* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Part of a Molecular Compound
Host–guest chemistry wikipedia , lookup
Chemical bond wikipedia , lookup
Depletion force wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Vapor–liquid equilibrium wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Stoichiometry wikipedia , lookup
History of molecular theory wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
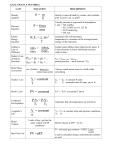
Chemical Quantities Chapter 7 Representative Particles • The smallest divisible unit of a substance • Elements – Atoms • Molecular Compounds – Molecules • Ionic Compounds – Formula Units • Part of a Molecular Compound – – Atom • Part of an Ionic Compound - ion The Mole • Quantity equal to 6.022 x 1023 representative particles. • 602200000000000000000000 particles • A.k.a Avogadro's Number How big is a mole? • A mole of hockey pucks is equal to the mass of the moon. How big is a mole? • A mole of rice would occupy a cube with sides of about 120 miles! How big is a mole? • A mole of rice grains is more grains then the number of grains of all grain grown since the beginning of time! How big is a mole? • If you had a mole of pennies, you could buy kite string at a million dollars an inch. With this string you could wrap it around the earth a million times and to the moon and back 25 times. You could sell the left over string back at a penny an inch ( a major loss) and take the remaining money and give everyone in the world about $10,000. O.K. seriously…How big is a mole? • How much is a mole of water molecules? • Let me show you! Now for some math… • How many moles is 3.78 x 1022 atoms of magnesium? Example 2 • How many moles is 3.78 x 1022 molecules of sulfur dioxide? Example 3 • How many moles is 1.80 x 1021 formula units of sodium sulfate? Example 4 • How many atoms in 0.750 moles of zinc? Example 5 • How many molecules in 0.750 moles of carbon dioxide? Example 6 • How many formula units in 1.22 moles of iron (III) chloride? Example 7 • How many atoms of hydrogen are in 5.15 mol of methane (CH4)? Example 8 • How many ions of potassium are in 0.80 mol of potassium carbonate? Example 9 • How many ions of nickel are in 0.80 mol of nickel (II) oxalate? Molar Mass Chapter 7 Molar Mass • The mass in grams of one mole of substance. • Older terms you might hear – Molecular weight – Formula weight Calculating Molar Mass • Use the atomic masses on the periodic table. • On many of them you can round to the nearest gram, except for elements that atomic masses end in 0.5 • Use as many significant figures needed to maintain the number of significant figures given in the problem. Example 1 • What is the molar mass of selenium? • mm = 78.96 g/mol Example 2 • What is the molar mass of nitrogen gas (N2)? • mm = 2 x 14.0 = 28.0 g/mol Example 3 • What is the molar mass of water (H2O)? – H 2 x 1.0 = 2.0 – O 1 x 16.0 = 16.0 – mm = 18.0 g/mol Example 4 • What is the molar mass of Tin (II) Chloride? – SnCl2 – Sn 1 x 119 = 119 – Cl 2 x 35.5 = 71 – mm = 190 g/mol Mole Map Periodic Table Mole Molar Mass Avogadro's Number 6.022 x 1023 Mass (g) Representative Particles Molecules Formula Units Atoms Ions Atoms Example 5 • How many atoms are in 5.6 mol of iron? Example 6 • How many grams are in 5.6 mol of iron? Example 7 • How many atoms are in 5.6 g of iron? Example 8 • How many grams does 3.4 x 1024 atoms of Sodium weigh? Example 9 • How many moles is 125 g of calcium? Empirical and Molecular Formulas Chapter 7 Empirical Formula • A formula with the lowest whole number ratio of elements. • Examples H2O2 (molecular formula) = HO (empirical formula) H2O = H2O C12H22O10 = C6H11O5 • Several different formulas can yield the same empirical formula. Steps for solving empirical formula problems 1. Convert grams to moles. (If in percent, treat as grams.) 2. Divide by lowest mole. 3. If not in whole number ratio, multiply by a common whole number to get a whole number ratio. Example 1 • Find the empirical formula of a compound that is 25.9g N and 74.1g O. Example 2 • 1.58 g of copper was reacted with sulfur to produce a compound with a mass of 1.98 g. What is the empirical formula for the compound? Example 3 • Find the empirical and molecular formula of a compound that is 67.6% Hg, 10.8% S and the balance O. The molar mass of the compound is 891 g/mol. Molar Volume Chapter 7 Molar Volume • The volume of one mole of a gas. • At STP, the volume of one mole of gas is 22.4 L • STP = standard temperature and pressure – Standard Temperature = 0oC – Standard Pressure = 1 atm • Atm = atmosphere Mole Map Volume (L) At STP 1 mole = 22.4L Periodic Table Mole Molar Mass Avogadro's Number 6.022 x 1023 Mass (g) Representative Particles Molecules Formula Units Atoms Ions Atoms Example 1 • What is the volume of 0.60 moles of SO2 at STP? Example 2 • What is the volume in mL of 90 g of SO2 at STP? Percent Composition Chapter 7 Percent Composition • Percent by mass of elements in a compound. Example 1 • Find the percent composition of potassium chromate. Example 2 • What is the percent sodium in sodium fluoride? Example 3 • How many grams of a 40 g sample of sodium fluoride is sodium?