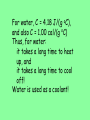* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Thermochemistry
X-ray photoelectron spectroscopy wikipedia , lookup
Marcus theory wikipedia , lookup
Thermodynamics wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Solar air conditioning wikipedia , lookup
Internal energy wikipedia , lookup
Heat transfer wikipedia , lookup
Transition state theory wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
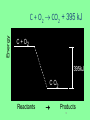
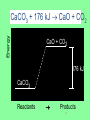
Thermochemistry • Heat • a form of energy. • can be transferred between samples • heat flows from matter at a higher temperature to matter at a lower temperature • Temperature • a measure of the average kinetic energy of the particles in a sample. Thermochemistry • Units of Heat • Joule (SI unit) • calorie • cal • the amount of energy required to raise the temperature of one gram of water one degree Celsius. • Calorie • Cal • a dietary calorie. • kilocalorie, kcal (1,000 calories) 1 cal = 4.184 Joules Thermochemistry • Enthalpy • the heat content of a system • represented by H • only changes in enthalpy can be measured • ∴ ΔH is used Thermochemistry • Specific heat capacity, cp • the amount of energy required to raise the temperature of one gram of a substance one degree Celsius • used in equation q = m x cp x ΔT Thermochemistry q = m x cp x ΔT Energy, or heat (J) mass (g) specific heat (J/g•°C) Δ temp (°C) • note that in the Metric System, Joules are the unit of measure for heat. Heat capacity, cp For water, C = 4.18 J/(g oC), and also C = 1.00 cal/(g oC) Thus, for water: it takes a long time to heat up, and it takes a long time to cool off! Water is used as a coolant! q = m x cp x ΔT 1. A 45.0-gram sample of iron is heated from 25.0°C to 50.0°C. How much energy is required? (cp iron = 0.449 J/g°C) q=? m = 45.0 g cp = 0.449 J/g°C ΔT = 50.0°C – 25.0°C = 25.0°C q = m cp ΔT q = 45.0g (0.449 J/g°C) (25.0°C) = 505.125 J q = 505 J 2. What is the specific heat capacity of an object if a 12.5-gram sample is heated from 12.0°C to 28.0°C using 100.0 joules? q = 100.0 J m = 12.5 g cp = ? ΔT = 28.0 – 12.0 = 16.0°C q = m cp ΔT q cp = mΔT 100 .0J cp = 12.5g(16.0 C) J cp = 0.500 gC Heat - represented by “q”, is energy that transfers from one object to another, because of a temperature difference between them. In studying heat changes, think of defining these two parts: the system – the part of the universe you focus your attention on the surroundings – everything else If heat flows into a system from the surroundings, the system gains energy, and the change is said to be endothermic. Heat has a positive value. If heat flows out of a system to the surroundings, the system loses heat, and the change is said to be exothermic. Heat has a negative value. Every reaction has an energy change associated with it. Exothermic reactions release energy, usually in the form of heat. Endothermic reactions absorb energy. Energy is stored in bonds between atoms. The Law of Conservation of Energy states that in any chemical or physical process, energy is neither created nor destroyed. All the energy is accounted for as work, stored energy, or heat. Calorimetry - the accurate and precise measurement of heat change for chemical and physical processes. For systems at constant pressure, the heat content is the same as a property called Enthalpy (H) of the system. Changes in enthalpy = H q = H These terms will be used interchangeably. Thus, q = H = m x C x T H is negative for an exothermic reaction. H is positive for an endothermic reaction. Energy C + O2 CO2 + 395 kJ C + O2 395kJ C O2 Reactants Products 16 THIS IS AN EXOTHERMIC REACTION. THE CHEMICAL BONDS OF THE PRODUCTS CONTAIN LESS CHEMICAL POTENTIAL ENERGY THAN THE BONDS OF THE REACTANTS. THE SYSTEM GIVES OFF ENERGY TO THE SURROUNDINGS. H IS NEGATIVE. ANOTHER WAY OF SHOWING THIS IS THE ENERGY CHANGE IS SHOWN AS A PRODUCT. Energy CaCO kJ +CaO CaCO CaO CO2+ CO2 3 + 3176 CaO + CO2 176 kJ CaCO3 Reactants Products 18 THIS IS AN ENDOTHERMIC REACTIION. THE CHEMICAL BONDS IN THE PRODUCTS HAVE MORE CHEMICAL POTENTIAL ENERGY THAN THE CHEMICAL BONDS IN THE REACTANTS. THE SYSTEM GAINS ENERGY FROM THE SURROUNDINGS. H IS POSITIVE. Chemistry Happens in MOLES An equation that includes energy is called a thermochemical equation CH4 + 2O2 CO2 + 2H2O + 802.2 kJ 1 mole of CH4 releases 802.2 kJ of energy. When you make 802.2 kJ you also make 2 moles of water Thermochemical Equations A heat of reaction is the heat change for the equation, exactly as written • The physical state of reactants and products must also be given. • Standard conditions for the reaction is 101.3 kPa (1 atm.) and 25 oC 21 CH4 + 2 O2 CO2 + 2 H2O + 802.2 kJ If 10. 3 grams of CH4 are burned completely, how much heat will be produced? 10. 3 g CH4 1 mol CH4 16.05 g CH4 802.2 kJ 1 mol CH4 = 514 kJ 22 SPECIAL THANKS TO: WWW. pa016.k12.sd.us/Chapter%2011%20revised.ppt THERMOCHEMISTRY THE STUDY OF ENERGY CHANGES THAT ACCOMPANY CHEMICAL AND PHYSICAL CHANGES