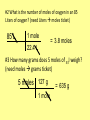* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The Moletro
Survey
Document related concepts
Transcript
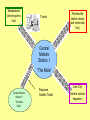
The Moletro Chemistry’s Metro Massadonia (where grams live) T-chart Particleville (where atoms and molecules live) Central Moletro Station 1 “The Mole” Central Moletro Station 2 “The Mole Ratio” Requires Golden Ticket Liter City (where volume happens) Let’s Go On a Trip! • Your Moletro tickets indicate your current location and where you want to go • For example – ticket #1 is used to go from Moles (central station) to atoms/molecule/particle (particleville) • Also on your ticket is the conversion factor (the relationship between one unit and another) Memorize the Conversion Factors!!!!! • 1 mole = 6.02 X1023 particles • 1 mole = molar mass in grams • 1 mole = 22.4 L (volume) • These are used in the 2nd column or your T-chart All Aboard the Moletro to??? Trip #1 - What is the volume of 3 moles of CH4? (need moles liters ticket) 3 moles CH4 22.4 Liters 1 mole = 67.2 Liters CH4 #2 What is the number of moles of oxygen in an 85 Liters of oxygen? (need Liters moles ticket) 85 L 1 mole = 3.8 moles 22.4 L #3 How many grams does 5 moles of 53I weigh? (need moles grams ticket) 5 moles 127 g 1 mole = 635 g #4 How many molecules are in 1.5 moles of glucose? (need moles molecules ticket) 1.5 moles 6.02 x1023 molecules 1 mole = 9.03 x 1023 molecules #5 How many moles is 88 g of CO2? (need g moles ticket) (molar mass of CO2 = 12 + 2 (16) = 44g) 88 g 1 mole 44 g = 2 moles #6 How many moles is 176 g of CO2? (need g moles ticket) – First I need to calculate grams of CO2. 12 + 2( 16) = 44 g 176 g 1 mole 44 g = 4 moles One Mole can equal 3 different things 1. 6.02 X 1023 atoms or molecules 2. The mass of any element or molecule in grams 3. 22.4 Liters of any gas • Since the mole can equal any one of these, it can also serve as a bridge to convert from one to another of them.