* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download From the first law of thermodynamics
Survey
Document related concepts
Negawatt power wikipedia , lookup
Low-carbon economy wikipedia , lookup
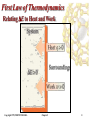
Energy efficiency in transport wikipedia , lookup
Alternative energy wikipedia , lookup
Energy policy of the European Union wikipedia , lookup
Internal energy wikipedia , lookup
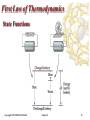
Conservation of energy wikipedia , lookup
Energy Independence and Security Act of 2007 wikipedia , lookup
Micro combined heat and power wikipedia , lookup
Gibbs free energy wikipedia , lookup
Transcript
Thermochemistry David P. White University of North Carolina, Wilmington Chapter 5 Copyright 1999, PRENTICE HALL Chapter 5 1 The Nature of Energy Kinetic and Potential Energy From Physics: • Force is a push or pull on an object. • Work is the product of force applied to an object over a distance: w=Fd • Energy is the work done to move an object against a force. • Kinetic energy is the energy of motion: 2 1 E k mv 2 Copyright 1999, PRENTICE HALL Chapter 5 2 The Nature of Energy Kinetic and Potential Energy • Potential energy is the energy an object possesses by virtue of its position. • Potential energy can be converted into kinetic energy. Example: a ball of clay dropping off a building. Copyright 1999, PRENTICE HALL Chapter 5 3 The Nature of Energy Energy Units SI Unit for energy is the joule, J: E k 1 mv 2 1 2 kg 1 m/s 2 2 2 1 kg m 2 / s 2 1J We sometimes use the calorie instead of the joule: 1 cal = 4.184 J (exactly) A nutritional Calorie: 1 Cal = 1000 cal = 1 kcal Copyright 1999, PRENTICE HALL Chapter 5 4 The Nature of Energy Systems and Surroundings System: part of the universe we are interested in. Surroundings: the rest of the universe. Copyright 1999, PRENTICE HALL Chapter 5 5 First Law of Thermodynamics Internal Energy • Internal Energy: total energy of a system. • Cannot measure absolute internal energy. • Change in internal energy, DE = Efinal Einitial Copyright 1999, PRENTICE HALL Chapter 5 6 First Law of Thermodynamics Relating DE to Heat and Work Energy cannot be created or destroyed. Energy of (system + surroundings) is constant. Any energy transferred from a system must be transferred to the surroundings (and vice versa). From the first law of thermodynamics: when a system undergoes a physical or chemical change, the change in internal energy is given by the heat added to or absorbed by the system plus the work done on or by the system: DE = q + w Copyright 1999, PRENTICE HALL Chapter 5 7 First Law of Thermodynamics Relating DE to Heat and Work Copyright 1999, PRENTICE HALL Chapter 5 8 First Law of Thermodynamics Relating DE to Heat and Work Copyright 1999, PRENTICE HALL Chapter 5 9 First Law of Thermodynamics Endothermic and Exothermic Processes Endothermic: absorbs heat from the surroundings. Exothermic: transfers heat to the surroundings. An endothermic reaction feels cold. An exothermic reaction feels hot. Copyright 1999, PRENTICE HALL Chapter 5 10 First Law of Thermodynamics State Functions State function: depends only on the initial and final states of system, not on how the internal energy is used. Copyright 1999, PRENTICE HALL Chapter 5 11 First Law of Thermodynamics State Functions Copyright 1999, PRENTICE HALL Chapter 5 12 Enthalpy Enthalpy, H: Heat transferred between the system and surroundings carried out under constant pressure. Can only measure the change in enthalpy: DH = Hfinal - Hinitial = qP Copyright 1999, PRENTICE HALL Chapter 5 13 Enthalpies of Reaction For a reaction DHrxn = H(products) - H (reactants) Enthalpy is an extensive property (magnitude DH is directly proportional to amount): CH4(g) + 2O2(g) CO2(g) + 2H2O(g) DH = -802 kJ 2CH4(g) + 4O2(g) 2CO2(g) + 4H2O(g) DH = -1604 kJ When we reverse a reaction, we change the sign of DH: CO2(g) + 2H2O(g) CH4(g) + 2O2(g) DH = +802 kJ Change in enthalpy depends on state: H2O(g) H2O(l) DH = -88 kJ Copyright 1999, PRENTICE HALL Chapter 5 14 Calorimetry Heat Capacity and Specific Heat Calorimetry = measurement of heat flow. Calorimeter = apparatus that measures heat flow. Heat capacity = the amount of energy required to raise the temperature of an object (by one degree). Molar heat capacity = heat capacity of 1 mol of a substance. Specific heat = specific heat capacity = heat capacity of 1 g of a substance. q = (specific heat) (grams of substance) T. Be careful of the sign of q. Copyright 1999, PRENTICE HALL Chapter 5 15 Calorimetry Heat Capacity and Specific Heat Copyright 1999, PRENTICE HALL Chapter 5 16 Calorimetry Constant-Pressure Calorimetry Atmospheric pressure is constant! DH = qP qrxn = -qsoln = -(specific heat of solution) (grams of solution) DT. Copyright 1999, PRENTICE HALL Chapter 5 17 Calorimetry Bomb Calorimetry (Constant-Volume Calorimetry) Reaction carried out under constant volume. Use a bomb calorimeter. Usually study combustion. Copyright 1999, PRENTICE HALL Chapter 5 18 Calorimetry Bomb Calorimetry (Constant-Volume Calorimetry) qrxn = -CcalorimeterT. Copyright 1999, PRENTICE HALL Chapter 5 19 Hess’s Law • Hess’s law: if a reaction is carried out in a number of steps, DH for the overall reaction is the sum of DH for each individual step. • For example: CH4(g) + 2O2(g) CO2(g) + 2H2O(g) DH = -802 kJ 2H2O(g) 2H2O(l) DH = -88 kJ CH4(g) + 2O2(g) CO2(g) + 2H2O(l) DH = -890 kJ Copyright 1999, PRENTICE HALL Chapter 5 20 Hess’s Law In the above enthalpy diagram note that DH1 = DH2 + DH3 Copyright 1999, PRENTICE HALL Chapter 5 21 Enthalpies of Formation • If 1 mol of compound is formed from its constituent elements, then the enthalpy change for the reaction is called the enthalpy of formation, DHof . • Standard conditions (standard state): 1 atm and 25 oC (298 K). • Standard enthalpy, DHo, is the enthalpy measured when everything is in its standard state. • Standard enthalpy of formation: 1 mol of compound is formed from substances in their standard states. • If there is more than one state for a substance under standard conditions, the more stable one is used. Copyright 1999, PRENTICE HALL Chapter 5 22 Enthalpies of Formation • Standard enthalpy of formation of the most stable form of an element is zero. Copyright 1999, PRENTICE HALL Chapter 5 23 Enthalpies of Formation Using Enthalpies of Formation to Calculate Enthalpies of Reaction We use Hess’ Law to calculate enthalpies of a reaction from enthalpies of formation. DHrxn = DH1 + DH2 + DH3 Copyright 1999, PRENTICE HALL Chapter 5 24 Enthalpies of Formation Using Enthalpies of Formation to Calculate Enthalpies of Reaction For a reaction: DH rxn nDH f products mDH f reactants Copyright 1999, PRENTICE HALL Chapter 5 25 Foods and Fuels Foods •Fuel value = energy released when 1 g of substance is burned. •1 nutritional Calorie, 1 Cal = 1000 cal = 1 kcal. •Energy in our bodies comes from carbohydrates and fats (mostly). •Intestines: carbohydrates converted into glucose: C6H12O6 + 6O2 6CO2 + 6H2O, DH = -2816 kJ •Fats break down as follows: 2C57H110O6 + 163O2 114CO2 + 110H2O, DH = -75,520 kJ •Fats: contain more energy; are not water soluble, so are good for energy storage. Copyright 1999, PRENTICE HALL Chapter 5 26 Foods and Fuels Foods Copyright 1999, PRENTICE HALL Chapter 5 27 Foods and Fuels Fuels U.S.: 1.0 x 106 kJ of fuel per day. Most from petroleum and natural gas. Remainder from coal, nuclear, and hydroelectric. Fossil fuels are not renewable. Copyright 1999, PRENTICE HALL Chapter 5 28 Foods and Fuels Fuels Fuel value = energy released when 1 g of substance is burned. Hydrogen has great potential as a fuel with a fuel value of 142 kJ/g. Copyright 1999, PRENTICE HALL Chapter 5 29 Thermochemistry End of Chapter 5 Copyright 1999, PRENTICE HALL Chapter 5 30