RNA synthesis on bacteriophage lambda DNA. In RNA Polymerase, R.R. Losick and M.J. Chamberlin, eds. (Cold Spring Harbor, New York: Cold Spring Harbor Laboratory), pp. 413–428. Kainz, M., and Roberts, J.W. (1992). Structure of transcription elongation complexes in vivo. Science 255, 838–841. Keilty, S., and Rosenberg, M. (1987). Constitutive function of a positively regulated promoter reveals new sequences essential for activity. J. Biol. Chem. 262, 6389–6395. Kenney, T.J., York, K., Youngman, P., and Moran, C.P.J. (1989). Genetic evidence that RNA polymerase associated with sA uses a sporulation-specific promoter in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 86, 9109–9113. Krumm, A., Hickey, L.B., and Groudine, M. (1995). Promoter-proximal pausing of RNA polymerase II defines a general rate-limiting step after transcriptional initiation. Genes Dev. 9, 559–572. Krummel, B., and Chamberlin, M.J. (1989). RNA chain initiation by Escherichia coli RNA polymerase. Structural transitions of the enzyme in early ternary complexes. Biochemistry 28, 7829–7842. Kumar, A., Malloch, R.A., Fujita, N., Smillie, D.A., Ishihama, A., and Hayward, R.S. (1993). The 235 recognition region of Escherichia coli sigma 70 is inessential for initiation of transcription at an “extended 210” promoter. J. Mol. Biol. 232, 406–418. Lee, H.S., Kraus, K.W., Wolfner, M.F., and Lis, J.T. (1992). DNA sequence requirements for generating paused polymerase at the start of HSP70. Genes Dev. 6, 284–295. McClure, W.R. (1985). Mechanism and control of transcription initiation in prokaryotes. Annu. Rev. Biochem. 54, 171–204. Riggs, D.L., Mueller, R.D., Kwan, H.-S., and Artz, S.W. (1986). Promoter domain mediates guanosine tetraphosphate activation of the histidine operon. Proc. Natl. Acad. Sci. USA 83, 9333–9337. Ring, B.Z. (1995). Roles for the nontranscribed DNA strand and sigma-70 initiation factor in E. coli RNA polymerase pausing. Ph.D. thesis, Cornell University, Ithaca, New York. Ring, B.Z., and Roberts, J.W. (1994). Function of a nontranscribed DNA strand site in transcription elongation. Cell 78, 317–324. Roberts, J. (1992). Antitermination and the control of transcription elongation. In Transcriptional Regulation, S. McKnight and K. Yamamoto, eds. (Cold Spring Harbor, New York: Cold Spring Harbor Laboratory), pp. 389–406. Roberts, C.W., and Roberts, J.W. (1996). Base-specific recognition of the nontemplate strand of promoter DNA by E. coli RNA polymerase. Cell, this issue. Ross, W., Gosink, K.K., Salomon, J., Igarashi, K., Zou, C., Ishihama, A., Severinov, K., and Gourse, R.L. (1993). A third recognition element in bacterial promoters: DNA binding by the a subunit of RNA polymerase. Science 262, 1407–1413. Rougvie, A., and Lis, J. (1990). Postinitiation transcriptional control in Drosophila melanogaster. Mol. Cell. Biol. 10, 6041–6045. Shimamoto, N., Kamigochi, T., and Utiyama, H. (1986). Release of the sigma subunit of Escherichia coli DNA-dependent RNA polymerase depends mainly on time elapsed after the start of initiation, not on length of product RNA. J. Biol. Chem. 261, 11859–11865. Siegele, D.A., Hu, J.C., Walter, W.A., and Gross, C.A. (1989). Altered promoter recognition by mutant forms of the sigma-70 subunit of Escherichia-coli RNA polymerase. J. Mol. Biol. 206, 591–604. Straney, D.C., and Crothers, D.M. (1987). A stressed intermediate in the formation of stably initiated RNA chains at the Escherichia coli lac UV5 promoter. J. Mol. Biol. 193, 279–292. Travers, A.A. (1980). Promoter sequence for stringent control of bacterial ribosomal RNA synthesis. J. Bact. 141, 973–976. Waldburger, C., Gardella, T., Wong, R., and Susskind, M.M. (1990). Changes in the conserved region 2 of Escherichia coli s 70 subunit affecting promoter recognition. J. Mol. Biol. 215, 267–276. Yarnell, W.S., and Roberts, J.W. (1992). The phage lambda gene Q transcription antiterminator binds DNA in the late gene promoter as it modifies RNA polymerase. Cell 69, 1181–1189. Zuber, P., Healy, J., Carter, H.L.I., Cutting, S., Moran, C.P.J., and Losick, R. (1989). Mutation changing the specificity of an RNA polymerase sigma factor. J. Mol. Biol. 206, 605–614.