* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Patterns in the structure of Asian and North American desert small
Survey
Document related concepts
Molecular ecology wikipedia , lookup
Unified neutral theory of biodiversity wikipedia , lookup
Introduced species wikipedia , lookup
Occupancy–abundance relationship wikipedia , lookup
Ecological fitting wikipedia , lookup
Biodiversity action plan wikipedia , lookup
Island restoration wikipedia , lookup
Habitat conservation wikipedia , lookup
Latitudinal gradients in species diversity wikipedia , lookup
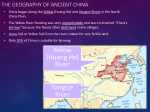
Biogeography wikipedia , lookup
Reconciliation ecology wikipedia , lookup
Transcript
Journal of Biogeography, 26, 825–841 Patterns in the structure of Asian and North American desert small mammal communities Douglas A. Kelt, Kontantı́n Rogovin, Georgy Shenbrot and James H. Brown Department of Wildlife, Fish, & Conservation Biology, University of California, Davis, CA 95616, U.S.A., Academy of Sciences of Russia, Institute of Animal Evolutionary Morphology and Ecology, Leninskyi pr. 33, Moscow 117071, Russia, Ramon Science Center, Ben Gurion University of the Negev, Mitpe Ramon, 80600, Israel, and Department of Biology, University of New Mexico, Albuquerque, NM 87131, U.S.A. Abstract Aim We compared assemblages of small mammal communities from three major desert regions on two continents in the northern hemisphere. Our objective was to compare these with respect to three characteristics: (1) species richness and representation of trophic groups; (2) the degree to which these assemblages exhibit nested community structure; and (3) the extent to which competitive interactions appear to influence local community assembly. Location We studied small mammal communities from the deserts of North America (N= 201 sites) and two regions in Central Asia (the Gobi Desert (N=97 sites) and the Turan Desert Region (N=36 sites), including the Kara-Kum, Kyzyl-Kum, NE Daghestan, and extreme western Kazakhstan Deserts). Method To provide baseline data we characterized each desert region in terms of alpha, beta, and gamma diversity, and in terms of the distribution of taxa across trophic and locomotory groups. We evaluated nestedness of these communities using the Nestedness Temperature Calculator developed by Atmar & Patterson (1993, 1995), and we evaluated the role of competitive interactions in community assembly and applied a null model of local assembly under varying degrees of competitive interaction (Kelt et al., 1995, 1996). Results All three desert regions have low alpha diversity and high beta diversity. The total number of species in each region varied, being highest in North America, and lowest in the Turan Desert Region. The deserts studied all present evidence of significant nestedness, but the mechanism underlying this structure appears different in North American and Asia. In North America, simulations strongly implicate interspecific competition as a dominant mechanism influencing community and assemblage structure. In contrast, data from Asian desert rodent communities suggest that these are not strongly influenced by competition; in fact, they have greater numbers of ecologically and morphologically similar species than expected. These results appear to reflect strong habitat selection, with positive associations among species that share similar habitat requirements in these communities. Our analyses support earlier reports suggesting that predation and abiotic forces may have greater influences on the assembly and organization of Asian desert rodent communities, whereas interspecific competition dominates assembly processes in North America. Additionally, we suggest that structuring mechanisms may be very different among the two Asian deserts studied. Gobi assemblages appear structured by trophic and locomotory strategies. In contrast, Turan Desert Region assemblages appear to be randomly structured with respect to locomotory strategies. When trophic and locomotory categories are combined, however, Turan species are positively and nonrandomly associated. Correspondence: Douglas A. Kelt, Department of Wildlife, Fish, & Conservation Biology, University of California, Davis, CA 95616, U.S.A. e-mail: [email protected] 1999 826 Douglas A. Kelt et al. Main conclusions Very different ecological dynamics evidently exist not only between these continents, but within them as well. These small mammal faunas differ greatly in terms of community structure, but also appear to differ in the underlying mechanisms by which communities are assembled. The underlying role of history and geography are strongly implicated as central features in understanding the evolution of mammalian faunas in different deserts of the world. Keywords Desert rodents, North American deserts, Asian deserts, community assembly, nested subsets, null model, competition, importance of history INTRODUCTION Desert small mammals have long held the attention of community ecologists, and have served as models for understanding both patterns of community structure and the proximate mechanisms underlying these patterns. This partly reflects the relative simplicity of arid zones, with reduced habitat complexity and more apparent underlying resources, at least when compared with shrub or forest habitats. In North America, numerous studies have suggested or demonstrated competitive interactions among desert rodent species (e.g. Rosenzweig & Winakur, 1969; Rosenzweig & Sterner, 1970; Brown, 1973, 1975; Rosenzweig, 1973; Rosenzweig et al., 1975; Schroder & Rosenzweig, 1975; Price, 1978, 1986; Munger & Brown, 1981; Freeman & Lemen, 1983; Frye, 1983; Brown & Munger, 1985; Brown, 1989a; Heske et al., 1994; Valone & Brown, 1996), and interspecific competition, both direct and indirect, has been implicated as a dominant mechanism of community assembly there. Subsequent studies have documented behavioural (Reichman, 1983; Reichman & Price, 1993), social (Jones, 1993), morphological (e.g. Rosenzweig & Sterner, 1970; Bowers & Brown, 1982), and biogeographical (Brown, 1975, 1987; Brown & Kurzius, 1987, 1989; Kelt and Brown, in press a, b) correlates to local co-occurrence of species. The patterns reported for North American rodent communities were so simple and intuitively appealing that they gradually reached the status of paradigms for the structure and assembly of desert small mammal communities. This generality was challenged by several authors (e.g. Mares, 1983; Morton, 1985; Kerley, 1992; 1993a; 1993b; Morton et al., 1994; Rogovin et al., 1994; Kelt et al., 1996), resulting in changes in our understanding of the patterns of community organization in different deserts. In particular, desert small mammal communities generally exhibit low alpha diversity (S=2–4 species) and high beta diversity, whereas gamma diversity varies from desert to desert, although this is confounded by differences in their geographical extent (Kelt et al., 1996). Species in most desert regions are distributed in a Gleasonian manner, responding to the spatial distribution of those variables that determine their individual niches (Brown & Kurzius, 1987; Morton et al., 1994; Shenbrot et al., 1994). As a result, local communities are fluidly structured with respect to species composition. Additionally, the trophic structure of communities is variable from desert to desert, and is only loosely predictable by the pool of species that are available to a site. Trophic characteristics of species in a given region appear strongly influenced by regional and phylogenetic history, and largely by the characteristics of species that initially colonized these regions (Kelt et al., 1996). Most of these earlier studies have been based on the statistical and graphical distribution of species and species characteristics across multiple sites. Some authors have compared the number of species per site, the number of trophic groups and their relative richness, and patterns of ecomorphological structure (Rogovin & Surov, 1990; Rogovin et al., 1991, 1994; Shenbrot, 1992; Rogovin & Shenbrot, 1993; Shenbrot et al., 1994), while others have addressed the autecology of specific species (e.g. Abramsky & Sellah, 1982; Schroder, 1987; Fox & Gullick, 1989; Kerley, 1989; Brown & Harney, 1993). A complementary approach to understanding the structure and assembly of large assemblages of communities is to incorporate null models (Caswell, 1976; Strong et al., 1979; Harvey et al., 1983; Colwell & Winkler, 1984; Kelt et al., 1995). Ecologists have used null models to address questions in a wide array of ecological and behavioural contexts (reviewed in Gotelli & Graves, 1995). These have greatly aided the search for effects of particular ecological processes by comparing observed structure to that obtained assuming nonindependence among or within species or between biotic and abiotic factors such as temperature, precipitation, etc. Earlier (Kelt et al., 1996) we evaluated patterns of species distribution and community structure in the desert small mammal faunas of seven deserts on four continents. In the present study we employ two null models to expand beyond pattern description and analysis to a more refined evaluation of the structure and assembly of desert small mammal communities in North America and Asia. Specifically, we ask if nocturnal terrestrial small mammal communities in North American and two Asian deserts are similarly structured, or if there are broad differences in the type of structure characterizing these faunas. The first null model that we apply evaluates the degree to which communities are nested subsets of more speciesrich communities (Patterson & Atmar, 1986). Significant nestedness is thought to reflect underlying hierarchical relationships among the species in a region (Patterson & Blackwell Science Ltd 1999, Journal of Biogeography, 26, 825–841 North American and Asian desert rodents 827 Brown, 1991). Such factors as differential immigration and/or extinction, hierarchical competitive relationships, or differential habitat relationships might result in a nested pattern. The second model implicitly assumes competitive interactions to occur between ecologically similar species. This model compares an observed assemblage of communities against simulated assemblages produced by random assembly (i.e. no interactions among species), thereby testing the null hypothesis of noninteractive assembly. Additionally, this model allows for an estimate of the mean strength of interaction among species, as well as the power of this estimate (Kelt et al., 1995). We discuss the results of these analyses in the context of earlier studies on the worlds desert small mammal communities, and the role that history has played in structuring contemporary communities. METHODS Terminology To avoid confusion, we define a community as the set of species that occur at a local site. In this paper we will use the term assemblage to refer to a set of communities. Thus, for example, we will refer to the assemblage of small mammal communities from the Gobi Desert. Taxonomy follows Wilson & Reeder (1993). several endemic taxa, they also share a number of species and genera (e.g. Mares, 1993a). Direct comparisons of these two hierarchical schemes are difficult at best. However, the Turanian and Mongolian regions served as the two main centres of radiation for Central Asian rodent faunas, and these are separated physically by the expansive (c. 2000 km east to west) Kazakhstan Desert (Heptner, 1945). As a result, higher taxonomic comparisons (e.g. genera, families) demonstrate much greater differences among Asian deserts than among North American deserts. Because these Asian deserts are physically separated from each other by the Kazakhstan Desert and high mountain ranges (e.g. the Altay and Sayan Mountains), are physiognomically and climatically very different (Walter & Box, 1983), remain relatively little studied (relative to North American deserts; e.g. Genoways & Brown, 1993), and exhibit substantial faunal differences above the level of species, we feel it is more informative to analyse the Turan and Gobi regions separately. For both data sets, only nocturnal and terrestrial species under 500 g in body weight were included; because we are not certain of the efficiency with which sciurids, gophers, and soricines were sampled, these groups have been excluded from these analyses. This results in only minor changes to the database used by Kelt et al. (1996); a single site in the Gobi Desert has been removed from the present analysis because it possessed only Rhombomys opimus (Lichtenstein 1823), a large diurnal prairie dog-like gerbilline rodent. Data Data for North American deserts were taken from Brown & Kurzius (1987) as corrected in Morton et al. (1994), and consist of a presence/absence matrix of forty-one species at 201 sites broadly distributed throughout the Great Basin, Sonoran, Chihuahuan, and Mojave Deserts of the western United States (Table 1). All sites experienced a minimum of 100 trap-nights of effort; in long-term studies only data from the first year were used. Details on the locations and characteristics of these sites are given in Brown & Kurzius (1987). Similar data for Asian deserts have been taken from Kelt et al. (1996), and were originally reported by Shenbrot et al. (1994) and Rogovin & Shenbrot (1995). These data include twenty species from ninety-seven sites in Mongolia, and fifteen species from thirty-six sites in the Turan Desert Region, which here is considered to include the Kyzyl-Kum, Kara-Kum, Daghestan and extreme western Kazakstan Deserts (Table 1). Many Asian species did not readily enter live traps, so this method was supplemented with visual surveys and capturing animals with hand-held nets. Although we have grouped all North American deserts in a single category while evaluating the two Asian deserts separately, it might be argued that faunal differences between North American deserts are nearly as extreme as those between Asian deserts, if not equally so, and that these also should be analysed separately. Hagmeier (1966) segregated North American deserts into four separate ‘super-provinces’, while the Turan and Gobi Deserts are considered distinct “provinces” within the same (Turano-Gobian) ‘subrealm’ (G. Shenbrot, unpublished observations). Although both Asian deserts possess Blackwell Science Ltd 1999, Journal of Biogeography, 26, 825–841 Analyses As background for interpretation of subsequent analyses we compared the three desert regions in terms of the species composition within functional groups occurring at local sites, as well as those in both habitat and regional species pools, using a one-way Model II analysis of variance, and employed Scheffe’s multiple-comparison test to assess which deserts deviated significantly. We then employed two statistical randomizations to evaluate internal structure. Null model of nested structure A series of communities is considered to be nested when each species is present in all communities richer than the most depauperate community in which that species occurs (Patterson & Atmar, 1986). Atmar & Patterson (1995) have recently expanded the generality and availability of this concept with the Nestedness Temperature Calculator, which resolves several problematic issues in earlier nestedness metrics (e.g. their earlier metric, N, emphasizes unexpected presences more than absences, all absences are given equal weight, the metric is dependent upon matrix size and therefore not comparable across data sets; for details, see Atmar & Patterson, 1993; Kelt, 1997). In their approach, an assemblage of communities is compared to that expected under maximum nestedness. The unexpected presence or absence of a species is similar to information surprise, and may be thought of conceptually as increased disorder or entropy. The system’s ‘characteristic temperature’ is a thermodynamic-like metric reflecting the degree to which communities deviate from nestedness. A 828 Douglas A. Kelt et al. Mode of locomotion Trophic code North America Heteromyidae, Dipodomyinae Dipodomys deserti Stephens, 1887 Dipodomys merriami Mearns, 1890 Dipodomys microps (Merriam, 1904) Dipodomys ordii Woodhouse, 1853 Dipodomys panamintinus (Merriam, 1894) Dipodomys spectabilis Merriam, 1890 Microdipodops megacephalus Merriam, 1981 Microdipodops pallidus Merriam, 1901 Heteromyidae, Perognathinae Chaetodipus baileyi Merriam, 1894 Chaetodipus fallax Merriam, 1889 Chaetodipus formosus Merriam, 1889 Chaetodipus hispidus Baird, 1858 Chaetodipus intermedius Merriam, 1889 Chaetodipus nelsoni Merriam, 1894 Chaetodipus penicillatus Woodhouse, 1852 Perognathus amplus Osgood, 1990 Perognathus flavus Baird, 1855 Perognathus longimembris (Coues, 1875) Perognathus parvus (Peale, 1848) Muridae, Arvicolinae Lemmiscus curtatus (Cope, 1868) Microtus longicaudus (Merriam, 1888) Microtus montanus (Peale, 1848) Muridae, Cricetinae Baiomys taylori (Thomas, 1887) Neotoma albigula Hartley, 1894 Neotoma lepida Thomas, 1893 Neotoma micropus Baird, 1885 Onychomys leucogaster (Wied-Neuwied, 1841) Onychomys torridus (Coues, 1874) Peromyscus boylii (Baird, 1855) Peromyscus crinitus (Merriam, 1891) Peromyscus eremicus (Baird, 1858) Peromyscus leucopus (Rafinesque, 1818) Peromyscus maniculatus (Wagner, 1845) Peromyscus pectoralis Osgood, 1904 Peromyscus truei (Shufeldt, 1885) Reithrodontomys fulvescens J. A. Allen. 1894 Reithrodontomys megalotus (Baird, 1858) Reithrodontomys montanus (Baird, 1855 Sigmodon arizonae Mearns, 1890 Sigmodon hispidus Say and Ord, 1825 Sigmodon ochrogaster Bailey, 1902 Gobi Desert Dipodidae, Allactaginae Allactaga balikunica Hsia and Fang, 1964 Allotaga bullata Allen, 1925 Allactaga sibirica (Forster, 1778) Pygeretmus pumilio (Kerr, 1792) Dipodidae, Cardiocraniinae Cardiocranius paradoxus Satunin, 1903 Salpingotus crassicauda Vinogradov, 1924 Salpingotus kozlovi Vinogradon, 1922 Dipodidae, Dipodinae Dipus sagitta (Pallas, 1773) Stylodipus andrewsii Allen, 1925 B B B B B B Q Q G G F G G G G G Q Q Q Q Q Q Q Q Q Q Q G G G G G G G G G G G Q Q Q F F F Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q Q O F F F I I O O O O O O O O G O F F F B B B B O O O F B B B G G I B B M M Table 1 List of species included in the analyses. Differences between this listing and that in Kelt et al. (1996) reflect different objectives. For example, Eolagurus przwalskii (Büchner 1889) and Meriones meridianus (Pallas 1773) are diurnal and are not included here. Microtus limnophilis (Büchner 1889) and Phodopus campbelli (Thomas 1905) were not collected at any sites and were not included in our earlier analysis. These species do contribute to the species pool for these sites, however, and are therefore included here for use in the null model. Taxonomy follows Wilson & Reeder (1993). Codes are: B=bipedal, Q=quadrupedal, F=folivore, G=granivore, I=insectivore, M=mixed folivore/omnivore, O=omnivore. continued Blackwell Science Ltd 1999, Journal of Biogeography, 26, 825–841 North American and Asian desert rodents 829 Table 1 continued. Mode of locomotion Trophic code Dipodidae, Euchoreutinae Euchoreutes naso Sclater, 1891 Muridae, Arvicolinae Microtus limnophilus Büchner, 1889 Muridae, Cricetinae Allocricetulus curtatus Allen, 1925 Cricetulus migratorius (Pallas 1773) Cricetulus sokolovi Orlov and Malygin, 1988 Phodopus campbelli (Thomas, 1905) Phodopus roborovskii (Satunin, 1903) Muridae, Gerbillinae Meriones meridianus (Pallas, 1773) Muridae, murinae Mus musculus Linnaeus, 1758 Turan Desert Region Dipodidae, Allactaginae Allactaga elater (Lichtenstein, 1828) Allactaga major (Kerr, 1792) Allactaga severtzovi Vinogradov, 1925 Allactodipus bobrinskii Kolesnikov, 1937 Pygeretmus platiurus (Lichtenstein, 1823) Pygeretmus pumilio (Kerr, 1792) Dipodidae, Dipodinae Dipus sagitta (Pallas, 1773) Jaculus blanfordi turcmenicus Vinogradov and Bondar, 1949 Stylodipus telem (Lichtenstein, 1823) Dipodidae, Paradipodinae Paradipus ctenodactylus (Vinogradov, 1929) Muridae, Cricetidae Allocricetulus eversmani (Brabdt, 1895) Cricetulus migratorius (Pallas, 1773) Muridae, Gerbillinae Meriones libycus Lichtenstein, 1823 Meriones meridianus (Pallas, 1773) Meriones tamariscinus (Pallas, 1773) perfectly ‘cold’ assemblage would be completely nested, whereas increasingly non-nested assemblages would include greater numbers of unexpected presences and absences, and would therefore have a higher ‘temperature’ (see Atmar & Patterson (1993) for methodological details). We calculated the ‘temperatures’ of North American and Asian desert rodent communities, as well as the probability that these are random subsets of the overall pools of species available. Earlier, Patterson & Brown (1991) analysed the nestedness of the granivore component of the North American data set, and reported these to be significantly nested. Subsequently, Kelt and Brown (in press a) expanded these analyses to include nongranivorous species. Here, we extend these analyses to include Asian deserts, and to compare the patterns there with those in North America. Null model of competitive assembly Fox (1987, 1989) presented a null model to describe the assembly of communities of locally interacting species. Readers familiar with this model, especially as advanced by Kelt et al. (1995), may wish to skip this section. Blackwell Science Ltd 1999, Journal of Biogeography, 26, 825–841 B I Q F Q Q Q Q Q I O O G G B M Q O B B B Q B B M O O F F F B B B M M M B F Q Q I O Q Q Q M M M Fox’s model assumes that competitive interactions occur between species that use their environment in a similar manner. Such species share some ecologically important attributes, and comprise the set of species in a given functional group (see Kelt et al., 1995 for details). According to the null model, communities should develop such that each new species is drawn from a different functional group, until all functional groups are represented, at which point the procedure is repeated. Functional groups often are based on diet, such that species are characterized as omnivores, folivores, carnivores, etc. In a community with these three functional groups, the first species to enter could be a member of any functional group. The second species, however, would be expected to come from one of the two functional groups that are not yet represented, and the third species would be a member of the final functional group. Therefore, a three species community would be predicted to possess one omnivore, one folivore, and one carnivore (we could denote this as [1,1,1]). A fourth species could be drawn from any of the functional groups, and so on. On the other hand, the model would not predict a three species community in which two species were from one functional 830 Douglas A. Kelt et al. group, one species was from a second functional group, and the third functional group was not represented (denoted as [2, 1,0]). Such a configuration of species would be unexpected, as competitive interactions would be greatest among the two species sharing functional group membership, and one of these would be expected to be competitively excluded from the community, until a member of the third functional group was present. At this point, there would be an equal competitive influence within any of the three functional groups, so a fourth species could come from any group. Unexpected combinations of species, such as the [2,1,0] configuration just described, are referred to as unfavoured states. In contrast, configurations conforming to the model are referred to as favoured states. Examples of favoured states include [1,1,1], [2,1,1], or [3,2,2]. Favoured states are those in which the difference between the number of species in any two categories is not greater than one. Fox’s (1987) model has three principal features (see Kelt et al., 1995). First, all species (both those present at observed sites and those comprising the pool of species that could enter these sites) are placed in functional groups. Second, communities at all sites are scored as being favoured or unfavoured, and a tally (Tobs) of the number of sites in a favoured configuration is computed. Third, Tobs is compared to the expected distribution of T under the null hypothesis of no competitive interaction. This distribution is obtained via a Monte Carlo simulation. For each site, a simulated community is assembled by randomly drawing species from the species pool for that site until the number of species in the simulated community equals that in the observed community. The simulated community is scored as being in a favoured or unfavoured state, and the process is repeated for the remaining sites in the assemblage. The number of simulated communities that are favoured is recorded (yielding one estimate of the value of T), and the entire process is repeated. Each such cycle represents one iteration of the model, and produces an additional estimate of T. This is repeated many times (here, n=2000) to produce a frequency distribution of the number of favoured states expected for this assemblage of communities. The observed community is then compared to this distribution. If the observed number of favoured states lies in the upper critical region (aupper) of this distribution then the observed assemblage is considered to be significantly more structured than expected by random assortment, and competition is inferred. Alternatively, the observed number of favoured states may lie in the lower critical region (alower) of the distribution, indicating that the observed assemblage is significantly more clumped than expected by random assortment, and some sort of positive association between species is inferred. Because either alternative is possible, we employ a two-tailed test with a=2.5%. Kelt et al. (1995) extended Fox’s model by incorporating explicit alternative hypotheses through the introduction of an ‘association coefficient’ (h,=‘interaction coefficient’ of Kelt et al., 1995) that produces a decrease in the probability of a species successfully establishing in a community if another species in the same functional group is already present. Note that this is not the same interaction coefficient used in classic Lotka–Volterra models (e.g. Roughgarden, 1979), as it does not reflect direct interaction between individuals. Rather, h is the reduction in the probability of establishment by a species that has immigrated to a site. Assembly proceeds by the immigration and establishment of species. Immigration is entirely dependent upon the pool of species that are available to enter a community. Species are drawn from functional groups according to the relative size of these groups, and species are drawn without replacement, so that no species may be entered twice. Immigration probabilities are recalculated after each successful establishment. The probability of establishment of a species depends upon the composition of the community. Each species present in the community decreases the probability of successful establishment of another species in the same functional group; the strength of this interaction is h, and theoretically may range from –x to 1. Positive values of h reflect negative associations among species, whereas negative values represent positive associations. When h =1 an incoming species is barred from establishing. In the null model of random assembly, interactions are zero, and h =0. Two factors that may produce positive associations among species (h < 0) are habitat choice or geographical structuring of species pools. Unfortunately, because h theoretically extends to –x it is difficult to evaluate the strength of such associations (e.g. is h =–1 ‘strong’?; how much ‘stronger’ is h=−3?), except to document that these are nonrandom (e.g. h≠h). Species pools were prepared from the literature and our personal knowledge of the ecological and geographical distribution of these species. For North America and Asia, the species pools consisted of those species whose geographical ranges overlapped a particular site. Thus, each site had a potentially unique pool of potential immigrants. Because this was generated strictly by the geographical distribution of species, we refer to this as the geographical species pool (GSP). Because two of us (KR and GS) collected all of the Asian data ourselves we also have detailed information on the particular habitats at each Asian site, and were therefore able to develop a second set of species pools based on the known habitat tolerances of these species; we refer to this as the habitat species pool (HSP). Because the North American data set was largely extracted from the literature we could not develop a series of HSPs that we believed was consistent across all sites. Finally, we developed species pools based on two different functional categories. Extensive research on North American desert rodents has suggested that both locomotory mode (bipedal vs. quadrupedal) and trophic habits are important factors influencing community structure. Additionally, these factors – especially locomotory mode – have been invoked to help explain structure in Asian desert rodent communities (e.g. Rogovin & Surov, 1990; Shenbrot, 1992; Rogovin & Shenbrot, 1993, 1995; Shenbrot et al., 1994; Shenbrot & Rogovin, 1995). Thus, we prepared GSPs and HSPs with each of these characteristics, as well as with both. Trophically based species pools segregated species as omnivores, folivores, carnivores, or granivores. Because many Asian species are both granivorous and folivorous (Shenbrot et al., 1994), we have included a fifth trophic category that we call mixed granivorous-folivorous. In Blackwell Science Ltd 1999, Journal of Biogeography, 26, 825–841 North American and Asian desert rodents 831 a separate analysis (Kelt et al., 1996) we allocated such species equally to both granivorous and folivorous categories. Because the model used in this paper requires that functional groups contain integer quantities of species, however, we could not consider a species to be 50% granivorous and 50% folivorous. In North America, many microtines undergo seasonal shifts in their dietary strategies, focusing largely on green vegetation when available (winter and spring), but switching to seeds and other items during summer and fall. This is fundamentally different from the mixed diets of such Asian taxa as Allactaga (F. Cuvier 1837), Dipus Zimmermann 1780, Jaculus Erxleben 1777, Meriones Illiger 1811, and Stylodipus Allen 1925, which forage on both food items whenever available. Therefore we have retained this category, although we recognize that some readers may find it awkward. To evaluate the sensitivity of these analyses to such allocations, however, we conducted an additional set of analyses in which we grouped mixed folivores/ omnivores with omnivores. Locomotory based species pools segregated species according to whether they were bipedal or quadrupedal. Finally, these categories were combined to form combination species pools, in which species were segregated as bipedal granivores, quadrupedal folivores, bipedal omnivores, quadrupedal carnivores, etc. The probability of a species successfully immigrating into a site, and subsequently establishing itself there, may be calculated as: P(yj = i | Xj−1) = (ni−Xi, j−1)(1−h)Xi, j−1 [(ni−Xi, j−1)(1−h)Xi, j−1] k i=1 (1) where i and j index functional groups and species, respectively, k is the number of functional groups, ni is the number of species in functional group n, yj is a random variable, indicating the functional group to which the jth species will be placed, Xj−1 is a vector of local species composition after the j−1th species has entered the community, and Xi, j−1 is a scalar which gives the number of species in functional group i after the jth species has entered. The numerator gives the combined probability of a species immigrating and becoming established. The denominator normalizes these probabilities so that they sum to one (Kelt et al., 1995). Finally, by varying the value of h we can determine the value of h that produces a distribution of expected number of favoured states that best agrees with the observed number of favoured states. We refer to this value as ĥ, and it constitutes a maximum likelihood estimate of the mean strength of nonindependence across all sites and all species. While this is clearly a general value with limited ability to predict the strength of interaction at any given site, or with any pair of species, its existence allows us to estimate the statistical power of our model. Power is the probability that a hypothesis is incorrect and therefore, should be refuted. If we assume that ĥ reflects the real strength of interaction among these species, then the proportion of the distribution of expected values (number of favoured states) produced with h=ĥ that lies in the 2.5% critical region (two-tailed test) of the distribution of expected values produced with h=0 (the null hypothesis) Blackwell Science Ltd 1999, Journal of Biogeography, 26, 825–841 constitutes an estimate of the power with which we can state that observed communities are significantly different from the null. Note that because the frequency distributions are discrete (not continuous) we cannot provide power at exactly a=0.05; rather, we bracket this a level with adjacent categories. RESULTS In order to provide sufficient background for interpreting the results of simulations, we compared these three desert regions in terms of local species richness (within and across functional groups) and we evaluated patterns of species co-occurrence and species combinations (sensu Brown & Kurzius, 1987; Morton et al., 1994; Kelt et al., 1996). Local communities and regional species pools The number of species present at local sites varies considerably across these deserts. The total number of species present is lower in the Turan Desert Region than in the Gobi or in North America, but the distribution of trophic and locomotory types is more complex (Table 2; Figs 1 and 2). North America and the Gobi Desert have high numbers of insectivores and omnivores, whereas the Turan Desert Region shares high representation of folivores with North America, and has the fewest number of granivores of these three regions. The Turan Desert Region also possesses more mixed omnivore/folivores than the Gobi. North America and the Gobi Desert have significantly more bipedal species, and significantly fewer quadrupedal species, than the Turan Desert Region. The number of species in the geographical species pools for these sites also varies greatly from desert to desert (Table 3). North American sites have the largest geographical species pools, and include significantly more folivorous, omnivorous, and granivorous species than Asian deserts. This result may be confounded by the much larger area of the North American deserts (Kelt et al., 1996), or by the subdivision of North American deserts by the many mountain ranges that occur there North America is intermediate in richness of insectivorous species, although exclusion of shrews (Insectivora, Soricidae) may have influenced this result. North America had significantly fewer bipedal species, and significantly more quadrupedal species, than either Asian region. The Gobi Desert has the greatest number of bipedal species, and of insectivorous species, and the fewest folivorous species. The Turan Desert Region has the fewest insectivores, omnivores, and granivores, and an intermediate number of folivores. This region had a greater number of mixed omnivore/folivores than the Gobi Desert. Finally, the numbers of species whose habitats are congruent with the characteristics of a given site (species in the habitat species pools) was only evaluated for Asian sites. There, sites in the Gobi Desert were ecologically suitable to more species than were sites in the Turan Desert Region. Habitat pools in the Gobi Desert contained more bipedal species, and more insectivorous and omnivorous species, than the Turan Desert Region, whereas these pools in the latter region, in turn, possessed more quadrupedal species and more folivorous and mixed omnivorous/folivorous taxa (Table 4). 832 Douglas A. Kelt et al. Table 2 Results of analysis of variance on the number of species found at sites. Desert regions with the same letters were not significantly different in a posteriori tests (A denotes greater richness than B, etc.). Trophic categories All species Model Error Folivores Model Error Insectivores Model Error Omnivores Model Error Granivores Model Error Mixed Folivores/Omnivores Model Error Bipedal species Model Error Quadrupedal species Model Error d.f. SS 2 331 23.16 1121.54 2 331 F P Gobi Turan Desert Region North America 3.42 0.0339 A,B B A 10.18 101.00 16.67 0.0001 B A A 2 331 4.31 96.29 7.41 0.0007 A B A 2 331 8.93 181.66 8.14 0.0004 A B A 2 331 243.22 440.61 91.36 0.0001 B C A 2 331 194.39 78.76 408.49 0.0001 B A C 2 331 45.75 694.31 10.90 0.0001 A B A 2 331 7.52 302.76 4.11 0.0173 B A B Species co-occurrences In both North America and Asia, species co-occurred with a large number of other species and in many different combinations (Fig. 3). Species generally occurred most frequently with a small number of species, and progressively less frequently with a large number of species. Community nestedness North American sites exhibited moderately high system ‘temperatures’, but these were significantly lower (more structured) than that expected by random (Table 5), indicating that these communities are significantly more nested than expected by random assortment of species. Most Asian deserts exhibit similar patterns. All three regions of the Gobi Desert, and the Gobi as a whole, are significantly more nested than expected. The deserts of the Turan Desert Region are also significantly more nested than expected assuming random distribution of species. The only exception to this pattern is the geographically restricted Daghestan + Kazakhstan region. The lack of a significantly nested structure there may reflect reduced power to discriminate, resulting from the relatively small pool of species (S=9 species) and small number of sites (N=14). Null assembly model Our analysis of forty-one species of small mammal at 201 sites throughout arid North America confirmed the results presented for more restrictive data sets by Fox & Brown (1993) and Kelt & Brown (in press a). When species were characterized by both trophic (granivore, omnivore, folivore, insectivore) and locomotory (bipedal v. quadrupedal) features, these communities appeared highly structured (Table 6). This pattern also held true for analyses using only locomotory mode to characterize species. When only trophic features were incorporated, however, no structure was indicated (Table 6). Moreover, species at these sites exhibit strong negative associations. Using locomotory categories, ĥ=0.41 (power > 99%), whereas using trophic and locomotory categories combined, ĥ=0.36 (power=89–92%; Table 6). Very different results were obtained for analyses on Asian deserts. With sixteen species and ninety-seven sites in the Gobi Desert, analyses based on trophic or locomotory modes, and using either regional or habitat species pools, demonstrated significant deviation from random assortment. However, in all cases the results of simulations indicated that the distribution of species in functional groups in these communities was significantly more clumped than expected by random; that is, there were fewer favoured states in the real world than in assemblages of simulated communities. This indicates that similar species (those occupying the same functional group) co-occurred more frequently than expected by chance. The power of these analyses was very high (Table 6), indicating that we are highly likely to be correct in stating that these are non-randomly assembled communities. The high power of these analyses also argues strongly for positive, rather than negative, associations among these species. When trophic and Blackwell Science Ltd 1999, Journal of Biogeography, 26, 825–841 North American and Asian desert rodents 833 Figure 1 Number of species found at each site (mean +1 SD), as well as in the habitat and regional species pools, across all species and two functional groups based on animals’ mode of locomotion. Habitat species pools were not determined for North American sites. It is evident that the greater regional diversity in North America reflects a radiation of bipedal species (Dipodomys). However, this increase is not observed at the local level, where observed diversity of bipedal species is similar across all deserts. Because these figures represent the mean number of species that occur at a site, or whose habitat or geographical affinities correspond to those at a given site, the number of species in the habitat pool may exceed that for the geographical pool at a given site (e.g. quadrupedal species in the Turan Desert Region). locomotory modes were combined, we found no evidence of non-random assortment. Finally, using fifteen species at thirty-six sites in the Turan Desert Region, most analyses indicated that these sites are not significantly different from random. When we analysed either species pools using combined functional groups, our analyses suggested that these assemblages were significantly less favoured than random collections of species, as in the analyses for the Gobi Desert. Similar to analyses for the Gobi Desert, species pools that demonstrated significant deviation from noninteractive assembly were very powerful (Table 6), and provide strong support for a conclusion that species sharing functional group membership are positively associated. In contrast, nonsignificant analyses also possessed relatively low statistical power. In order to evaluate the influence of the ‘mixed omnivore/ folivore’ category on these results we conducted a separate simulation in which we combined this category with other omnivorous species. In the Turan Desert Region, using the habitat species pool and analysing structure with the combined trophic and locomotory categories resulted in an increase in ĥ from less than −2.0 to −0.4 (a reduction in the degree of positive associations), and these results were not significantly Blackwell Science Ltd 1999, Journal of Biogeography, 26, 825–841 Figure 2 Number of species found (mean +1 SD) in functional groups based on diet. Habitat species pools were not determined for North American sites. North American sites do not have any mixed omnivores/folivore species, whereas the Turan Desert Region lacks granivores entirely. The regional diversification of granivores in North America is also expressed locally, with more species per site than in the Gobi Desert. North American pools also contain more omnivores and folivores than Asian deserts, but these do not result in greatly elevated diversity of these groups locally. Because these figures represent the mean number of species that occur at a site, or whose habitat or geographical affinities correspond to those at a given site, the number of species in the habitat pool may exceed that for the geographical pool at a given site (e.g. folivores and granivores in the Gobi Desert). different from random association (Table 6, values in brackets). For the other seven simulations that were repeated, however, both ĥ and the probability that these communities were randomly assembled declined when these trophic categories were combined (Table 6), indicating increasingly positive associations and statistical significance. Thus, segregating ‘mixed omnivorous/folivorous’ species provided a relatively conservative analysis for comparison with patterns from North America. DISCUSSION Similarities and differences between North American and both Asian desert rodent communities have been demonstrated in 834 Douglas A. Kelt et al. Table 3 Results of analysis of variance on the number of species occurring in regional species pools. Desert regions with the same letters were not dignificantly different in a posteriori tests (A denotes greater richness than B, etc). All species Model Error Folivores Model Error Insectivores Model Error Omnivores Model Error Granivores Model Error Mixed Folivores/Omnivores Model Error Bipedal species Model Error Quadrupedal species Model Error d.f. SS F P Gobi Turan Desert Region North America 2 331 3957.08 3646.52 179.59 0.0001 B B A 2 331 764.11 345.72 365.79 0.0001 C B A 2 331 74.67 54.95 224.87 0.0001 A C B 2 331 427.11 882.63 80.09 0.0001 B C A 2 331 2273.50 1070.20 428.91 0.0001 B C A 2 331 1014.55 21.44 7830.31 0.0001 B A C 2 331 1069.03 340.11 520.19 0.0001 A B C 3 331 9001.44 2754.43 540.85 0.0001 B B A Table 4 Results of analysis of variance on the number of species occurring in habitat species pools (North American deserts excluded from this analysis). Letters (A, B) indicate which region had greater richness, with A denoting higher richness than B. All species Model Error Folivores Model Error Insectivores Model Error Omnivores Model Error Mixed Folivores/Omnivores Model Error Bipedal species Model Error Quadrupedal species Model Error d.f. SS 1 131 20.64 405.75 1 131 F P Gobi Turan Desert Region 6.66 0.0109 A B 20.51 32.80 81.89 0.0001 B A 1 131 32.72 36.80 116.45 0.0001 A B 1 131 3.36 67.46 6.53 0.0117 A B 1 131 159.77 48.53 431.22 0.0001 B A 1 131 91.87 302.39 39.80 0.0001 A B 1 131 25.42 130.10 25.60 0.0001 B A Blackwell Science Ltd 1999, Journal of Biogeography, 26, 825–841 North American and Asian desert rodents 835 Figure 3 Left panels give the numbers of sites occupied (solid bars) by the most commonly encountered species in three deserts, and numbers of different combinations involving those species (hatched bars) as a function of the number of coexisting species per site. Right panels give the frequency with which each species occurs with other species. Species presented are the most commonly encountered species in North American deserts (Dipodomys merriami) and the two most commonly encountered species in the Gobi Desert (Dipus sagitta and Allactaga sibirica) and the Turan Desert Region (Meriones meridianus and Allactaga elator). Table 5 Results of an analysis on the degree of nestedness in communities from three desert regions. Presented for the original data are the number of species in the region and the number of sites analysed, the observed system temperature (see text), and the percentage fill of the data matrix. Simulation results, based on of 500 randomizations, include the probability of the observed system temperature (and the number of standard deviations between the observed and the mean of the 500 simulations), and the mean (‘characteristic’) temperature and standard deviation of 500 simulated assemblages. Characteristic temperature System Desert Southern Gobi Western Gobi Eastern Gobi Gobi desert Daghestan + Kazakhstan Kyzyl-Kum Turan Desert Region North America Number of species/sites Temp Fill 11/32 12/29 10/36 16/97 9/14 10/22 15/36 41/201 26.72° 39.34° 38.18° 32.39° 48.60° 38.82° 31.87° 13.84° 28.6% 31.0% 39.7% 22.6% 34.9% 34.8% 22.4% 9.7% terms of species diversity, guild structure, and ecomorphology (Rogovin & Surov, 1990; Shenbrot et al., 1994; Rogovin & Shenbrot, 1995; Kelt et al., 1996). As treated here, North American deserts possess twice as many terrestrial nocturnal small mammal species (S=41) as either the Gobi Desert (S= 20) or the Turan Desert Region (S=15). Although this difference may be partially confounded by differences in the Blackwell Science Ltd 1999, Journal of Biogeography, 26, 825–841 P(TΖTobs) 9.48e-5 (–3.74 r) 1.30e-2 (–2.235 r) 7.10e-4 (–3.20 r) 2.71e-12 (–6.90 r) 0.655 (+0.43 r) 0.0105 (–1.26 r) 0.00462 (–2.61 r) 4.32e-47 (–15.84 r) Mean SD 51.18° 53.80° 56.16° 55.42° 44.63° 48.77° 47.88° 36.44° 6.54° 6.49° 5.63° 3.33° 9.17° 7.93° 6.14° 1.42° areal extent of our sampling regions (greatest in North America, smallest in the Turan Region; see Kelt et al., 1996), this should not influence the simulations conducted here, with the single exception of nestedness in the Daghestan + Kazakhstan portion of the Turan Desert Region (see below). The composition of these deserts were also very different (Figs 1 and 2). Species pools for North American sites were dominated by quadrupedal 836 Douglas A. Kelt et al. Table 6 Results of 2000 Monte Carlo simulations. Values of hˆ in boldface print are significantly different from 0. Power is given for critical values bracketing a=0.05 (see text). Values in brackets are for analyses in which mixed omnivores/folivores were combined with omnivores; power was not evaluated for these simulations. Gobi Desert Regional species pool Trophic categories Locomotion categories Combination categories Habitat species pool Trophic categories Locomotion categories Combination categories Turan Desert Region North America P(h=0) ĥ Power P(h=0) ĥ Power P(h=0) ĥ Power 0.0215 [0.0024 0.0005 0.1760 [0.0040 –0.45 c. –0.55 –0.69 –0.27 c. –0.95 0.5560–0.6490 0.0005 0.9445–0.9615 0.2410–0.3240 0.0070 0.1555 < –2.00] 0.3605 0.0005 c. –1.9] –0.51 0.1820–0.3165 0.0925 –0.30 0.4530–0.5385 –0.11 –1.90 0.0755–0.1495 0.8400–0.9195 0.0005 0.0001 0.41 0.36 0.9925–0.9950 0.8865–0.9185 0.0025 [0.0035 0.0005 0.5645 [0.0015 –0.97 –0.8 –0.75 –0.07 c. –0.95 0.8780–0.9280 0.0195 0.9950–0.9900 0.0810–0.1215 0.3290 0.3400 –2.00] 0.0580 0.0050 –0.40] –0.44 0.1780–0.2950 –0.52 –2.20 0.2550–0.3925 0.8135–0.8945 species with folivorous, omnivorous, or granivorous diets, whereas the pools for Gobi Desert sites were dominated by nonfolivorous species, with a slightly greater representation of bipedal than quadrupedal species. Species pools for sites in the Turan Desert Region were moderately biased towards bipedal species, and were dominated by folivorous or omnivorous species (or mixed folivorous/omnivorous species); this region lacked granivores entirely. In spite of these functional differences, alpha diversity remained low in all deserts (Fig. 1), with three to four species typically occurring at a site. Beta diversity also remained high, as demonstrated by the great variability in the number and combinations of species with which the most widespread species occurred (Fig. 3). Most analyses provided strong indications of nested structure (Table 5), but there were notably different patterns in North America and Asia. Perhaps most apparent is that both the temperature and fill of the North American deserts was much lower than that of the two Asian deserts studied. The reduced fill is a consequence of combining all North American deserts into one analysis, so that less of the species pool occurs at any one site. The statistics effect of this, however, is to reduce the characteristic temperature for North American deserts. That this is still significantly greater than the observed temperature (Table 5) further underscores the difference between North American and Asian faunas. Additionally, when analysed separately each North American desert retains a cooler temperature than any Asian desert studied (Great Basin, 13.55°, Mojave, 23.16°, Sonoran, 13.59°, Chihuahuan, 19.37°; all are significantly cooler than expected, P < < 10–9). Thus, it is likely that nestedness in North American and Asian assemblages is a result either of different underlying mechanisms or of differential influence of similar mechanisms. For reasons discussed below, we believe that different processes operate to produce nestedness in North American and Asian deserts. Results of an iterative model of local assembly suggested major differences in patterns of community assembly in these deserts. On the basis of much empirical data (reviewed in Price, 1986; Brown & Harney, 1993; Kelt & Brown, in press a), North American communities provide strong evidence for competitive interactions. Notably, use of trophic categories alone did not demonstrate significant structuring, but when the locomotory category was used, or when both categories were combined, associations were significantly negative (e.g. h > 0). In contrast, Asian assemblages provide no evidence for a significant role of interspecific competition in community organization, in agreement with other pattern-based analyses using these data or subsets of them (Rogovin & Surov, 1990; Shenbrot et al., 1994). However, the results of simulations for the two Asian deserts were also very different from each other. Assemblages from the Turan Desert Region exhibited random associations of species when analysed with trophic and locomotory categories, but were significantly positively associated when these categories were combined (Table 6). When we combined omnivorous and mixed omnivore/folivore species, however, significantly positive associations characterized all analyses except those based on locomotory categories. Finally, analyses on the Gobi Desert demonstrated positive associations among species, with respect to both trophic and locomotory functional groupings, but the combined groupings (both trophic and locomotory) were significant only when omnivores were combined with mixed omnivore/folivore species (Table 6). Thus, these simulations suggest either that the processes or dynamics underlying the assembly of small mammal communities in these deserts are very different, or that similar dynamics are occurring, but that these are either very contextdependent or are controlled by different parameter values, such that the final result appears very different. For example, both Abrams (1990) and Leibold (1996) have suggested that, under appropriate conditions, competitive interactions may lead to the coexistence of ecologically similar species. In any case, the three desert regions studied appear to constitute a structural continuum from significantly dispersed (North America) to Blackwell Science Ltd 1999, Journal of Biogeography, 26, 825–841 North American and Asian desert rodents 837 random or moderately clumped (Turan) to significantly clumped (Gobi), at least with respect to this null model and these functional groups. The factors underlying such variation merit consideration, although only further research will allow us to fully evaluate the intrinsic dynamics producing these patterns. Factors producing positive associations Four factors that could produce positive associations among species are local endemism, shared distributional strategies, shared geographical origins, and shared habitat (Gilpin & Diamond, 1982). Endemism is not a factor in these continental faunas, since many species occur over most or all of the regions concerned. By shared distributional strategies, Gilpin & Diamond (1982) were referring to their tramp-supertramp continuum, which reflects the habitat specificity and dispersal abilities of each species. However, this is not relevant to continental rodent communities in which the geographical ranges of most species span the entire desert, as is the case in both the Gobi Desert and the Turan Desert Region. Although Asian desert rodent assemblages consist of species with different geographical origins (e.g. Asian dipodid (jerboas) and cricetid rodents likely radiated within northern Asia, whereas gerbilline rodents (jirds and gerbils) evolved in the Sahara-Sindian Desert region; Pavlinov et al., 1991; Shenbrot et al., 1995), they have occupied similar regions of temperate Asia since the late Miocene (Pavlinov et al., 1995), and such a long period of co-occurrence may contribute to the positive associations we observed. However, North American deserts and taxa have been present since the late Miocene or early Pleistocene, and desert adapted rodents in the Heteromyidae may have a longer history than Asian desert rodent taxa (for fuller discussions of the history of these deserts and faunas, see Shenbrot et al., 1994; Kelt et al., 1996). We believe that it is not so much the ages of these deserts and lineages, but the geomorphology and eco-climatic history that has differed between them, that has led to the differences that we observe today. We elaborate upon this below. Shenbrot et al. (1994) argued that Asian desert rodents were organized into spatial guilds that were separated primarily by soil and vegetative characteristics. Thus, some species are found only on rocky soils (e.g. Allactodipus bobrinskii Kolesnikov 1937), whereas other species occur primarily or exclusively on sandy soils (e.g. Salpingotus crassicaudata Vinogradov 1922, and S. kozlovi Vinogradov 1922, in the western and eastern Gobi, respectively). Such habitat guilds possess species that cooccur more frequently than a random assortment would suggest, and we believe that the positive associations that we observe in Asian deserts are largely a reflection of the similar habitat requirements of the functional group members. When communities are assembled in our simulations, individual habitat associations are ignored, and functional groups are more evenly represented than in real communities. At a smaller spatial scale, variation in microhabitat use (e.g. Rosenzweig & Winakur, 1969; Brown, 1975), spatial variation in resource abundance (Brown, 1989b), and seasonal shifts in foraging efficiencies (Brown, 1989b) have been invoked most Blackwell Science Ltd 1999, Journal of Biogeography, 26, 825–841 commonly to explain the local coexistence of species in North American deserts (see also Brown & Harney, 1993). In a community of three rodent species in the Negev Desert, Brown et al. (1994) demonstrated that habitat heterogeneity, promoted by daily winds that re-distributed sand and food resources, when combined with differing foraging tactics of the three species, appeared to explain the coexistence of these common species. Factors leading to nestedness in non-insular communities Although our analyses support the overriding importance of competition in structuring North American desert communities, competition is only one type of interaction involved in structuring ecological communities, and the relative strength of this may vary from place to place and from time to time (e.g. Kotler & Holt, 1989). Nested faunal structure neither depends upon, nor implies the action of, competition among the constituent species (Patterson & Brown, 1991), although it is consistent with a mechanism of competition (Kelt & Brown, in press a). Our analyses for central Asian communities offer no evidence that competition structures these communities, agreeing with studies on ecomorphology (Rogovin & Surov, 1990), niche packing (Shenbrot et al., 1994), and geographical ecology of these deserts (Rogovin & Shenbrot, 1995). In fact, our results suggest that species in these communities either are noninteractive or are positively interactive, with species being found more frequently with similar species than expected at random. Yet, these assemblages are highly nested. The mechanisms underlying nestedness in Asian assemblages appear to have a very different basis than in North America. Patterson & Brown (1991) proposed three necessary conditions for the development of nested subset structure: (1) a common biogeographic history (2) generally similar contemporary environments, and (3) hierarchical organization of niche relationships. These factors all appear to be operational both in North America and in central Asia, but the mechanisms that operate locally to produce the observed communities appear to be very different in these geographically isolated desert regions. The overwhelming evidence for the importance of competition in North American desert rodent communities, and the fact that these are much more strongly nested structure than are Asian communities, supports an argument that interspecific competition, rather than area-dependent extinction, is responsible for the observed nestedness. However, this is not the case for Asian communities. We believe that the differences observed are at least partly due to the different ages and geomorphologies of North American and Asian deserts, and the different ages and origins of the mammal lineages in these deserts. In this study we have minimized the variability in these factors by studying desert regions that share several features which have been implicated to influence desert evolution and contemporary structure across a global scale. Earlier we reviewed the arguments for historical influences on 838 Douglas A. Kelt et al. local structure (Kelt et al., 1996), but it is worth reiterating briefly to place these communities in a broader perspective. The hand of history – again The deserts considered in this manuscript are all relatively high latitude deserts, occurring >35–76°N. Additionally, they share similar patterns of precipitation and of variation in precipitation (Kelt et al., 1996). Thus, differences in community structure cannot be explained in terms of strategies for dealing with extended droughts, as has been postulated for Australia (Morton, 1985, 1993; Morton et al., 1994; Kelt et al., 1996). However, differences are also apparent between these deserts. The Gobi and Turan Desert Regions both occur at relatively high elevations within large continents, whereas North American deserts vary over a broad range of elevations, and range from the coast of Mexico to the interior of the continent. Asian deserts are thought to have originated in the Cretaceous, but did not become widespread until the Miocene (Sinitzin, 1962), whereas North American deserts likely originated sometime between the late Miocene and the Pleistocene (Axelrod, 1958; Webb, 1977; Van Devender & Spaulding, 1979; Wells, 1979; Thompson & Mead, 1982; Riddle, 1995). Asian deserts are extensive and present little topographic relief, whereas those in North America are embedded within a Basin and Range topography that provides a much greater variety of habitats, as well as barriers to gene flow that have varied in severity with the elevational advance and retreat of forests during pluvial/interpluvial cycles. These barriers likely have favoured isolation of local populations (e.g. Schmidly et al., 1993), providing greater opportunities for speciation than in the more topographically homogeneous Asian deserts. Additionally, and a consequence of the Basin and Range topography plus the pluvial/interpluvial history of North America, species found in forested regions of North America have evolved in proximity to arid regions, and some taxa (e.g. Reithrodontomys Giglioli 1874, Peromyscus Gloger 1841, Neotoma Say & Ord 1825) have since colonized desert communities. Finally, the pluvial/interpluvial phases just mentioned have resulted in significant temporal changes in the areal extent of North American deserts, whereas Asian deserts are thought to have persisted as arid regions since their formation (Sinitzin, 1962). Thus, North American deserts probably are younger than Asian deserts, but more importantly, are more heterogeneous (both spatially and temporally), and have had greater and more recent opportunities for radiation of the taxa present. We do not wish to imply that Asian rodents do not compete. Competitive interactions likely have helped mold the current suites of coexisting species, via mechanisms such as microhabitat selection and use of different foraging tactics in a heterogenous environment (e.g. Abramsky, 1989; Brown et al., 1994; Rosenzweig & Abramsky, 1997; Rosenzweig et al., 1997). However, the strength of competition, and the degree to which it has been supplanted by other more visible mechanisms, may have lowered the detectability of this interaction beyond the resolution of this study (Abramsky, 1989). It is tempting to speculate that past competitive interactions may have sorted the species pools for Asian communities, and that species presently constituting this fauna represent those species that were successful competitors and/or those species that shifted their niche requirements so as to minimize competitive interactions. This would result in highly structured regional faunas, predetermining some degree of structure locally (the ‘Narcissus’ effect of Colwell & Winkler, 1984). The greater age and persistence of Asian deserts has allowed much more time to sort out competitive regimes. This hypothesis emphasizes the pivotal influence of historical processes in structuring contemporary ecological communities (e.g. Connell, 1980; papers in Ricklefs & Schluter, 1993), and may in fact be correct (see, e.g. Diamond, 1986; Van Devender, 1986; for similar arguments). Unfortunately, it is essentially ad hoc, and is probably not testable in this system (see Connell, 1980; Lawton, 1984; Strong, 1984). CONCLUSION Our analyses document important similarities and significant differences between the three desert regions studied, and underscore the importance of regional and historical influences on the structure of local communities. Competitive interactions appear to strongly influence the assembly of rodent faunas in North American deserts. In contrast, our simulations provide no indication that competition is a central feature in the assembly of temperate Asian deserts. We agree with earlier authors that the distribution and extent of major habitat features appear to have greater influence on the assembly of small mammal communities there (see also, Rogovin & Surov, 1990; Shenbrot et al., 1994). While this idea is not novel to this paper, ours is the first attempt to model such regionally specific assembly dynamics. Additionally, this study indicates that there may be important differences in the mechanisms by which communities in the Gobi and Turan Desert Region are organized. The hand of history likely has played a role in forming these assemblages, but how this has differentially influenced Gobi and Turan faunas is not clear. If temperate Asian desert assemblages are structured to some extent by the macrohabitats that are available, then further emphasis on the dynamics within these macrohabitats might be expected to elucidate fundamental structuring forces at the level of habitat patches not visible at the broader scale of the present analyses. ACKNOWLEDGMENTS We thank Barry Fox, Rick Ostfeld, Bruce Patterson, Mary Price, and three anonymous reviewers for numerous comments that improved and clarified the presentation. REFERENCES Abrams, P.A. (1990) Adaptive responses of generalist herbivores to competition: convergence or divergence. Evol. Ecol. 4, 103–114. Abramsky, Z. (1989) Communities of gerbilline rodents in sand dunes of Israel. Patterns in the structure of mammalian communities (ed. by D. W. Morris, Z. Abramsky, B. J. Fox and M. R. Willig), pp. 205–217. Special Publication 28, The Museum, Texas Tech University, Lubbock. Blackwell Science Ltd 1999, Journal of Biogeography, 26, 825–841 North American and Asian desert rodents 839 Abramsky, Z. & Sellah, C. (1982) Competition and the role of habitat selection in Gerbillus allenbyi and Meriones tristrami: a removal experiment. Ecology, 63, 1242–1247. Atmar, W. & Patterson, B.D. (1993) The measure of order and disorder in the distribution of species in fragmented habitat. Oecologia, 96, 373–382. Atmar, W. & Patterson, B.D. (1995) The nestedness temperature calculator: a visual basic program, including 294 presence-absence matrices. AICS Research, Inc., University Park, NM, and The Field Museum, Chicago. Axelrod, D.I. (1958) Evolution of the Madro-Tertiary geoflora. Bot. Rev. 24, 433–509. Bowers, M.A. & Brown, J.H. (1982) Body size and coexistence in desert rodents: chance or community structure. Ecology, 63, 391–400. Brown, J.H. (1973) Species diversity of seed-eating desert rodents in sand dune habitats. Ecology, 54, 775–787. Brown, J.H. (1975) Geographical ecology of desert rodents. Ecology and evolution of communities (ed. by M. L. Cody and J. M. Diamond), pp. 315–341. Belknap Press, Cambridge, Mass. Brown, J.H. (1987) Variation in desert rodent guilds: patterns, processes, and scales. Organization of communities: past, present (ed. by J. H. R. Gee and P. S. Giller), pp. 185–203. Blackwell Scientific Publishers, Oxford. Brown, J.S. (1989a) Coexistence on a seasonal resource. Am. Nat. 133, 168–182. Brown, J.S. (1989b) Desert rodent community structure: a test of four mechanisms of coexistence. Ecol. Monogr. 59, 1–20. Brown, J.H. & Harney, B.A. (1993) Population and community ecology of heteromyid rodents in temperate habitats. Biology of the Heteromyidae (ed. by H. H. Genoways and J. H. Brown), pp. 618–651. Special Publication, American Society of Mammalogists, 10. Brown, J.S., Kotler, B.P. & Mitchell, W.A. (1994) Foraging theory, patch use, and the structure of a Negev Desert granivore community. Ecology, 75, 2286–2300. Brown, J.H. & Kurzius, M.A. (1987) Composition of desert rodent faunas: combinations of coexisting species. Ann. Zool. Fenn. 24, 227–237. Brown, J.H. & Kurzius, M.A. (1989) Spatial and temporal variation in guilds of North American granivorous desert rodents. Patterns in the structure of mammalian communities (ed. by D.W. Morris, Z. Abramsky, B.J. Fox and M.R. Willig), pp. 71–90. Texas Tech University Press, Lubbock. Brown, J.H. & Munger, J.C. (1985) Experimental manipulation of a desert rodent community: food addition and species removal. Ecology, 66, 1545–1563. Caswell, H. (1976) Community structure: a neutral model analysis. Ecol. Monogr. 46, 327–354. Colwell, R.K. & Winkler, D.W. (1984) A null model for null models in biogeography. Ecological communities: conceptual issues and the evidence (ed. by D.R. Strong, Jr, D. Simberloff, L.G. Abele and A.B. Thistle), pp. 344–359. Princeton University Press, Princeton. Connell, J.H. (1980) Diversity and the coevolution of competitors, or the ghost of competition past. Oikos, 35, 131–138. Diamond, J. (1986) Evolution of ecological segregation in the New Guinea montane avifauna. Community ecology (ed. by J. Diamond and T. J. Case), pp. 98–125. Harper & Row, New York. Fox, B.J. (1987) Species assembly and the evolution of community structure. Evol. Ecol. 1, 201–213. Fox, B.J. (1989) Small-mammal community pattern in Australian heathland: a taxonomically based rule for species assembly. Patterns in the structure of mammalian communities (ed. by D.W. Morris, Z. Abramsky, B.J. Fox and M.R. Willig), pp. 91–103. Special Publication 28, The Museum, Texas Tech University, Lubbock. Blackwell Science Ltd 1999, Journal of Biogeography, 26, 825–841 Fox, B.J. & Brown, J.H. (1993) Assembly rules for functional groups in North American desert rodent communities. Oikos, 67, 358–370. Fox, B.J. & Gullick, G. (1989) Interspecific competition between mice: a reciprocal field manipulation experiment. Aust. J. Ecol. 14, 357–366. Freeman, P.W. & Lemen, C. (1983) Quantification of competition among coexisting heteromyids in the southwest. Southwest. Nat. 28, 41–46. Frye, R.J. (1983) Experimental field evidence of interspecific aggression between two species of kangaroo rat (Dipodomys). Oecologia, 59, 74–78. Genoways, H.H. & Brown, J.H. (eds) (1993) Biology of the Heteromyidae. Special Publication, American Society of Mammalogists 10, 1–719. Gilpin, M.E. & Diamond, J.M. (1982) Factors contributing to nonrandomness in species co-occurrences on islands. Oecologia, 52, 75–84. Gotelli, N.J. & Graves, G.R. (1995) Null models in ecology. Smithsonian Institution Press, Washington, D.C. Hagmeier, E.M. (1966) A numerical analysis of the distributional patterns of North American mammals. II. Re-evaluation of the provinces. Syst Zool. 15, 279–299. Harvey, P. H., Colwell, R. K., Silverton, J. W. & May, R. M. (1983) Null models in ecology. Annu. Rev. Ecol. Syst. 14, 189–211. Heptner, V.G. 1945) Desert-steppe fauna of Palearctic and centers of its formation. Bull. Moscow Soc. Naturalists, Biol. Section, 50, 17–38. Heske, E.J., Brown, J.H. & Mistry, S. (1994) Long-term experimental study of a Chihuahuan desert rodent community: 13 years of competition. Ecology, 75, 438–445. Jones, T. (1993) Social systems of heteromyid rodents. Biology of the Heteromyidae (ed. by H. H. Genoways and J. H. Brown), pp. 575–595. Special Publication, American Society of Mammalogists 10. Kelt, D.A. (1997) [Review of] The nestedness temperature calculator: a visual BASIC program, including 294 presence-absence matrices (by Wirt Atmar and Bruce D. Patterson), 1995. Bull. Ecol. Soc. Am. 78, 63–65. Kelt, D.A., Brown, J.H., Heske, E.J., Marquet, P.A., Morton, S.R., Reid, J.R.W., Rogovin, K.A. & Shenbrot, G. (1996) Community structure of desert small mammals: comparisons across four continents. Ecology, 77, 746–761. Kelt, D.A. & Brown, J.H. in press (a) Community structure and assembly rules: confronting conceptual and statistical issues with data on desert rodents. Ecological assembly rules – perspectives, advances, retreats (ed. by E. Weiher and P.A. Keddy). Cambridge University Press, Cambridge. Kelt, D.A. & Brown, J.H. in press (b) Diversification of body sizes: patterns and processes in the assembly of terrestrial mammal faunas. Biodiversity dynamics: origination and extinction of populations, species, communities, and higher taxa (ed. by M. L. McKinney and J. A. Drake). Columbia University Press. Kelt, D.A., Taper, M.L. & Meserve, P.L. (1995) Assessing the impact of competition on the assembly of communities: a case study using small mammals. Ecology, 76, 1283–1296. Kerley, G.I.H. (1989) Diet of small mammals from the Karoo, South Africa. S. Afr. J. Wildlife Res. 19, 67–72. Kerley, G.I.H. (1992) Trophic status of small mammals in the semiarid Karoo, South Africa. J. Zool. (Lond.) 226, 563–572. Kotler, B.D. & Holt, R.D. (1989) Predation and competition: the interaction of two types of species interactions. Oikos, 54, 256–260. Lawton, J.H. (1984) Non-competitive populations, non-convergent communities, and vacant niches: the herbivores of bracken. Ecological communities: conceptual issues and the evidence (ed. by D.R. 840 Douglas A. Kelt et al. Strong, Jr, D. Simberloff, L.G. Abele and A.B. Thistle), pp. 67–100. Princeton University Press, Princeton, NJ. Leibold, M.A. (1996) A graphical model of keystone predators in food webs: trophic regulation of abundance, incidence, and diversity patterns in communities. Am. Nat. 147, 784–812. Mares, M.A. (1983) Desert rodent adaptation and community structure. Biology of desert rodents (ed. by O. J. Reichman and J. H. Brown), pp. 30–43. Great Basin Naturalist Memoirs 7. Mares, M.A. (1993a) Heteromyids and their ecological counterparts: a pandesertic view of rodent ecology and evolution. Biology of the Heteromyidae (ed. by H.H. Genoways and J.H. Brown), pp. 652–719. Special Publication, American Society of Mammalogists 10. Mares, M.A. (1993b) Desert rodents, seed consumption, and convergence. Bioscience, 43, 373–379. Morton, S.R. (1985) Granivory in arid regions: comparison of Australia with North and South America. Ecology, 66, 1859–1866. Morton, S.R. (1993) Determinants of diversity in animal communities of arid Australia. Species diversity in ecological communities: historical and geographical perspectives (ed. by R.E. Ricklefs and D. Schluter), pp. 159–169. University of Chicago Press, Chicago. Morton, S.R., Brown, J.H., Kelt, D.A. & Reid, J.R.W. (1994) Comparisons of community structure among small mammals of North American and Australian deserts. Aust. J. Zool. 42, 501–525. Munger, J.C. & Brown, J.H. (1981) Competition in desert rodents: an experiment with semipermeable exclosures. Science, 211, 510–512. Patterson, B.D. & Atmar, W. (1986) Nested subsets and the structure of insular mammalian faunas and archipelagos. Island biogeography of mammals (ed. by L.R. Heaney and B.D. Patterson), pp. 65–82. Academic Press, London. Patterson, B.D. & Brown, J.H. (1991) Regionally nested patterns of species composition in granivorous rodent assemblages. J. Biogeogr. 18, 395–402. Pavlinov, I.A., Dubrovsky Yu, A., Rossolimo, O. L. & Potapova, E.G. (1991) Gerbils of the World fauna. Nauka Press, Moscow. Pavlinov, I., Ya, E.L., Yakhontov & Agadjanian, A.K. (1995) Mammals of Eurasia, Vol. 1. Rodentia. Moscow University Publishers, Moscow. Price, M.V. (1978) The role of microhabitat in structuring desert rodent communities. Ecology, 59, 910–921. Price, M.V. (1986) Structure of desert rodent communities: a critical review of questions and approaches. Am. Zool. 26, 39–49. Reichman, O.J. (1983) Behavior or desert heteromyids. Biology of desert rodents (ed. by O. J. Reichman and J. H. Brown), pp. 77–90. Great Basin Naturalist Memoirs 7. Reichman, O.J. & Price, M.V. (1993) Ecological aspects of heteromyid foraging. Biology of the Heteromyidae (ed. by H. H. Genoways and J.H. Brown), pp. 539–574. Special Publication, American Society of Mammalogists, 10. Ricklefs, R.E. & Schluter D. (eds) (1993) Species diversity in ecological communities: historical and geographical perspectives. University of Chicago Press, Chicago. Riddle, B.R. (1995) Molecular biogeography in the pocket mice (Perognathus and Chaetodipus), and grasshopper mice (Onychomys): the Late Cenozoic development of a North American aridlands rodent community. J. Mammal. 76, 283–301. Rogovin, K.A. & Shenbrot, G.I. (1993) Structural aspects of community organization of terrestrial vertebrates: an example of Mongolian desert rodents. Uspechi Sovremennoy Biologii (Adv. Current Biol) 113, 198–222 (in Russian). Rogovin, K.A. & Shenbrot, G.I. 1995) Geographical ecology of Mongolian desert rodent communities. J. Biogeogr. 22, 111–128. Rogovin, K.A., Shenbrot, G.I. & Surov, A.V. (1991) Analysis of spatial organization of a desert rodent community in Bolson de Mapimi, Mexico. J. Mammal. 72, 347–359. Rogovin, K.A., Shenbrot, G.I., Surov, A.V. & Idris, M. (1994) Spatial organization of a rodent community in the Western Rajasthan desert (India). Mammalia, 58, 234–260. Rogovin, K.A. & Surov, A.V. (1990) Morpho-ecological structure of desert rodent communities in Central Asia and southwestern North America: a multivariate approach. Acta Theriologica, 35, 225–239. Rosenzweig, M.L. (1973) Habitat selection experiments with a pair of coexisting heteromyid rodent species. Ecology, 54, 111–117. Rosenzweig, M.L. & Abramsky, Z. (1997) Two gerbils of the Negev: a long-term investigation of optimal habitat selection and its consequences. Evol. Ecol. 11, 733–756. Rosenzweig, M.L., Abramsky, Z. & Subach, A. (1997) Safety in numbers: sophisticated vigilance by Allenby’s gerbil. Proc. Natl. Acad. Sci. (USA), 94, 5713–5715. Rosenzweig, M.L., Smigel, B. & Kraft, A. (1975) Patterns of food, space and diversity. Rodents in desert environments (ed. by I. Prakash and P.K. Ghosh), pp. 241–268. Dr W. Junk, The Hague. Rosenzweig, M.L. & Sterner, P.W. (1970) Population ecology of desert rodent communities: body size and seed husking as bases for heteromyid coexistence. Ecology, 51, 217–224. Rosenzweig, M.L. & Winakur, J. (1969) Population ecology of desert rodent communities: habitats and environmental complexity. Ecology, 50, 558–572. Roughgarden, J. (1979) Theory of population genetics and evolutionary ecology: an introduction. MacMillan Publishing Co, Inc., New York. Schmidly, D.J., Wilkins, K.T. & Derr, J.N. (1993) Biogeography. Biology of the Heteromyidae (ed. by H.H. Genoways and J.H. Brown), pp. 319–356. Special Publication, American Society of Mammalogists, 10. Schroder, G.D. (1987) Mechanisms for coexistence among three species of Dipodomys: habitat selection and an alternative. Ecology, 68, 1071–1083. Schroder, G.D. & Rosenzweig, M.L. (1975) Perturbation analysis of competition and overlap in habitat utilization between Dipodomys ordii and Dipodomys merriami. Oecologia, 19, 9–28. Shenbrot, G.I. (1992) Spatial structure and niche patterns of a rodent community in the south Bukhara desert (Middle Asia). Ecography, 15, 347–357. Shenbrot, G.I. & Rogovin, K.A. 1995) Temporal variation in spatial organization of a rodent community in the Southwestern Kyzylkum Desert (Middle Asia). Ecography, 18, 370–383. Shenbrot, G.I., Rogovin, K. A. & Heske, E.J. (1994) Comparison of niche-packing and community organization in Asia and North America. Aust. J. Zool. 42, 479–499. Shenbrot, G.I., Sokolov, V.E., Heptner, V.G. & Kowalskaya Yu, M. (1995) Mammals of Russia and adjacent regions. Dipodid rodents. Nauka Press (in Russian). Sinitzin, V.M. (1962) Palaeogeography of Asia. USSR Academy of Sciences Press, Moscow, (in Russian). Strong, D.R. Jr (1984) Exorcising the ghost of competition past: phytophagous insects. Ecological communities: conceptual issues and the evidence (ed. by D.R. Strong, Jr, D. Simberloff, L.G. Abele and A.B. Thistle), pp. 28–41. Princeton University Press, Princeton. Strong, D.R., Szyska, L.A. & Simberloff, D. (1979) Tests of communitywide character displacement against null hypotheses. Evolution, 33, 897–913. Thompson, R.S. & Mead, J.I. (1982) Late Quaternary environments and biogeography in the Great Basin. Quat. Res. 17, 39–55. Valone, T.J. & Brown, J.H. (1996) Desert rodents: long-term responses to natural changes and artificial perturbations. Long-term studies of vertebrate communities (ed. by M.L. Cody and J.A. Smallwood), pp. 555–583. Academic Press, Orlando. Van Devender, T.R. (1986) Climatic cadences and the composition of Blackwell Science Ltd 1999, Journal of Biogeography, 26, 825–841 North American and Asian desert rodents 841 Chihuahuan Desert communities: the late Pleistocene packrat midden record. Community ecology (ed. by J. Diamond and T.J. Case), pp. 285–299. Harper & Row, New York. Van Devender, T.R. & Spaulding, W.G. (1979) Development of vegetation and climate in the southwestern United States. Science, 204, 701–710. Walter, H. & Box, E.O. (1983) Overview of Eurasian continental deserts and semi-deserts. Temperate deserts and semideserts (ed. by N.E. West), pp. 3–7. Elsevier Scientific, New York. Webb, S.D. (1977) A history of savanna vertebrates in the New World. Part I: North America. Annu. Rev. Ecol. Syst. 8, 355–380. Wells, P.V. (1979) An aquable glaciopluvial in the west: pleniglacial evidence of increased precipitation on a gradient from Great Basin to the Sonoran and Chihuahuan Deserts. Quat. Res. 12, 311–325. Wilson, D.E. & Reeder D. M. (eds) (1993) Mammal species of the world: a taxonomic and geographic reference, 2nd edn. Smithsonian Institution Press, in association with The American Society of Mammalogists, Washington, D.C. BIOSKETCHES Douglas A. Kelt (Ph.D., University of New Mexico), is an Assistant Professor at the University of California, Davis. His research interests emphasize macroecology, biogeography, and community ecology, primarily of desert and Neotropical small mammals. Konstantin A. Rogovin (Ph.D., Moscow State University), is Principal Investigator at the Severtzov Institute of Ecology and Evolution, Russian Academy of Science. He has studied the ecology and behaviour of rodents in Central Asia deserts for many years, and is currently studying terrestrial vertebrates in Kalmykia (Russian Federation). Georgy Shenbrot (Ph.D., Moscow State University) is a Researcher at the Ramon Science Center, Ben-Gurion University of the Negev. He has over 25 years of experience in desert rodent community ecology and taxonomy, and is currently writing a book on ‘Spatial ecology of desert rodent communities’. James H. Brown (Ph.D., University of Michigan) is a Regents’ Professor of Biology at the University of New Mexico, and past President of the American Society of Mammalogists, the American Society of Naturalists, and the Ecological Society of America. Current research projects include long-term experimental studies in the Chihuahuan Desert, investigations into macroecological patterns of biotic structure, the influence of body size and biological scaling on biodiversity, and applied efforts to preserve ranching livelihoods and biodiversity in the American South-west. Blackwell Science Ltd 1999, Journal of Biogeography, 26, 825–841