* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download lipid raft
Survey
Document related concepts
Transcript
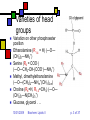
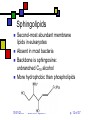
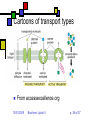
Lipids II Andy Howard Introductory Biochemistry, Fall 2009 01 October 2009 Biochem: Lipids II 10/01/2009 Plans for Today Lipids Membranes Glycerophospholipids Plasmalogens Sphingolipids Isoprenoids Steroids Other lipids 10/01/2009 Biochem: Lipids II Fatty acids Triacylglycerol Glycerophospholipids Plasmalogens Sphingolipids Isoprenoids Steroids Other lipids p. 2 of 37 Varieties of head groups Variation on other phosphoester position Ethanolamine (R1-4 = H) (—O— (CH2)2—NH3+) Serine (R4 = COO-) (—O—CH2-CH-(COO-)—NH3+) Methyl, dimethylethanolamine (—O—(CH2)2—NHm+(CH3)2-m) Choline (R4=H, R1-3=CH3) (—O— (CH2)2—N(CH3)3+) Glucose, glycerol . . . 10/01/2009 Biochem: Lipids II p. 3 of 37 Phospholipids aren’t interchangeable! Phosphatidylcholine and phosphatidylethanolamine are the major components of eukaryotic membranes Phosphatidylserine and P-inositol tend to be on the inner leaflet only, and are more prevalent in brain tissue than other tissues Good reference: http://www.lipidlibrary.co.uk/ 10/01/2009 Biochem: Lipids II p. 4 of 37 Chirality in common lipids Fatty acyl chains themselves are generally achiral Glycerol C2 is often chiral (unless C1 and C3 fatty acyl chains are identical) Phospholipid polar groups are achiral except for phosphatidylserine and a few others 10/01/2009 Biochem: Lipids II p. 5 of 37 iClicker quiz question 3 What is the most common fatty acid in soybean triglycerides? (a) Hexadecanoate (b) Octadecanoate (c) cis,cis-9,12-octadecadienoate (d) all cis-5,8,11,14-eicosatetraeneoate (e) None of the above 10/01/2009 Biochem: Lipids II p. 6 of 37 iClicker quiz, question 4 Which set of fatty acids would you expect to melt on your breakfast table? (a) fatty acids derived from soybeans (b) fatty acids derived from olives (c) fatty acids derived from beef fat (d) fatty acids derived from bacteria (e) either (c) or (d) 10/01/2009 Biochem: Lipids II p. 7 of 37 iClicker quiz question 5 Suppose we constructed an artificial lipid bilayer of dipalmitoyl phosphatidylcholine (DPPC) and another artificial lipid bilayer of dioleyl phosphatidylcholine (DOPC). Which bilayer would be thicker? (a) the DPPC bilayer (b) the DOPC bilayer (c) neither; they would have the same thickness (d) DOPC and DPPC will not produce stable bilayers 10/01/2009 Biochem: Lipids II p. 8 of 37 Plasmalogens Ether phospholipids have an ether link to C1 instead of an ester linking Plasmalogens are ether phospholipids with C1 linked via cis-vinyl ether linkage. They constitute the other major category of phospholipids besides esterified glycerophospholipids Ordinary fatty acyl esterification at C2… platelet activating factor has R2 = CH3 Usually PE or PC at C3 position 10/01/2009 Biochem: Lipids II p. 9 of 37 Specific plasmalogens 10/01/2009 Biochem: Lipids II p. 10 of 37 Roles of phospholipids Most important is in membranes that surround and actively isolate cells and organelles Other phospholipids are secreted and are found as extracellular surfactants (detergents) in places where they’re needed, e.g. the surface of the lung 10/01/2009 Biochem: Lipids II p. 11 of 37 Sphingolipids Second-most abundant membrane lipids in eukaryotes Absent in most bacteria Backbone is sphingosine: unbranched C18 alcohol More hydrophobic than phospholipids 10/01/2009 Biochem: Lipids II p. 12 of 37 Varieties of sphingolipids Ceramides Sphingomyelin Image on steve.gb.com sphingosine at glycerol C3 Fatty acid linked via amide at glycerol C2 QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Sphingomyelins C2 and C3 as in ceramides C1 has phosphocholine 10/01/2009 Biochem: Lipids II p. 13 of 37 Cerebrosides Ceramides with one saccharide unit attached by glycosidic linkage at C1 of glycerol Galactocerebrosides common in nervous tissue 10/01/2009 Biochem: Lipids II p. 14 of 37 Gangliosides Anionic derivs of cerebrosides (NeuNAc) Provide surface markers for cell recognition and cell-cell communication 10/01/2009 Biochem: Lipids II p. 15 of 37 Isoprenoids Huge percentage of non-fatty-acid-based lipids are built up from isoprene units Biosynthesis in 5 or 15 carbon building blocks reflects this Steroids, vitamins, terpenes Involved in membrane function, signaling, feedback mechanisms, structural roles 10/01/2009 Biochem: Lipids II p. 16 of 37 Isoprene units: how they’re employed in real molecules Can be linked head-to-tail … or tail-to-tail (fig. 8.16, G&G) 10/01/2009 Biochem: Lipids II p. 17 of 37 Steroids Molecules built up from ~30-carbon four-ring isoprenoid starting structure Generally highly hydrophobic (1-3 polar groups in a large hydrocarbon); but can be derivatized into emulsifying forms Cholesterol is basis for many of the others, both conceptually and synthetically Cholesterol: Yes, you need to memorize this structure! 10/01/2009 Biochem: Lipids II p. 18 of 37 Other lipids Waxes nonpolar esters of long-chain fatty acids and long-chain monohydroxylic alcohols, e.g H3C(CH2)nCOO(CH2)mCH3 Waterproof, high-melting-point lipids Eicosanoids QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. oxygenated derivatives of C20 polyunsaturated fatty acids Involved in signaling, response to stressors Non-membrane isoprenoids: vitamins, hormones, terpenes 10/01/2009 Biochem: Lipids II Image courtesy cyberlipid. org QuickTime™ TIFF (Uncompressed) d Image are needed to see th Courtesy Oregon State Hort. & Crop Sci. p. 19 of 37 Example of a wax Oleoyl alcohol esterified to stearate (G&G, fig. 8.15) 10/01/2009 Biochem: Lipids II p. 20 of 37 Membranes Fundamental biological mechanism for separating cells and organelles from one another Highly selective barriers Based on phospholipid or sphingolipid bilayers Contain many protein molecules too (50-75% by mass) Often contain substantial cholesterol too: cf. modeling studies by H.L. Scott 10/01/2009 Biochem: Lipids II p. 21 of 37 Solvent Bilayers Self-assembling roughly planar structures Bilayer lipids are fully extended Aqueous above and below, apolar within Solvent 10/01/2009 Biochem: Lipids II p. 22 of 37 Fluid Mosaic Model Membrane is dynamic Protein and lipids diffuse laterally; proteins generally slower than lipids Some components don’t move as much as the others Flip-flops much slower than lateral diffusion Membranes are asymmetric Salmonella ABC transporter MsbA PDB 3B60 3.7Å 2*64 kDa QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Newly synthesized components added to inner leaflet Slow transitions to upper leaflet (helped by flippases) 10/01/2009 Biochem: Lipids II p. 23 of 37 Fluid Mosaic Model depicted Courtesy C.Weaver, Menlo School 10/01/2009 Biochem: Lipids II p. 24 of 37 Physical properties of membranes Strongly influenced by % saturated fatty acids: lower saturation means more fluidity at low temperatures Cholesterol percentage matters too: disrupts ordered packing and increases fluidity (mostly) 10/01/2009 Biochem: Lipids II p. 25 of 37 Chemical compositions of membranes (fig. 9.10, G&G) 10/01/2009 Biochem: Lipids II p. 26 of 37 Lipid Rafts Cholesterol tends to associate with sphingolipids because of their long saturated chains Typical membrane has blob-like regions rich in cholesterol & sphingolipids surrounded by regions that are primarily phospholipids The mobility of the cholesterol-rich regions leads to the term lipid raft 10/01/2009 Biochem: Lipids II p. 27 of 37 Significance of lipid rafts: still under discussion May play a role as regulators Sphingolipid-cholesterol clusters form in the ER or Golgi and eventually move to the outer leaflet of the plasma membrane There they can govern protein-protein & protein-lipid interactions Necessary but insufficient for trafficking May be involved in anaesthetic functions: Morrow & Parton (2005), Traffic 6: 725 10/01/2009 Biochem: Lipids II p. 28 of 37 Membrane Proteins Many proteins associate with membranes But they do it in several ways Integral membrane proteins: considerable portion of protein is embedded in membrane Peripheral membrane proteins: polar attachments to integral membrane proteins or polar groups of lipids Lipid-anchored proteins: protein is covalently attached via a lipid anchor 10/01/2009 Biochem: Lipids II p. 29 of 37 Integral (Transmembrane) Proteins Drawings courtesy U.Texas Span bilayer completely May have 1 membrane-spanning segment or several Often isolated with detergents 7-transmembrane helical proteins are very typical (e.g. bacteriorhodopsin) Beta-barrels with pore down the center: porins 10/01/2009 Biochem: Lipids II p. 30 of 37 Peripheral Membrane proteins Also called extrinsic proteins Associate with 1 face of membrane Associated via H-bonds, salt bridges to polar components of bilayer Easier to disrupt membrane interaction: salt treatment or pH 10/01/2009 Biochem: Lipids II QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. Chloroflexus auracyanin PDB 1QHQ 1.55Å 15.4 kDa p. 31 of 37 Lipid-anchored membrane proteins Protein-lipid covalent bond Often involves amide or ester bond to phospholipid Others: cys—S—isoprenoid (prenyl) chain Glycosyl phosphatidylinositol with glycans 10/01/2009 Biochem: Lipids II p. 32 of 37 N- Myristoylation & S-palmitoylation 10/01/2009 Biochem: Lipids II p. 33 of 37 Membrane Transport What goes through and what doesn’t? Nonpolar gases (CO2, O2) diffuse Hydrophobic molecules and small uncharged molecules mostly pass freely Charged molecules blocked 10/01/2009 Biochem: Lipids II p. 34 of 37 Transmembrane Traffic: Types of Transport (Table 9.3) Type Protein Saturable Carrier w/substr. Diffusion No No ChannelsYes No & pores Passive Yes Yes transport Active Yes Yes 10/01/2009 Biochem: Lipids II Movement Rel.to conc. Down Down Energy Input? No No Down No Up Yes p. 35 of 37 Cartoons of transport types From accessexcellence.org 10/01/2009 Biochem: Lipids II p. 36 of 37 Thermodynamics of passive and active transport • If you think of the transport as a chemical reaction Ain Aout or Aout Ain • It makes sense that the free energy equation would look like this: • Gtransport = RTln([Ain]/[Aout]) • More complex with charges; see eqns. 9.4 through 9.6. 10/01/2009 Biochem: Lipids II p. 37 of 37 Example Suppose [Aout] = 145 mM, [Ain] = 10 mM, T = body temp = 310K Gtransport = RT ln[Ain]/[Aout] = 8.325 J mol-1K-1 * 310 K * ln(10/145) = -6.9 kJ mol-1 So the energies involved are moderate compared to ATP hydrolysis 10/01/2009 Biochem: Lipids II p. 38 of 37