* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download File - Prairie Science
Survey
Document related concepts
Transcript
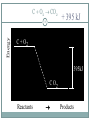
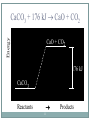
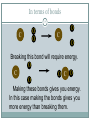
Chapter 9 - Thermochemistry Heat and Chemical Change PRAIRIE SCHOOL C HE M I S T RY SPRING 2015 The Flow of Energy - Heat OBJECTIVES: Explain the relationship between energy and heat. The Flow of Energy - Heat OBJECTIVES: Distinguish between heat capacity and specific heat. Energy and Heat Thermochemistry - concerned with heat changes that occur during chemical reactions Energy - capacity for doing work or supplying heat Energy and Heat 5 Heat - represented by “q”, is energy that transfers from one object to another, because of a temperature difference between them. How does heat travel? Exothermic and Endothermic Processes In studying heat changes, think of defining these two parts: the system the surroundings Exothermic and Endothermic Processes The Law of Conservation of Energy states that in any chemical or physical process, energy is neither created nor destroyed. All the energy is accounted for as work, stored energy, or heat. Examples? Exothermic and Endothermic Processes Review: if heat is flowing into a system from it’s surroundings: defined as positive q has a positive value (we will discuss this later) called endothermic system gains heat as the surroundings cool down Exothermic and Endothermic Processes Review: When heat is flowing out of a system into it’s surroundings: defined as negative q has a negative value called exothermic system loses heat as the surroundings heat up C + O2 CO2 Energy 10 + 395 kJ C + O2 395kJ C O2 Reactants Products Energy CaCO CaO CaCO CaO + CO+2 CO2 3 + 176 3 kJ CaO + CO2 176 kJ CaCO3 Reactants 11 Products Heat Capacity and Specific Heat 12 A calorie is defined as the quantity of heat needed to raise the temperature of 1 g of pure water 1 oC. Used except when referring to food a Calorie, written with a capital C, always refers to the energy in food 1 Calorie = 1 kilocalorie = 1000 cal. Heat Capacity and Specific Heat 13 Joule-- the SI unit of heat and energy 4.184 J = 1 cal Specific Heat Capacity - the amount of heat it takes to raise the temperature of 1 gram of the substance by 1 oC (abbreviated “C”) This is found in a chart in your books Heat Capacity and Specific Heat 14 For water, C = 4.18 J/(g oC), and also C = 1.00 cal/(g oC) Thus, for water: it takes a long time to heat up, and it takes a long time to cool off! Heat Capacity and Specific Heat 15 To calculate, use the formula: q = mass (g) x T x C heat abbreviated as “q” T = change in temperature C = Specific Heat Units are either J/(g oC) or cal/(g oC) Calorimetry Calorimetry - the accurate and precise measurement of heat change for chemical and physical processes. Calorimetry For systems at constant pressure, the heat content is the same as a property called Enthalpy (H) of the system Calorimetry Changes in enthalpy = H q = H These terms will be used interchangeably in this textbook Thus, q = H = m x C x T H is negative for an exothermic reaction H is positive for an endothermic reaction In terms of bonds 19 C O O O C O Breaking this bond will require energy. O C O C O O Making these bonds gives you energy. In this case making the bonds gives you more energy than breaking them. Chemistry Happens in 20 MOLES An equation that includes energy is called a thermochemical equation CH4 + 2O2 CO2 + 2H2O + 802.2 kJ 1 mole of CH4 releases 802.2 kJ of energy. When you make 802.2 kJ you also make 2 moles of water CH4 + 2 O2 CO2 + 2 H2O + 802.2 kJ 21 If 10. 3 grams of CH4 are burned completely, how much heat will be produced? 10. 3 g CH4 1 mol CH4 16.05 g CH4 802.2 kJ 1 mol CH4 = 514 kJ CH4 + 2 O2 CO2 + 2 H2O + 802.2 kJ 22 How many grams of O2 would be required to produce 23 kJ of heat? How many grams of water would be produced with 506 kJ of heat? Homework Read Section 9.1 and complete the section review (problems 1-8) due Wednesday This Week… Tomorrow: Activity over Thermodynamics Wednesday: Lab over Thermodynamics Thursday: Finish lab, work on chemistry demonstrations Born Haber Cycle A particular set of equations known as the Born- Haber Cycle demonstrate how chemists are able to use the first law of thermodynamics to find an unknown energy value that is impossible to get from chemical reactions in a lab. Lattice energy Election affinity Born-Haber Cycle There is more than one path to the formation of a substance to a particular state. Because of this, we can calculate unknown values using known values Each Physical or chemical change has… A chemical equation A definition about the type of energy change A ΔH (enthalpy value), expressed in kJ Directions 1. Cut out the cards for names, equations, definitions, and symbols with energy values Arrange them with their correct type of reaction and enthalpy value Glue to a piece of paper, to make a poster of each reaction. Find the unknown lattice energy for the three reactions (show your work) Some terms to help… Sublimation: solid to gas formation Ionization energy: breaking an election from an element (free electron in products) ½ bond energy: 1/2F2F Election affinity: Amount of energy needed to gain an election (electron on reactant side) Lattice energy: energy required for ions to combine together (strength of ionic bond) Standard enthalpy of formation: energy required for reaction to be complete, using the ½ bond energy. Chapter 9: Calories and Finding your energy needs The energy content of food is found from burning the dry food sample in a bomb calorimeter. Calories can be converted into kJ 1 Cal= 4.184 kJ Using this conversion, you can find the amount of J your body takes in by looking at the calories 310 Calorie burger=1297kJ of energy You get energy from carbohydrates, fats, and proteins. Also get nutrients from vitamins, water and minerals You can also look at a food label… Carbohydrates: 4 calories=17kJ/g Proteins: 4 calories=17kJ/g Fat: 9 calories=38 kJ/g Look at the food label and add the values for each of the components above to find total kJ of energy produced. Fats have more energy per gram than carbohydrates and proteins. Energy storing compounds in the body What are your energy needs? Your daily energy needs are calculated by your basal metabolic rate, your activity level, and your thermal effect of food. Basal metabolic rate: the energy needed to sustain life. Not constant: varies with age, gender, stress level, general health. When you measure this, it needs to be taken before eating any food. This is also called the resting metabolic rate