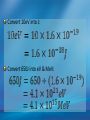* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Photoelectric effect
Buck converter wikipedia , lookup
Voltage optimisation wikipedia , lookup
Mercury-arc valve wikipedia , lookup
Mains electricity wikipedia , lookup
Cavity magnetron wikipedia , lookup
Alternating current wikipedia , lookup
Resistive opto-isolator wikipedia , lookup
Shockley–Queisser limit wikipedia , lookup
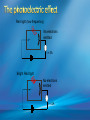
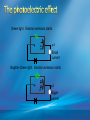
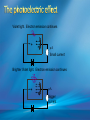
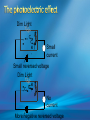
Photoelectric Effect Basically, the photoelectric effect is the ejecting of electrons from a metal by shining light of a particular frequency on it. It doesn’t work for all wavelengths/frequencies It was during 1887 that Hertz was conducting the experiment in which the photoelectric effect was discovered. Light was shone onto the cathode, which at certain frequencies emitted electrons, which were detected by the ammeter. These emitted electrons were called photoelectrons. Named to show that light was responsible for emitting them. The flow of these photoelectrons is called the photocurrent. In the initial set up of the experiment, the cathode is made negative and the anode is made positive. When the photoelectrons are released from the metal, they will naturally go to the positively charged anode. A sensitive ammeter will measure this photocurrent flow With this arrangement, the maximum current flowing will be recorded by the ammeter. All of the photoelectrons flowing across the gap will have a certain amount of kinetic energy. The amount depends on the location of the metal that the electron came from At or close to the surface – high energy Well below the surface – low energy When the anode and cathode had zero potential, there was still a current being recorded flowing across the gap. Though not as high as when the anode was made to be positive. For electrons to be emitted, the light had to have a certain frequency. The minimum frequency that would cause this is called the threshold or cut-off frequency, f0 Anything below this, would not provide enough energy to eject electrons. The German physicist Lenard found out the following things: The rate at which electrons are emitted depends on the intensity of the light High intensity – high current Low intensity – low current Cont’d.. Metals would only emit electron above certain frequencies Threshold frequency, f0 Electrons were emitted without any apparent time delay The time delay is actually about 10-9s The intensity of light doesn’t affect the threshold frequency. Only frequency affects it Lenard continued his investigations to find out more about the kinetic energy of the electrons being emitted. In particular the maximum kinetic energy of the released electrons This was done by reversing the potential around the circuit The anode was made more and more negative As this occurred, the current being recorded was observed to decrease. This showed that a decreasing number of photoelectrons had enough energy to overcome the opposing electric potential of the anode. Electrons with little or no KE were stopped as soon as the anode becomes negative Once a certain potential was reached, no more photoelectric current was recorded. This point is called the stopping voltage, V0 The photoelectrons with the most KE being stopped by Vo volts Light with a higher frequency, was found to have a higher stopping voltage. Red light (low frequency) No electrons emitted + i = 0A Bright Red light No electrons emitted + i = 0A Green light. Electron emission starts + e- μA Small current Brighter Green light. Electron emission starts + e- μA Bigger current Violet light. Electron emission continues + e- μA Small current Brighter Violet light. Electron emission continues μA + e- Bigger current Dim Light + - e - Small current Small reversed voltage Dim Light + e -- No current More negative reversed voltage Photoelectric effect animation http://www.ifae.es/xec/phot2.html A convenient unit of energy when dealing with electrons is the electron volt (eV). An electron gains 1eV of energy when it is accelerated across a potential difference of 1 volt. 1 eV = 1.6 × 10-19 J To convert from J to eV: divide the energy (in Joules) by 1.6 10-19. Since an electron gains 1eV of energy when it is accelerated across a potential difference of 1 volt, it can be said that: An electron accelerated through a potential difference of 50V, will gain 50eV of kinetic energy Should a stopping voltage of 10V be required, the maximum kinetic energy of any electron would be 10eV. Convert 10eV into J: Convert 650J into eV & MeV: The voltage at which the current is reduced to zero, the ‘Stopping voltage’ Vo, gives a measure of the maximum kinetic energy of the emitted photoelectrons. Ek max = ½mvmax2= eVo e - Charge on an electron V0 – stopping voltage m – mass of an electron = 9.11 x 10-31kg vmax – maximum speed of the electron A photoelectron is released though doesn’t make it to ‘the other side’ as the stopping voltage is 6.0V. What is the maximum kinetic energy of this photoelectron and at what speed must it be travelling? If the stopping voltage is 6.0V, the maximum kinetic energy must be 6.0eV After further work by Philip Lenard between 1899 and 1902, Albert Einstein concluded that light does in fact behave like a particle. The wave model could not explain the photoelectric effect.