* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Specific Heat
Vapor-compression refrigeration wikipedia , lookup
Thermal conductivity wikipedia , lookup
Hypothermia wikipedia , lookup
Space Shuttle thermal protection system wikipedia , lookup
Insulated glazing wikipedia , lookup
Dynamic insulation wikipedia , lookup
Building insulation materials wikipedia , lookup
Solar water heating wikipedia , lookup
Solar air conditioning wikipedia , lookup
Thermoregulation wikipedia , lookup
Heat exchanger wikipedia , lookup
Intercooler wikipedia , lookup
Heat equation wikipedia , lookup
R-value (insulation) wikipedia , lookup
Cogeneration wikipedia , lookup
Copper in heat exchangers wikipedia , lookup
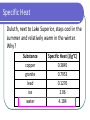
Unit 5: Thermochemistry Specific Heat Specific Heat specific heat: willingness of an object to change temperature, with the symbol Cp (the p means "under constant pressure") specific heat: the amount of energy required to change the temperature of one gram of a substance by 1°C Specific Heat Units • • • 1 calorie = energy required to heat 1 gram of water by 1°C 1 Calorie (food labels) = 1 kilocalorie 1 calorie = 4.184 joules Specific Heat Duluth, next to Lake Superior, stays cool in the summer and relatively warm in the winter. Why? Substance copper granite lead ice water Specific Heat (J/g°C) 0.3845 0.7953 0.1276 2.06 4.184 Calculating Heat (Energy Transfer) q = Cp x m x T specific heat (units: J/g°C) change in temperature (units: °C) mass (units: g) Calculating Heat Example #1 q = Cp x m x T 3.05 kg of aluminum is heated from 22.1C to 67.5C. Calculate the heat absorbed in both J and kJ by the metal. The specific heat of aluminum is 0.900 J/gC. Calculating Heat Example #2 A 200-g block of copper at temperature of 90°C is dropped into 400 g of water at 27°C. What is the final temperature of the mixture? The specific heat of copper is 0.386 J/gC.