* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Effects of Stapedius-Muscle Contractions on Masking of Tone
Survey
Document related concepts
Transcript
Effects of Stapedius-Muscle Contractions on
Masking of Tone Responses in
the Auditory Nerve
RLE Technical Report No. 544
May 1989
Xiao Dong Pang
Research Laboratory of Electronics
Massachusetts Institute of Technology
Cambridge, MA 02139 USA
a
e
a
a
-2-
EFFECTS OF STAPEDIUS-MUSCLE CONTRACTIONS
ON MASKING OF TONE RESPONSES IN THE AUDITORY NERVE
by
XIAO DONG PANG
Submitted to the Department of Electrical Engineering and
Computer Science on April 29, 1988 in partial fulfillment of the
requirements for the Degree of Doctor of Science
ABSTRACT
The stapedius muscle in the mammalian middle ear contracts under various conditions, including vocalization, chewing, head and body movement, and sound stimulation.
Contractions of the stapedius muscle' modify (mostly attenuate) transmission of acoustic
signals through the middle ear, and this modification is a function of acoustic frequency.
This thesis is aimed at a more comprehensive understanding of (1) the functional benefits
of contractions of the stapedius muscle for information processing in the auditory system,
and (2) the neuronal mechanisms of the functional benefits.
The above goals were approached by investigating the effects of stapedius muscle
contractions on the masking by low-frequency noise of the responses to high-frequency
tones of cat auditory-nerve fibers. The following considerations led to the approach. (1)
Most natural sounds have multiple spectral components; a general property of the auditory system is that the responsiveness of individual auditory-nerve fibers and the whole
auditory system to one component can be reduced by the presence of another component,
a phenomenon referred to as "masking". (2) It is known that low-frequency sounds mask
auditory responses to high-frequency sounds much more than the reverse. (3) Noise in
natural acoustic environments is predominantly of low frequency. (4) Contractions of the
stapedius muscle attenuate low-frequency sounds much more than high-frequency
sounds. (5) There have been human psychoacoustic studies which suggest that contractions of the stapedius muscle significantly improve (A) the detection of high-frequency
tones masked by low-frequency noise and (B) the identification of speech signals at high
intensities (at high intensities, masking of the high-frequency components of speech by
its low-frequency components becomes considerable).
There were four aspects of the investigation: (1) measurement of the stapediusinduced attenuation of sound and the determination of whether the attenuation depends
on sound level; (2) measurement of the masking of auditory-nerve fiber responses to 6
-3and 8 kHz tones by continuous low-frequency narrow-band noise (300Hz bandwidth centered at 500Hz); (3) measurement of the effects of stapedius contractions on the masking
of auditory-nerve fiber responses; and (4) a test of a mechanistic model which attempts
to explain the results of (3) from the results of (1) and (2). The measurements were
made with the stapedius muscle activated by artificial electric stimulation (activation of
the stapedius muscle by the central nervous system was blocked pharmacologically).
Results show that (1) For a given level of stapedius contraction, the attenuation of
sound does not depend on sound level. (2) The stapedius-induced attenuation of lowfrequency sound reduces masking by low-frequency noise of auditory-nerve fiber
responses to high-frequency tones. Unmasking effects up to 40 dB were observed; the
data suggest that unmasking up to 75 dB might occur in some fibers. (3) The observed
unmasking effects of the stapedius contractions on auditory-nerve fiber responses can be
completely explained by the mechanistic model which predicts the unmasking based only
on the growth-rate of neuronal masking and the stapedius-produced linear attenuation of
sounds reaching the inner ear. (4) The average growth-rate of neuronal masking was
2 dB/dB for the maskers and signals studied. (5) Maximum growth-rate of masking and
maximum stapedius-unmasking occurs in auditory-nerve fibers whose characteristic frequencies are near the test-signal frequency.
It is concluded that (1) The determination of linearity of stapedius effects on
middle-ear transmission makes it possible to (A) predict stapedius effects on auditory
responses in general and (B) simulate stapedius filtering at any stage before the cochlea.
(2) The observed stapedius-unmasking in auditory-nerve fibers is adequate to explain the
stapedius-unmasking reported from psychophysical experiments. (3) The quantitative
model of the stapedius-unmasking is equally applicable to neuronal as well as to
psychoacoustic responses, and is in particular applicable when the stapedius muscle is
activated through the acoustic reflex (i.e., with "closed-loop" regulation of acoustic
transmission through the middle ear). (4) Stapedius-unmasking on the order of 40 dB is
achievable with physiological activation of the stapedius muscle such as with vocalization. (5) An important function of the stapedius muscle is to improve acoustic communications both in noisy environments and for high-intensity acoustic signals with low and
high frequency components (such as human speech). Such a function might be of important survival value. Furthermore, a simulation or augmentation of "stapedius filtering" in
hearing-aid devices might provide significant help in dealing with some wide-spread
hearing-impairment problems.
Thesis Supervisor:
Dr. John J. Guinan, Jr.
Title: Principal Research Scientist in Electrical Engineering and Computer Science
-4-
To
my parents and my wife
Sw
-5-
Acknowledgments
There are many people whom I want to thank.
I thank MIT (as a collective of people) for giving me a good education in many dimensions.
Many members of the Eaton-Peabody Laboratory, where the experimental work of this
thesis was conducted, helped me in various ways. I thank you all. While it would be difficult to
enumerate all of the ways they helped, some deserve special mention. Monique Bourgeois, Paul
Davis, Leslie Dodds, Vijay Gandevia, and Debbie Learson performed the lengthy and demanding
surgical preparations with great care, and taught me about various aspects of animal surgery and
care. Monique also contributed to the histology work. Anna Graybeal participated in data processing. John Ledwith summarized the thesis for me by helping prepare the summary figure. I would
like to express my gratitude to the engineers, Bob Brown, Mark Curby, Charlie Gage, Ishmael
Stefanov-Wagner, and Dave Steffens, who provided prompt and quality technical support upon
every request, and many times more assistance than I requested. They not only fixed hardware
problems or came up with the software needed but also frequently went on to tell me how they
did it, thus increasing the benefits I received by many dB. My special thanks go to Drs. Jim
Kobler and Sylvette Vacher, who taught me the surgical approach to the stapedius muscle. I
would like to thank Barbara Kiang, not only for her generous assistance with the histology work,
but also for her general support and care. Dr. John Rosowski gave me introductions to both
Chamber III and Americanisms (sorry I can't remember the Polish words you told me). I am
thankful to Dr. Bertrand Delgutte for inspiring discussions and Dr. Charlie Liberman for instructions on various aspects of electrophysiological experiments. My thanks also go to Dr. Ruth Ann
Eatock for her spiritual support in the early and most difficult phase of this research by sharing
with me her experience with the lizard auditory nerve when I was struggling in those long nights
trying to figure out how to record from cat auditory-nerve fibers. I wish to thank my fellow gra-
-6-
duate students, Scott Dynes, Mike McCue, Jennifer Melcher, and Houston Warren, for good company, each in his or her own way. While the exchange of 2:00 AM jokes with Houston through
the computer helped to keep both my wife and me awake, his contribution in my "de-efferented"
animal experiments was crucial. I was especially touched when I learned afterwards that Houston
gave up his classes to help with my experiment (his medical-school classes, his dream classes).
Jennifer's support always came in when I was most unsatisfied with my experiments, by persistently pointing out to me the "bright side". I sincerely appreciated all the warm help from
Mike, from chamber wiring to MacDraw, among other things.
Each member of my Thesis Committee, Drs. John Guinan, Steve Colburn, Larry Frishkopf, Nelson Kiang, and Bill Peake, has contributed in a unique way.
I am grateful to my thesis supervisor, Dr. John Guinan, for helpful criticisms, advice,
assistance and support in every phase of this thesis research. I learned much from him both in
technical capabilities and in the knowledge of the whole auditory system.
Thanks are due to Dr. Larry Frishkopf and Dr. Steve Colburn for their encouragement and
insightful suggestions in the preparation of this thesis. They broadened my view.
There is, of course, Dr. Nelson Kiang, Director of the Laboratory. He has had a great
influence on my training in scientific thinking, and his care for my overall well-being was felt in
many ways.
I am indebted to Dr. Bill Peake for showing me an example of a great teacher in every
capacity. It is such a privilege to have been in close association with him, and his impact on me
will remain for many years to come.
My gratitude is due to the late Chairman of the General Chamber of Commerce of Hong
Kong, Mr. K. C. Wong. It was his kindness and generosity that made my coming to the United
States in 1981 possible.
I would like to express my appreciation of the continuous support and friendship of Nat
Durlach, my first supervisor at MIT, and my appreciation of the spiritual support of Dr. Francis
Lee.
I*
)r
P
C
r*
pi
U
·LI
-7-
The friendship of Helen Peake is greatly appreciated. She has added much to my student
life at MIT.
I wish to thank Ed Strachan and his family of Groton, MA for their friendship.
I want to thank my college teacher and friend, Prof. Zhou Xian-Yi, for his special contribution to my mathematical background.
I thank Prof. and Mrs. Liang Wen-Hai for their encouragement, friendship and help.
Finally there are my folks. I warmly thank my parents for all they have done for me -their love, their teaching, their sacrifice. My aunt-in-law, Li Yu-Hua, initiated the financial
arrangement for my coming to the U.S. The support from my special friends, Shao Yun and Wei
Min of Hangzhou, has meant so much to me in the past seven years. My wife, Qing-Qing,
directly shared the load of this thesis work during every step. I want to thank her for her love,
understanding, patience and sacrifice. Her contributions have made a difference.
Financial support for this thesis research was provided in part by a Sherman Fairchild Fellowship in Computational Neuroscience (three years).
-8-
Table of Contents
Title Page...............................................................................................................................
A bstract ..................................................................................................................................
2
Dedication.............................................................................................................................. 4
Acknowledgments ................................................................................................................... 5
Table of Contents ..............................................................
.........
......88
List of Figures...................................................................................................................... 11
Chapter I. Introduction..............................................................................................
Chapter II.
14
Background (I): Stapedius Muscle ........................................................ 19
2.1 Effect of Stapedius Contractions on Acoustic Transmission
Through the Middle Ear ...........................................................................................
19
2.2 Activation of the Stapedius Muscle .............................................................................. 19
2.2.1 Activation with signals of external origin ........................................
22
2.2.2 Activation with signals of internal origin .......................................
23
2.3 Functional Significance of Stapedius Activity .
.......................................26
2.3.1 Protection from damaging sounds ................................................................... 26
2.3.2 Improving auditory performance ......................................
28
2.4 Concluding Remarks ..................................................................................................... 35
Chapter III. Background (II): Auditory-Nerve Fiber Activity .............................. 38
3.1 General Description of Auditory-Nerve Fiber Activity ............................................... 38
3.2 Noise Masking and Rate Coding of Signals in the Auditory Nerve ........................... 46
52
3.3 Concluding Remarks .....................................................
Chapter IV.
Outline of Thesis Research .....................................................
53
53
4.1 Working Hypothesis .....................................................
1_·___11_1___;_1_1_111___411- ·-·
----- ----
I_-
-94.2 General Experimental Approach ......................................... ........................................ 59
4.3 Specific Experimental Approaches ........................................
60
4.4 Statistical Measures for Rate-Response Threshold Detection .................................... 63
4.5 Concluding Remarks ..................................................................................................... 65
Chapter V.
Methods ...................................................................................................... 67
5.1 Animal Preparation........................................................................................................
67
5.2 Generation of Acoustic Stimuli .......................................
68
5.3 Recording and Measurement of Responses .......................................
.................... 71
5.4 Electric Stimulation of the Stapedius Muscle ........................
5.5 Experimental Procedures and Paradig
.
7........2.....
m.....................................................................
s
82
5.6 Data-Processing Schemes ........................................
89
5.7 Controls for A rtifacts .................................................................................................... 91
Chapter VI.
Results (I): Masking Patterns of Auditory-Nerve Fiber
R esponses .......................................................................... 106
6.1 Masking Measured with Elevation of Tuning Curves ........................................
1.......06
6.2 Masking Patterns Observed from Rate-Level Functions ......................................... 118
6.3 Masking Measured with Elevation of Detection Threshold ...................................... 129
6.4 Summary and Conclusions ..........................................................................................
Chapter VII.
156
Results (II): Effects of Stapedius Contractions
on Auditory-Nerve Fiber Responses ......................... 158
7.1 Stapedius Effects on Auditory-Nerve Fiber Responses to Single Tones .................. 158
7.2 Stapedius Effects on Auditory-Nerve Fiber Responses to Tones in Noise ..............173
7.3 Summary and Conclusions ......................................
Chapter VIII.
Summary, Discussion and Conclusions
207
...................
209
8.1 Summary of Major Results ......................................................................................... 209
8.2 Masking Patterns of Auditory Responses ..........................
*
_
209
-
10-
8.3 Effects of Stapedius-Muscle Contractions ............................................
.................... 217
8.3.1 Linearity of the middle ear with stapedius contractions .................................. 217
8.3.2 Stapedius unmasking ................................................
8.4 Clinical Implications ..................................................................................
219
224
8.5 Comparison of Stapedius Effects with Olivocochlear Effects ................................... 227
Appendix ................................................
231
Literature Cited .................................................................................................
233
._______ _·__ii_
______I___II______1_11____
_
_____
I^
____ Il_------LI-II_
_ 11-1_ --_1
1*1
S
A*s
re
c
--·
- 11
List of Figures
Figure 1.1
Schematic drawing of the cat middle ear ...................................................... 15
Figure 2.1
Stapedius-induced change in acoustic transmission ...................................... 20
Figure 2.2
Stapedius-unmasking of human psychophysical responses ........................... 31
Figure 3.1
Discharge patterns of auditory neurons .......................................................... 39
Figure 3.2
Pulse-number distributions of auditory-nerve fiber discharges ..................... 41
Figure 3.3
Auditory-nerve fiber rate-level functions ....................................................... 43
Figure 3.4 Auditory-nerve fiber tuning curves .............................................................. 45
Figure 3.5
Rate-change in response to an 8kHz tone in noise vs. CF ........................... 49
Figure 4.1
Block diagram of the signal-flow pathways in the auditory system ............. 54
Figure 4.2
Diagrams illustrating the working hypothesis................................................57
Figure 5.1
Block diagram of the experimental apparatus ............................................... 69
Figure 5.2
Sinusoidal stapedius-shock threshold vs. shock frequency ........................... 75
Figure 5.3
Stability and repeatability of stapedius contractions ..................................... 78
Figure 5.4
Stable-contraction period of the stapedius vs. shock intensity ..................... 80
Figure 5.5
Stapes-head displacement vs. shock intensity ................................................ 83
Figure 5.6
Shock effects on CM tone responses with & without stapedius tendon ...... 85
Figure 5.7 Control for electric artifacts: sinusoidal shocks (I) ....................................... 92
Figure 5.8
Control for electric artifacts: sinusoidal shocks (II) ..................................... 94
Figure 5.9
Control for electric artifacts: pulse shocks .................................................... 96
.
Figure 5.10 Shock generated sounds
99
.......................................................
Figure 5.11 Control for shock-related mechanical artifacts .......................................... 101
Figure 5.12 CM-level functions recorded from different sites....................................... 104
Figure 6.1
Masking observed from tuning curves ......................................................... 107
Figure 6.2
All masking functions obtained from tuning curves ................................... 110
Figure 6.3
Tuning-curve growth-rate of masking at 6 & 8kHz vs. CF ........................ 112
1__1_1 _^^_____1____14__·__
_1_
1______1_
-·UIII.·--·YI-·
·Ill·--lpl_-·--l-·_I
II
-I
-
- 12-
Figure 6.4
Tuning-curve growth-rate of masking vs. test frequency............................ 114
Figure 6.5
Noise-induced change in Q10 vs. change in TC threshold .................
Figure 6.6
Masking observed from rate-level functions in a low-SR fiber ..................119
Figure 6.7
Masking observed from rate-level functions in a high-SR fiber ................. 23
Figure 6.8
Masking from rate-level functions vs. from tuning curves .................
Figure 6.9
Masking functions obtained from rate-level measurements ........................ 127
116
1........
1.......
125
Figure 6.10 Masking functions from a cat with severed olivocochlear efferents ..........130
a functions in a high-SR fiber ............................. 134
from a functions in a low-SR fiber .............................. 136
Figure 6.11 Masking observed from
Figure 6.12 Masking observed
Figure 6.13 All masking functions obtained from a measurements ............................... 138
Figure 6.14 The average masking function from
measurements ................................ 141
Figure 6.15 Growth-rate of masking from d functions vs. CF...................................... 144
Figure 6.16 Tuning curves from two fibers with similar tuning properties ................... 1 6
Figure 6.17 Masking of tuning curves from the two fibers of Fig. 6.16 ....................... 148
Figure 6.18 Masking of rate functions from the two fibers of Fig. 6.16....................... 150
Figure 6.19 Masking of a functions from the two fibers of Fig. 6.16 .......................... 152
Figure 6.20 Comparison of masking functions of the two fibers of Fig. 6.16 .............. 15
Figure 7.1
Time course of effects of burst-shock stimulation to the stapedius ........... 159
Figure 7.2
Effects of stapedius contractions on tuning curve & CM functions ...........
Figure 7.3
Stapedius effects on fiber & CM low-frequency tone-level functions ....... 164
Figure 7.4
Stapedius effects on fiber & CM high-frequency tone-level functions ...... 167
Figure 7.5
Stapedius effects on low- & high-frequency CM-level functions .............. 169
Figure 7.6
Stapedius effects: single-fiber measurements vs. CM measurements ......... 171
Figure 7.7
Stapedius effects on masking of tuning curve ............................................. 174
Figure 7.8
Stapedius effects on masking of rate-level function .................................... 177
Figure 7.9
Stapedius effects on masking of a-level function ........................................ 179
162
Figure 7.10 Stapedius-attenuation of sound corresponding to Fig. 7.9.......................... 181
- 13 -
Figure 7.11 Stapedius-unmasking from mean-rate and a measures ............................... 184
Figure 7.12 Stapedius-unmasking when olivocochlear efferents were severed ............. 186
Figure 7.13 Effects of stapedius contractions on masking functions .....................
1.......188
Figure 7.14 Effects of stapedius contractions on ROC .................................................. 191
Figure 7.15 Measured vs. predicted stapedius unmasking: tuning-curve data ............... 193
Figure 7.16 Measured vs. predicted stapedius unmasking: rate-function data .............. 195
Figure 7.17 Measured vs. predicted stapedius unmasking: d-function data ................... 197
Figure 7.18 Measured vs. predicted stapedius unmasking: all data ............................... 199
Figure 7.19 Stapedius-unmasking as a function of fiber CF.......................................... 203
Figure 7.20 Growth-rate of masking corresponding to Fig. 7.19 ................................... 205
Figure 8.1
Schematic integration of the main-line results .............................
210
S
a
a
a·
I
- 14 -
Chapter I
Introduction
The mammalian peripheral auditory system consists of the outer ear, the middle ear, and
the inner ear (Figure 1.1). Sound propagates through the outer ear to reach the tympanic membrane and causes it to vibrate. This vibration in turn causes the three ossicles in the middle ear to
vibrate. These three ossicles, the malleus, incus, and stapes, form a chain in which the malleus is
connected to the tympanic membrane and the footplate of the stapes is connected to the membrane of the oval-window of the inner ear (the cochlea), with the incus in the middle of the
chain. The cochlea is filled with fluid; when this fluid is set in motion by vibration of the ovalwindow membrane, the hair cells in the cochlea transduce this motion into electric signals in the
primary auditory neurons, which send inner-ear output to the brain via the auditory nerve.
Besides the afferent acoustic signal pathway from the auditory periphery to the brain as
outlined above, various efferent signals from the brain to the auditory periphery influence acoustic
information processing. Mammals frequently experience involuntary modifications of acoustic
transmission through their middle-ears; external sounds are often processed differently by the
middle ear under different circumstances, including anticipation of vocalization, chewing, coughing, head and body movement, cutaneous stimulation, and previous sound stimulation. The primary mechanism by which such modifications of acoustic transmission through the middle ear are
performed is contraction of the middle-ear muscles. There are two muscles in most mammalian
middle-ears, the stapedius muscle and the tensor tympani muscle, attached via tendons to the
stapes and malleus, respectively. The motoneurons of these muscles are located in the brain (e.g.,
Joseph et al., 1985). Contractions of these muscles displace the ossicles and their attachments,
thus changing their impedance (Pang & Peake, 1985). This change in impedance of the ossicles
results in a change of acoustic transmission through the middle ear. The change in acoustic
transmission induced by contractions of the middle-ear muscles is mostly an attenuation; for a
- 15-
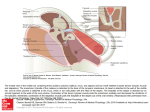
Figure 1.1
A somewhat schematic view of the cat middle ear. In response to sound the stapes
vibrates in the medial-lateral direction. When the stapedius muscle contracts, the stapes
is pulled in a posterior direction. The symbols are: AN: Auditory Nerve; C: Cochlea;
EAM: External Auditory Meatus; I: Incus; M: Malleus; OW: Oval Window; RW: Round
Window; S: Stapes; SM: Stapedius Muscle; TM: Tympanic Membrane; TTM: Tensor
Tympani Muscle.
MED. and POST. stand for the medial and posterior directions,
respectively. (From Pang & Peake, 1985; a modified version of part of a drawing by A.
Greene).
-16-
W%
TTM
I
I
N
MED.
!
POST.
SM'
- 17-
given status of middle-ear muscle contraction, the amount of attenuation depends on the frequency of the sound.
Among the questions unanswered in our understanding of the auditory system as a whole,
there are two fundamental ones pertaining to the middle-ear muscles: First, what is the functional
significance, or the "benefits" to the auditory system and its owner, of modifying acoustic
transmission by contractions of the middle-ear muscles? There have been wide speculations upon
the answer to this question, but there has not been conclusive evidence. Existing evidence points
at two distinct kinds of possibilities: protecting the inner ear from damaging sounds and improving auditory performance. While the meaning of the "protective role" (with careful definition) by
the attenuation of acoustic transmission is relatively clear, "improvement of auditory performance" by middle-ear muscles requires some explanation. It has been shown that the functioning
of human middle-ear muscles significantly improves the detection of high-frequency tones
masked by low-frequency noise and the identification of speech signals at high intensities (Borg
& Zakrisson, 1973, 1974). There then arises the second question: What are the physiological
mechanisms underlying such improvement of the overall auditory performance with contractions
of the middle-ear muscles? To answer this question, one has to probe beyond the middle ear
because the middle ear is known to be a largely linear processor of auditory signals; phenomena
such as masking are nonlinear in general and are known to take place at stages beyond the middle ear, and are also known to be inherent in neuronal responses to acoustic stimulation. Since
the activity of the auditory nerve constitutes the first stage of the neural processes that underlie
hearing (it represents simultaneously the output of the cochlea and the input to the brain), the
auditory nerve seems to be a suitable stage for starting direct study of the effects of middle-ear
muscle contractions on neural responses to acoustic signals. So far, however, there have been few
data reported on the effects of middle-ear muscle contractions on auditory-nerve fiber responses.
In this thesis research, the effects of stapedius-muscle* contractions on the masking of
* There is evidence indicating that in the cat the stapedius in general plays a more significant role in affecting acoustic
transmission than the tensor tympani (Simmons, 1959; Hilding, 1960; Cawmel & Starr, 1963; M6ller, 1965; and observations by this author). In fact, it is widely accepted that for humans under intense acoustic stimulation, the stapedius muscle is dominant in modifying acoustic transmission (Djupesland, 1964; Anderson, 1976; Borg et al., 1984). For this rea-
- 18 -
auditory-nerve fiber responses to high-frequency tones by low-frequency background noise were
studied in the cat, with the aim of achieving a more comprehensive understanding of the functional significance of the stapedius contractions in the processing of acoustic signals by the auditory system. A quantitative hypothesis was proposed which explains the mechanism of the stapedius effects on the masking of neuronal responses. This hypothesis was tested by comparing
the measured stapedius effects on the neuronal masking with the effects predicted by the
hypothesis based on the neuronal masking functions without stapedius contractions and the
stapedius-induced attenuations of the noise and tones. Results show that the experimental measurements are consistent with the hypothesis. In addition, the effects of stapedius contractions on
auditory-nerve fiber responses can explain the reported effects of stapedius contractions on
psychoacoustic responses.
son and others, I chose to focus my effort on the study of the effect of stapedius-muscle contractions.
1
O
a0
P
- 19 -
Chapter II
Background (I): Stapedius Muscle
2.1 Effect of Stapedius Contractions on Acoustic Transmission Through the Middle Ear
When the stapedius muscle contracts, the head of the stapes is pulled along the direction
of the stapedius tendon, which is approximately perpendicular to the direction of stapes vibration
in transmitting sound (see Fig. 1.1). In the cat, this displacement of the stapes head can be as
much as 40 m under intense binaural acoustic stimulation and as much as 60 gm under electric
stimulation of the stapedius muscle, as observed by this author (also see Pang & Peake, 1985).
Such displacements are large compared to the displacement of the stapes in transmitting sound,
for it is known that the peak-to-peak displacement of the stapes in response to a tone at
100 dB SPL is 0.2 tm or less (Guinan & Peake, 1967). As a result of stapedius-muscle contractions, the magnitude of the acoustic impedance of the stapes (including its associated structures)
is increased and the stapes motion in response to a given sound is reduced (a reduction in acoustic transmission). For a given stapes-head displacement, the amount of reduction in acoustic
transmission varies with the frequency of the sound. Figure 2.1 shows the reduction in acoustic
transmission* produced by various stapes-head displacements in a cat. It can be seen that for
every stapes-head displacement the reduction in transmission is larger in the low-frequency region
(below 1.2 kHz) than in the high-frequency region. The reduction of acoustic transmission in the
low-frequency region can reach 25 dB (versus 10-12 dB in the high-frequency region). In fact
transmission reductions of as much as 30 dB were observed in the low frequency region (Pang &
Peake, 1985; also will be shown in Chapter VII).
2.2 Activation of the Stapedius Muscle
* Acoustic transmission through the middle ear is by definition the ratio of the output of the middle ear over the input
to the middle ear.
- 20 -
Figuie 2.1'.
Change in the magnitude of acoustic transmission (20loglT(SHD)/T(0)I) in a cat
middle ear as a function of acoustic frequency for six different stapes-head displacements
(SHDs). The cochlear-microphonic potential was used as a measure of the output of the
middle ear, and sound pressure near the tympanic membrane was used as the measure of
the input to the middle ear. Each point is an average of six measurements. Each measurement was made during an interval ( 30 seconds) after the onset of the electric stimulation of the stapedius muscle. During this interval SHD, cochlear potential, and sound
pressure at the tyinpanic membrane were constant. Measurements were made at equal
intervals of 1/7 decade in log frequency. The horizontal line marks 0 dB. The sound
pressure level used for the measurements was about 70 dB SPL. Measurements in other
ears gave similar results. (From Pang & Peake, 1985).
-21-
Changes in Middle-Ear Transmission (T)
Produced by Stapes-Head Displacement (SHD)
I
I
I, I
I,, , , I
I
I I
I
I,1 I
I,
I,
I
I I I
_
Dashed line marks zero SHD (No change in T)
0o0
M
e-
1-
-10-
o*o-
SHD=22um
SHD=36um
SHD=42um
+-
SHD=44um
h-
SHD=47um
-20-
- SHD=54um
XDP8
-30I
0.01
I
·
I
I
I
_____
I
l I I
0.1
I
,I
I
I
11
I
I
1
I
I '
I I
10
Acoustic Frequency (kHz)
____1_____1111_____--l___l·--1L-
- ---_ 1·I-_I I I -
II·I·-
- 22 -
2.2.1 Activation with signals of external origin
A majority of the studies on the effects of stapedius contractions has been carried out in
connection with a phenomenon known as the acoustic reflex, which refers to the involuntary contraction of the middle-ear muscles evoked by intense external sound stimulation. This reflex
forms a closed-loop feedback control of the sound intensity reaching the inner ear in that it is
activated by an intense sound and it results in a reduction of the sound energy transmitted
through the middle ear. The sound pressure required to elicit the reflex is generally rather high
(above 80 dB SPL for a tone), and the strength of the reflex increases with stimulus strength over
a range of about 30 dB (Anderson, 1976; Guinan & McCue, 1987). The latency between the
onset of the sound and the contraction of the stapedius muscle ranges from a few milliseconds to
a couple of hundred milliseconds, depending on the physical characteristics of the stimulus and
physiological conditions of the subject/animal. The fact that the earliest reflex response (e.g..
measured by surface electromyographic recordings from the stapedius) has a latency of only a
few milliseconds (e.g., 6 ms) shows that the reflex only requires the lower parts of the brain,
though higher centers may contribute with a longer latency. The reflex is consensual in that a
monaural acoustic stimulus can evoke muscle contractions in both ears. It is also known that barbiturates in doses used in most animal experiments abolish or greatly reduce the reflex (Simmons,
1960; Carmel & Starr, 1963; Borg & M6ller, 1967, 1975).
The change in sound transmission when the stapedius is activated through the acoustic
reflex has been assessed in various ways. In a cat, M6ller (1965) indirectly measured with a
strong contralateral tone a reduction in transmission of 9 dB below 1 kHz. In human subjects,
Borg and Zakrisson (1974) estimated an ipsilateral transmission reduction of up to 20 dB at
0.5 kHz. Rabinowitz (1977) inferred, using in the human a method different from that of Borg,
transmission reductions of up to 10 dB at frequencies below 0.6 kHz. Attenuation of acoustic
transmission caused by stapedius acoustic reflex in the high frequency region (up to 8 kHz) has
also been reported (Borg, 1972).
- 23 -
Recent findings suggest that the acoustic reflex does not "completely" activate the stapedius muscle, or more specifically, there seems to be channels "reserved" for modes of stapedius
activation other than that through the acoustic reflex. Pang and Peake (1985) showed that in the
cat electric stimulation of the stapedius muscle could produce an attenuation of acoustic transmission at least 12 dB larger than any reported attenuation produced by the stapedius acoustic reflex.
This difference in the magnitude of the effect is consistent with the author's observation in three
cats that the saturation level of stapes-head displacement produced by binaural tonal stimulation
was no more than 40 gm whereas that produced by electric stimulation of the muscle could be as
much as 60 gm (in general), and that as shown in Fig. 2.1 there could easily be a difference of
over 12 dB between the transmission changes associated with 40 gm and 60 gm stapes-head displacements. McCue and Guinan (1983) found that in the cat a significant number of stapedius
motoneurons could be destroyed without noticeably affecting the acoustic reflex response.
Results from cats also indicate that there are stapedius motoneurons which do not respond to unilateral sound stimulation (Kobler et al., 1987).
There are also some less-well-studied situations of stapedius activation with signals of
external origin (signals not generated by the animal itself) other than intense sound. Djupesland
(1964) cited an example of "conditioned-reflexive" contractions of the stapedius produced by
informing the subject of an imminent intense noise (although in this case it is possible that the
contraction was voluntary, rather than "reflexive"). Klockhoff and Anderson (1959) reported that
stapedius contractions could be elicited by cutaneous stimulation in the external ear-canal in deaf
subjects, and that in normal subjects subthreshold cutaneous and acoustic stimulation could add to
evoke stapedius contractions, whereas simultaneous cutaneous and acoustic stimulation of high
intensities could eliminate the additive effects of the two stimulations on stapedius contractions.
2.2.2 Activation with signals of internal origin
Although stapedius-muscle contractions produced by the acoustic reflex have received the
_____·_
____1_1_11____1__4___11_1111
·1
·
- 24 -
most attention in the literature, the stapedius is probably active more often without intense external sound stimulation. For both humans and cats, there have been reports suggesting that in the
absence of any apparent stimulus, the stapedius is spontaneously active and has a "resting tonus",
which is presumed to be related to the "mental tension" in the subject/animal (Simmons, 1964;
Djupesland, 1967). In support of this "resting tonus" notion, Simmons (1959) reported sizable
differences in acoustic transmission through the middle ear between actively awake (presumably
tense) and anesthetized (presumably with at least partially relaxed stapedius muscles) cats at
moderate sound levels. In addition, data reported by Kobler et al. (1987) suggest that in anesthetized and paralysed cat preparations, about 13% of the stapedius motoneurons are spontaneously
active.
More clearly documented stapedius activation with signals generated internally (by the
animal itself) are from reports on the stapedius contractions associated with vocalization, mastication (chewing), and head and body movement. Carmel and Starr (1963) reported that in the
awake cat, with bodily movement there was attenuation of acoustic transmission through the middle ear "roughly proportional to the extent of bodily movement" in magnitude through contractions of the stapedius muscle (an example was a 7 dB attenuation in the cochlear microphonic
response to a free-field broadband noise stimulus). In the same series of experiments, they also
concluded that contractions of the stapedius muscle in response to nonacoustic activation developmentally precede the appearance of the acoustic reflex.
The most systematic study relating stapedius activity with vocalization is probably that by
Borg and Zakrisson (1975a) in man; Carmel and Starr (1963) and Simmons (1964) made similar
observations in cat. Borg and Zakrisson recorded the electromyographic (EMG) activity of the
stapedius and observed the motion of the stapedius tendon when their subjects vocalized. Among
their findings are the following points: (1) The threshold for stapedius activity is close to the
lowest vocal intensity that the subject could produce; (2) With normal vocal effort the stapedius
muscle is activated to about 50% of the contraction level produced by maximum vocalization
effort; (3) This maximum level of contraction is about equal to the reflex contraction level evoked
Ap
__
- 25 -
by maximum contralateral acoustic stimulation; and (4) The stapedius contraction is often
activated shortly (e.g., 75 ms) before the vocalization, indicating that the activation is from the
central nervous system as a part of the vocalization process.
With respect to changes in acoustic transmission, Irvine and Wester (1974) found that
contractions of the stapedius muscle in the cat produced reductions in the cochlear-microphonic
response to air-conducted sound and bone-conducted sound with equal effectiveness. This result
suggests that activation of the stapedius muscle by signals of internal origin such as in the case of
chewing, which produces strong low-frequency noise, can effectively attenuate some lowfrequency noise generated by the animal itself independent of, at least to some extent, the acoustic pathway to the cochlea.
In summary, the stapedius muscle in humans and animals is active under a variety of circumstances; it can be activated by signals of either external or internal origin, and under either
closed- or open-loop conditions with respect to acoustic transmission; it causes a frequencyselective reduction in acoustic transmission through the middle ear with reduction of transmission
in the low-frequency region larger than that in the high-frequency region.
One recurrent question is whether the attenuation of a sound by a given stapedius contraction depends on the level of the sound. It is known that when the middle-ear muscles are in their
"resting" state the middle ear is a linear processor of auditory signals in that middle-ear transmission is independent of the level of the sound (e.g., Guinan & Peake, 1967). It has not been
determined, however, whether the middle ear remains so when the stapedius contracts. There
have been some psychoacoustic studies which suggest that for a given stapedius contraction the
amount of the attenuation of a sound depends on the level of the sound. In humans Morgan et al.
(1978) measured the effects of middle-ear muscle (presumably the stapedius) contractions on the
hearing threshold at 0.25, 0.5 and 1.5 kHz and found no evidence of a change in the hearing
threshold accompanying middle-ear muscle contractions elicited by a 3 kHz contralateral tone at
105 dB SPL. Similar conclusions (that contractions of middle-ear muscles in humans have little
effect on threshold hearing but considerable effect on intense sounds) have been drawn by some
_____il__l_________·11_111___1_4____1_
·_ __
__
- 26 -
other investigators, while few mechanistic explanations have been offered (Loeb & Riopelle,
1960; Ward, 1961, 1967; Morgan & Dirks, 1975; Humes, 1978; Sesterhenn & Breuninger, 1978).
On the other hand, also in human studies involving the acoustic reflex, Rabinowitz (1977)
observed no level-dependent attenuation of moderate-level sounds with stapedius contractions. In
the cat Wever and Bray (1942) observed linear relations between the magnitude of the cochlear
microphonic potential and the level of a 1 kHz tone in the region above hearing threshold while
they had different weights attached to the stapedius tendon to displace the stapes. Nedzelnitsky
(1979) obtained in the cat linear relations between the sound pressure inside the cochlea and that
delivered to the tympanic membrane at very intense sound levels (up to 140 dB SPL) while he
electrically stimulated the stapedius to produce stable contractions. None of the physiological
studies, however, provides conclusive evidence on the linearity issue: Wever and Bray displaced
the stapedius tendon in a "non-physiological" way, which may result in modes of stapes displacement different from that produced by contractions of the muscle; and Nedzelnitsky only measured
acoustic transmission at very intense sound levels. While this issue will be addressed directly
later in this thesis, the remainder of this chapter will be devoted to a discussion on the possibilities for the functional significance of stapedius activity.
2.3 Functional Significance of Stapedius Activity
From experimental studies on the functional significance of stapedius activity, two major
and consistent benefits have been pointed out. These are protection of the cochlea from damaging sounds and improvement of auditory performance. Some representative experimental results
are reviewed here for each of these.
2.3.1 Protection from damaging sounds
It is known that intense sound stimulation can damage hearing (by damaging the sensory
receptors in the inner ear (e.g., Liberman et al., 1984)). For both cats and humans, it has been
- 27 -
clearly shown that contraction of the stapedius muscle helps to protect the nner ear from damage
under continuous stimulation of intense sound. Simmons (1960) compared the hearing loss in
cats with normal and severed stapedius muscles after the cats were exposed to a 1 kHz
135 dB SPL tone for 2 hours. He found that for cats with normal stapedius muscles the maximum temporary hearing loss was about 17 dB and there was no permanent hearing loss, while
for cats with their stapedius muscles cut, both the temporary and permanent hearing loss were as
much as 60 dB or more. Simmons (1963) also showed that cats with stronger stapedius-reflex
contractions (as measured by the difference between the onset and steady-state level of the
cochlear microphonic response to intense sound stimulation) tend to experience less soundinduced hearing damage.
Since experiments leading to permanent hearing loss cannot be readily performed in
human subjects, most experiments on the protective function of the stapedius for the human focus
on the temporary (hearing) threshold shift (TTS). Zakrisson (1975) reported that, for subjects
with unilateral stapedius paralysis, the TTS at 0.75 kHz was on the average 20 dB larger in the
affected ear than in the ear with intact stapedius reflex, after the subjects were exposed to a
narrow-band low-frequency noise (300Hz bandwidth centered at 500Hz) of 120 dB SPL for 5
minutes. In another report (Zakrisson et al., 1980), industrial noise from a shipyard was used as
sound stimulus (15 minutes duration), and an average TTS difference of 10 dB at 2 kHz was
seen between the subject's ears with and without functioning stapedius reflex.
A few additional points should be noted regarding the protective role of the stapedius: (1)
The stapedius muscle cannot protect the inner ear from single impulsive intense sound because of
the latency of the stapedius reflex (typically 25 ms or more to be effective). It can, however, provide protection if the listener is conditioned to anticipate the sound (Hilding, 1960; Fletcher,
1962; Djupesland, 1964). (2) Since the attenuation of acoustic transmission effected by stapedius
contractions is a function of frequency (see Fig. 2.1), the protection offered is also a function of
frequency. It has been shown in both cats and humans that the least 'protection provided by stapedius contractions is around 3 kHz (Sokolovski, 1973; Zakrisson, 1975), which agrees well with
- 28 -
Fig. 2.1. The fact that the protection produced by contractions of the 'stapedius is mainly restricted to the low-frequency region also challenges the notion that protection of the cochlea from
damaging sounds is the primary function of the stapedius, for in that case a uniform attenuation
of acoustic transmission in the entire frequency region would certainly be more effective.*
2.3.2 Improving auditory performance
As has been discussed in the first two sections of this chapter, signals of either external
origin (as in the case of acoustic reflex) or internal origin (as with vocalization) can activate the
stapedius muscle to effect a frequency-selective attenuation of acoustic transmission through the
middle ear. In order to see how a frequency-selective attenuation of acoustic transmission can
improve auditory performance, one has to first look into the nonlinear interactions between acoustic components in different frequency regions in contributing to the response of the auditory sstem.
It is well known that, in general, the principle of superposition does not apply to
responses of the auditory system to acoustic stimuli.** For example, the psychoacoustic detection
of a high-frequency sound can be masked (made difficult or impossible) by the presence of a
low-frequency sound and vice versa. Such masking of the response to one sound (say, a "signal")
by the presence of another sound (say, a "noise") is highly asymmetrical at high sound levels in
that low-frequency sounds are much more effective in masking responses to high-frequency
sounds than the reverse (e.g., Wegel & Lane, 1924). One can measure the effect of masking by
defining a masking function whose value is the increment in the signal (the sound being masked)
level necessary to maintain a constant sensation (typically a "threshold", or just-detectable, sensation) of the signal in relation to an increment in the masking sound (or masker) level. Typically,
when the masker is much lower in frequency than the signal, a 10 dB increment in masker level
* Evolutionally there is no obvious reason that a "flat-attenuation" mechanism would be impossible or more difficult to
implement
** Along the auditory signal pathway from the periphery to central the principle of superposition does not hold at least
as early as at the stage of auditory-nerve responses (e.g., "two-tone suppression", Sachs & Kiang, 1968).
- 29 -
produces a 20-40 dB increase in the signal threshold (a growth-rate of the masking function of
2-4 dB/dB); if the masker is much higher in frequency than the signal, however, a 40 dB increment in the masker raises the signal threshold by only 5 or 10 dB (Green & Wier, 1984).*
The effect of stapedius contractions on acoustic transmission also has an asymmetry: lowfrequency sounds are attenuated more than high-frequency sounds (see Fig. 2.1). Therefore, if
there is a high-frequency signal in a background of low-frequency noise, with stapedius contractions, the following can be predicted: first, both the signal and the noise will in general be
attenuated; second, the signal will be attenuated less than the noise, thus the masking of the
response to the signal by the noise will decrease (the signal-to-noise ratio in the response will
increase). To a first-order approximation, the condition for the effect of the reduction in masking
to offset that of the attenuation of the signal is: AN x GR > AS, where AN is the attenuation of
the noise in the effective masking range (above the masking threshold), GR is the average growth
rate ((dB signal)/(dB noise)) of the masking function (measured in the absence of stapedius contraction) in the range between the attenuated and un-attenuated noise level, and AS is the attenuation of the signal by the stapedius (in dB). The difference between the two sides of the inequality
is the gain in the signal-to-noise ratio.**
Experimental results supporting the above predictions have, indeed, been reported from
psychophysical studies. Borg and Zakrisson (1974) measured the masking produced by continuous narrow-band low-frequency noise (300 Hz bandwidth centered at 500 Hz) on the detection of
pulsating high-frequency tones (6 and 8 kHz) in subjects with unilateral stapedius paralysis. The
measurement was made both from the ear with stapedius paralysis and from the ear with a
* A simple interpretation of this asymmetry in psychoacoustic response can be offered from the point of view of
cochlear mechanics: In the mammalian cochlea, each part of the basilar membrane, on which the receptor cells are located, is maximally sensitive to (tuned for) a particular frequency for displacement, and the iso-displacement contour of the
basilar membrane is asymmetrical with respect to frequency in that the average slope of the contour in terms of the
stimulus increment required per unit frequency increment is steeper on the high-frequency side than on the lowfrequency side of the extreme (e.g., B6k6sy, 1960; Robles et al., 1986). Thus with a given stimulus intensity a lowfrequency signal displaces the parts of the basilar membrane tuned for high frequencies much more than the reverse.
This point will be further discussed in connection with auditory-nerve fiber tuning curves in Chapter III.
** As an example with realistic numbers, if we assume that a certain strength of the stapedius contraction effects a
15 dB attenuation of the low-frequency noise and a 5 dB attenuation of the high-frequency signal, and a mean growthrate of 2 dB/dB for the noise-on-signal masking function, then the gain in signal-to-noise ratio in the response would be
(15x2)-5-25 dB as a result of the stapedius contraction.
______I
-----II
-- -X
- 30 -
normal stapedius reflex, during the acute stage of the paralysis and after recovery (There was no
statistically significant difference between the hearing thresholds of these ears). Figure 2.2 shows
the results. It was found that the masking was consistently greater in the ear with stapedius
paralysis for sound levels above acoustic-reflex threshold, while it was equal in both ears below
reflex threshold. The difference in masking increased as the stimulus intensity increased (presumably because the strength of the stapedius contractions and the attenuation of transmission
increased in the normal ear). The greatest difference in masking between the normal and affected
ears was approximately 47 dB at 6 kHz with a 115 dB SPL noise masker. On the other hand, the
ipsilateral attenuation of the masker through the acoustic reflex was estimated to be 20 dB with a
115 dB SPL sound stimulation. The key element for a 20 dB attenuation of the noise to effect a
47 dB reduction in the masking is thus the growth-rate of the masking function at 6 kHz in the
absence of the acoustic reflex, which has a value of about 2.56 dB/dB in the noise intensity
region between 115 dB and 95 dB SPL (Fig. 2.2B, the upper-most curve). In other words, the
47 dB difference in masking can be explained from the observation that 2.56x20 > 47 dB (the
difference of 4.2 dB is presumably the stapedius-attenuation of the 6 kHz tone).
Since low-frequency noise is frequently present in natural environments and since lowfrequency sounds mask high-frequency sounds more effectively than the reverse, some mechanisms for reducing the masking effect produced by low-frequency noise, such as contractions of
the stapedius muscle, may be of great functional significance to the auditory system. A direct
example for the value of such an un-masking effect by the stapedius was also provided by Borg
and Zakrisson (1973) when they examined the perception of "natural" acoustic stimuli in subjects
with unilateral stapedius paralysis. It was found that when heard through the ear with stapedius
paralysis, the discrimination score for monosyllabic nonsense speech phrases decreased rapidly
compared to that when heard through the ear with normal stapedius reflex, once the intensity of
the speech phrases exceeded 100 dB SPL (there was no difference in the score below this level).
The higher the intensity level of the stimulus above 100 dB SPL, the larger the difference in the
score; and the largest difference in the score recorded was over 50% at 120 dB SPL.
- 31 -
Figure 2.2
Data from human psychoacoustic experiments on the masking of high-frequency
tones by low-frequency noise, with and without functioning stapedius muscle. (From
Borg and Zakrisson, 1974).
(A) Hearing thresholds of a subject obtained by fixed-frequency Bekesy audiometry at
6 kHz without masking (--) and with ipsilateral narrow-band low-frequency masking
noise (300 Hz bandwidth centered at 500 Hz) at various levels. Left side: Ear with stapedius paralysis (affected ear). Right side: Ear with normal stapedius reflex (non-affected
ear). Masking is defined as rise in threshold (M) during presentation of the masking
noise.
(B) Masking at 6 kHz (2 subjects) as a function of the level of the masking noise. Solid
lines are for the affected ears during stapedius paralysis; dashed lines are for the nonaffected ears. Each symbol is for one subject and arrows with symbols show estimated
ipsilateral stapedius reflex thresholds for the masking noise. "D" indicates shift of the
masking function produced by the functioning of stapedius reflex in the subject from
whom the data shown in (A) were obtained.
.
____ ___ ______111_·___1__1_1__^II
1_1
-32-
-0
AFFECTED
EAR
NONAFFECTED
EAR
SP l_
-
(A)
t,,~_
z
________
ls MISIG
!I
I
.
NOISE
it ISW
-
i_
_ __S
,_
m
(B)
z
a
4
o
i
i
MASKING
·- I
i
.
NOISE
INTENSITY
115
(dS SPL)
- 33 -
Alternatively, for the same performance, the ear with stapedius reflex could handle sournd 1520 dB more intense than the other ear. Furthermore, the stimulus intensity range over which there
was a significant difference in speech discrimination between the ear with and without stapedius
reflex corresponded well with the normal acoustic reflex activation of the stapedius muscle in the
subject, and the difference in the sound level (15-20 dB) between the ears for the same performance corresponded well with the amount of attenuation of transmission that would be provided
by a normal stapedius reflex in the low-frequency region. Borg and Zakrisson suggested as an
explanation of their findings that since speech sound has maximum power at low frequency and
the weaker high-frequency components are more important for intelligibility of speech, it is the
selective attenuation of transmission by stapedius contractions that reduced the masking of highfrequency components in the speech by the low-frequency "noise", leading to an enhancement in
the perception of the "signal".*
The validity of attributing the above results to the functioning of the stapedius is supported by a study in normal human subjects for whom the possible involvement of neural pathology other than stapedius paralysis, such as auditory-nerve block which may occur concomitantly
with stapedius paralysis, can be ruled out. Dorman et al. (1986) presented vowel signals of 50
and 300 ms duration at 72 dB SPL (below acoustic-reflex threshold) and 106 dB SPL (above
reflex threshold) to their subjects. The minimum latency for an externally measurable acousticreflex response (change in acoustic impedance at the tympanic membrane) to a contralateral
1 kHz tonal stimulus at 105 dB SPL was 50 ms or more for all subjects, therefore the experimenters assumed that at 106 dB SPL the 50 ms duration vowels were little attenuated by the
acoustic reflex. It was found that when the vowels were presented at 72 dB SPL, there was no
difference between the recognition scores for the 50 and 300 ms duration vowels; when the
vowel intensity reached 106 dB SPL, however, the recognition score for the 50 ms duration
vowels was on the average 30% worse than that for the 300 ms duration vowels (The maximum
* Analysis of the errors in the discrimination tasks for vowels and consonants by Borg et al. suggests that it was primarily direct (simultaneous) masking, rather than backward or forward (temporal) masking, that was responsible for the
decline in discrimination scores since the majority of the errors concerned vowels.
_I___
_ 1_1-11_11_ __111
i_l__._.lL_ ---11_1_-1_1111
_-_1-_1111_-111---_il.l
-._^-.
-1-·1_1_(.^1_1
-_---·111^---^_
. . - - _._----l111_1-1
- 34 -
sound level used here was 14 dB less than that used in the study by Borg & Zakrisson, 1973). It
was also found that when the acoustic reflex was pre-activated by a tonal stimulus at
106 dB SPL, the recognition score for the 50 ms duration vowels at 106 dB SPL was 20% higher
than that without pre-activation of the reflex.
In another study of the effects of stapedius contractions on speech discrimination,
Mahoney et al. (1979) delivered speech signals intense enough to elicit an acoustic reflex to cats
before and after their middle-ear muscles were pharmacologically paralysed, recorded the
corresponding cat cochlear potentials on a magnetic tape, and played the tape recording at a
"most comfortable" sound level (below acoustic reflex threshold in human) to normal human
listeners for speech discrimination. The same procedure was also carried out for speech signals
with competing messages. Results showed that discrimination scores by human listeners for both
the cases of speech only and speech with competing message were significantly higher with an
active stapedius reflex in the cat than without. It was also reported that the cat cochlear-potential
signals contained less energy in the low frequency region of 0.4-0.9 kHz with stapedius reflex
than without. Besides the data, this report is also interesting in that the experimenters developed a
way to measure the effect of cat stapedius contractions on the processing of human speech signals by the cat middle ear and cochlea, thus providing an "interface" for comparing the effects of
stapedius contractions measured in anesthetized cats with that measured in awake human subjects.
One kind of low-frequency noise that an animal or a human frequently encounters is the
noise generated when the animal or human is eating or vocalizing. As was mentioned in Section
2.2.2, the stapedius muscle is activated under these circumstances. The un-masking mechanism
should also be operative when the stapedius is activated by signals of internal origin. There is little direct evidence, however, on the functional significance of the stapedius when it is activated
by signals of internal origin. By combining the results from their studies of stapedius activation
with vocalization and of the effects of stapedius contractions on speech discrimination, Borg and
Zakrisson (1975b) arrived at the conclusion that "Most likely the significance of the stapedius
activity for perception of external sounds is greatest when one is talking". On the other hand,
I
__
_
_
- 35 -
Irvine et al. (1983) failed to find an un-masking effect of middle-ear muscles in the cat during
chewing. They delivered short clicks of moderate intensity binaurally to cats with unilateral
middle-ear muscle tenotomy, fed meat to the cats, and recorded the gross auditory-nerve action
potentials with an electrode implanted on the round-window of each cochlea. While they
observed a masking effect on the action-potential response to clicks by chewing, they could not
find a significant difference between the masking in the normal and tenotomized ears. They did
not, however, take into account in processing their data the fact that hearing in the tenotomized
ears was worse than in the unoperated ears (as shown in every waveform they presented). By
taking that fact into account, it can easily be shown that for all five actual recordings presented in
their paper (four of which constitute two-thirds of the total data points in one of their summary
plots and the fifth is the only actual recording shown for the other summary plot) there is an unmasking effect by the middle-ear muscles. The average un-masking effect is such that the percentage ratio of the masked response with muscle over that without muscle, normalized by the percentage ratio of unmasked response with muscle over that without muscle, is larger than 120%.
Moreover, since these waveform recordings provided the data points that, according to the plots
in the paper, were most "against" an un-masking effect, it is justified to associate a positive unmasking effect to every data point in their plots.
In summary, a built-in anti-masking mechanism, such as that affordable (at least conceptually) by contractions of the stapedius, in the ascending acoustic signal pathway before the cochlea
can clearly, from a signal-processing point of view, be beneficial to the overall performance of
the auditory system not only in the case of speech recognition but also in virtually all cases of
acoustic communication for many orders of mammals. Such a mechanism can be viewed as
complementary to the design of the cochlea and can conceivably be of great survival value for
some animals.
2.4 Concluding Remarks
_
_I
_______YL__^_II___YILY-··----LI·-
___-
-
·II
II-
--.I_
__
i
·I_
_
- 36 -
From the above discussion it can be seen that even though the acoustic reflex is only one
way of evoking stapedius contractions, it has been most widely employed in experimental studies
of stapedius function. The reason for this is several-fold: under most circumstances, especially in
humans, the acoustic reflex is the only way to produce controllable stapedius contractions. This
reflex is involuntary and its study can be non-invasive. Restricting the study of stapedius function
to the acoustic reflex, however, causes several problems: since very intense acoustic stimuli are
used to elicit the reflex, the experimentation itself can produce hearing damage in the subject.
More importantly, since the stapedius is often activated in the open-loop fashion without intense
sound stimulation, and in nature there are probably more low and moderate level sounds than
intense ones, a complete understanding of the stapedius function will have to include knowledge
on the effect of stapedius contractions (activated in open-loop) on auditory responses to sound at
low and moderate levels.* Results obtained with closed-loop activation of the stapedius at high
sound intensities (as with acoustic reflex) are, however, not necessarily applicable to situations
with open-loop activation of the stapedius (as with vocalization), because of the unresolved issue
of middle-ear linearity with stapedius contractions. Since, as will be seen in Chapter IV, this
issue of the linearity of stapedius effect is of great importance to this thesis study, a part of the
results portion of the thesis is directed to resolving this issue with physiological measurements for
at least the sound level range that is of concern to this study. In a more general sense, this thesis
is intended to be a direct study of the effects of stapedius contractions (when activated in openloop) on the physiological responses of the auditory system to sounds.
A problem of perhaps more significance than the lack of knowledge on the functional
significance of the stapedius at low and moderate sound levels is the complete lack of data on the
effect of stapedius-muscle contractions on responses of auditory-nerve fibers, and consequently
the lack of direct knowledge on neuro-physiological mechanisms underlying the improvement of
auditory performance produced by stapedius contractions. It is this problem that is the core of
* It may be interesting to note that in the visual system the iris muscles are active at all levels of light input to regulate
the flux.
-- -------------
- 37 -
this thesis. Before the core part of the thesis is discussed, however, a brief review of background
knowledge on auditory-nerve fiber activity should be helpful, and that is the subject of the next
chapter.
1
1_1_1____1_11_1_111__111___II_
·LULIOI
II1WI
-1I.--I_---··--I··111111·1^1_-
1
_··1111_-
-^--
1_
I
rC
P
1,
M.
- 38 -
Chapter III
Background (II): Auditory-Nerve Fiber Activity
3.1 General Description of Auditory-Nerve Fiber Activity
As was mentioned earlier, the activity of the auditory nerve represents both the output of
the inner ear and the input to the brain, and constitutes the first stage of the neural processes that
underlie hearing. It has been estimated that there are approximately 30,000 and 50,000 fibers in
the auditory nerve of the human and cat, respectively (Rasmussen, 1940; Gacek & Rasmussen,
1961). In auditory-nerve fibers, auditory information is thought to be carried by the occurrences
of the all-or-none neuronal spikes and by the temporal patterns of the occurrences (the spike
waveform is largely invariant). The spike discharge is probabilistic in that a fiber's responses to
repeated identical stimuli are not, in general, identical (see Fig. 3.1). The presence of an effective
acoustic stimulus produces changes in some statistical measures of the spike-discharge process.
With well-defined stimulus conditions, such changes become more stable as sample size
increases, thus enabling characteristic properties to be specified for each nerve fiber on the basis
of average responses. Both the technique for recording from auditory-nerve fibers and a variety
of data-processing schemes that lead to descriptions of responses have been well developed (e.g.,
Kiang et al., 1965). Commonly used data-processing schemes estimate either the underlying probability function for spike occurrence, or some parameters of the function. The post-stimulus time
(PST) histogram and the spike-number distribution (SND, or pulse-number distribution, PND) are
examples of the first category, while the average rate-level functions and the tuning curves fall
into the second category. The choice of processing schemes depends on what aspects of the
response are of interest. The PST histogram displays the number of spikes for successive time
intervals after a stimulus is presented and can therefore provide an estimate of the instantaneous
discharge rate and other information on the time structure of spike response (see Fig. 3.1); the
spike-number distribution estimates the probability of having a certain number of spikes in a time
_ __LII___II1_I111__1_·__·----^111_^·_1
-LI-.I·I-···IIC·III)
. II1II_-ltl -- · 1II-1IIII1-___-1yl(llllllllll-----·
-39-
DISCHARGE PATTERNS OF AUDITORY NEURONS
K636- 10
CF: 2100Hz
1 NOSTIMLUS
~~~~Il
1
iI I'lI
I
2
CLICKS
3
TON BLRSTS
4
TONE
I
_
I
I1111
.:
I'
~:~'~"
1
I
I
I
Il l
H
(
-
:i
I
I"
I
.
.,
'IT
_
1I
11
4h~
S
.
Sp's
I
31 -
!
1
'!
!!'
1
Iii
I
11
LS
I
V'S
SPEECH
Fig. 3.1
1
_
i1,1 1
I
~~~~~~~~~~~~~~~I
~ ~ ~ ~i ~ ~~~I I
I
~
I
_
'1
5
I
I
Ii
l
0
0
I
-1
C
C
- -
A
The basic method for studying spike discharge patterns of auditory neurons in response to acoustic
stimuli. Upper traces: a I-sec sample of single unit discharges recorded with a micropipette placed in the auditory nerve of an anesthetized cat. Amplitude of the positive (upward) deflections in the top trace is I mV and
serves to calibrate all spike recordings shown. Lower traces: the waveform of the electrical input to a l-in.
condenser earphone sealed into the external auditory meatus (7). The pairs of traces are for 1, spontaneous
activity; 2, activity during presentation of 100-/sec clicks at I dyne/cm-; 3, activity during presentation of
50-msec-long, 2,100-Hz tone bursts with rise-fall times of 2.5 msec and a peak level of 70 dB SPL; 4, activity
during presentation of a 2,100-Hz tone at 70 dB SPL; and 5, activity during presentation of a speech stimulus,
the utterance "SHOO CAT," at a moderate level (-37 dB re 200 V p - p into a I-in. condenser earphone). To the
right of each pair of traces is shown a poststimulus time (PST) histogram based on a -min sample of the recorded activity. Vertical scales on the histograms, instantaneous rates of discharge in spikes per second. Horizontal scales, chosen to illustrate particular features of the responses. Zero time for the histograms are, for clicks,
the onset of the electric stimulus to the earphone (0.07 msec travel time to the tympanic membrane); for tone
bursts, 2.5 msec before the onset of the bursts; for the tone, every other positive zero crossing of the sinusoidal
input to the earphone; and for the speech stimulus, approximately 0.2 sec before the beginning of the trace on
the left. (From Kiang, 1975).
..
- 40 -
interval as a function of that number (see Fig. 3.2); and the rate-level function gives
description
of the dependence of some "mean" discharge rate on the level of an acoustic stimulus (see
Fig. 3.3). Auditory-nerve fiber responses are nonlinear in general; for example, the rate-level
function displays a "threshold" and a "saturation" behavior. The particular kind of nonlinearity
that will be a subject of this study is the masking of auditory-nerve fiber responses to highfrequency tones by low-frequency background noise, a topic to be discussed in more detail in the
next section.
Auditory-nerve fibers respond selectively with respect to the frequency content of an
acoustic stimulus. Different fibers are tuned to different frequencies. The tuning curve, which
relates stimulus level (usually the level of sound pressure) to frequency through an iso-rate
response criterion (usually a just-detectable increase of the "mean" discharge rate above background level), is a measure that provides information on both sensitivity and frequency selectivity
of the fiber under the stimulus condition (see Fig. 3.4). The frequency at which a fiber "normally" has the lowest response threshold (highest sensitivity) with respect to stimulus level is
usually called the characteristic frequency (CF) of the fiber, and the corresponding stimulus level
provides one definition for the threshold of the fiber at CF.* The tuning curve is in general asymmetrical in that for most fibers the average slope of the tuning curve on the high frequency side
is steeper than that on the low frequency side.** It is also known that auditory-nerve fibers are
tonotopically organized, that is, the fibers are spatially arranged in an orderly way according to
their CFs (Liberman, 1982).
Auditory-nerve fibers can also be classified according to their behavior in the absence of
sound stimulation, i.e., their spontaneous discharges. It has been shown (e.g., Sachs & Abbas,
1974; Liberman, 1978) that the spontaneous discharge rate is correlated with the sensitivity and
dynamic response range of a fiber. Typically, a fiber with a low spontaneous rate (SR) has a
* A typical definition for a "normal condition" of tuning-curve measurement is that the curve is measured with a tonal
stimulus in a quiet background. It will be seen later that other factors such as the presence of a masker can "shift" the
"CF' and the "threshold".
I. The footnote on the tuning in the basilar membrane response in earlier discussion of the asymmetry in psychoacoustic masking is highly relevant here with respect to the origin of the tuning-curve asymmetry in auditory-nerve fibers.
_________lil
- 41 -
Figure 3.2
Pulse-number or spike-number distributions observed in two auditory-nerve fibers
with high and low spontaneous discharge rate (the discharge rate in the absence of sound
stimulation), respectively (From Teich & Khanna, 1985).
(A) The pulse-number distribution (PND) of a fiber with a high spontaneous activity (54
spikes/sec). The fiber was most sensitive to sound with frequency content near 6 kHz
(such as a 6 kHz tone; 6 kHz in this case is called the characteristic frequency, or CF, of
the fiber) and it started to respond to a 6 kHz tone when the tone level reached
20 dB SPL (its response threshold). The PNDs were measured with a 50 ms sampling
window at three levels of a 6 kHz tonal stimulus: 20 dB below response threshold, at
threshold and 20 dB above threshold. Each PND consisted of 1000 samples. As the
level of the sound stimulus changed, the mean and variance of the distribution changed,
but the shape of the distribution remained approximately symmetrical about the peak and
did not seem to change qualitatively.
(B) Similar to (A) except that this fiber had zero spontaneous rate and its CF was at
1.4 kHz with a threshold of 30 dB SPL. The PNDs were measured at three levels of a
1.4 kHz tone above the response threshold and the shape of the PNDs also appeared
approximately symmetrical about the peak.
0
-42-
0.3
P
r
b
6113 Hz
(C F)
/
(A)
.0
0.0
n
14.0
gl
,,,,
I
.
I
(B)
- 43 -
Figure 3.3
Mean discharge rate plotted versus stimulus level for 13 fibers from eight cats.
Stimuli were tones at fiber CF (characteristic frequency, the frequency which a fiber is
most sensitive to), which is given in kilohertz below each curve. Notice the difference
between the two columns in the change of the slopes of the curves as a function of the
stimulus level. (From Sachs & Abbas, 1974).
I
-44-
4
U
00
0
4:c
u
n
STIMULUS LEVEL (dB SPL)
0
-45-
0
~m
fo
o.
°
_ _
,
tE
m~
]l
..
MC.
Z s ,OC
-
:.~~~;
''_
i.
'o.c'
:
C,
,,
:
E3
iE
5.
E
.-
2e
v
,,,.
.
-I
o-o
I
I' ,
.F
,
-,
~
Z~ .
E: .
ar,E'
I-
e!
I
~
Z
I.
I-
..-I
I
F
E
C NC Y
IN
t
-
*K I
I
_
-.
k
1 IN1· \ 7_k 7`
11
k -Z
.
L
'-
c~~~~¥;
t
Fig. 3.4 Representative tuning curves in an untreated cat taken by the
autonmated procedure. Graph (A) shows the tuning curves plottcd in
terms of voltage into the earphone. These curves are equal-response
contours obtained by using a computer-controlled oscillator and attenuator to set the frequency and adjust the level of tone bursts to produce
a specific rate of unit discharge above the rate of spontaneous activity.
The So-ms bursts are presented at a rate of lo per second with rise-fall
times of 2'5
- ms. The tone frequency is held constant so long as level is
changing rapidly, and the direction of frequency change is always from
high to low. This procedure provides a faithful representation of the
steep slope found on the high-frequency side of most tuning curves while
permitting rapid determination of the extensive low-frequency side.
Each tuning curve has arbitrary initial and final points. Curve (a) shows
the sound-pressure level at the tympanic membrane for a constant
voltage into the earphone. This curve was used to convert graph (A)
into graph (B), which shows the tuning curves in terms of sound-pressure
level at the tympanic membrane. Similarly curve (b) shows stapes
displacement for constant sound-pressure level at the tympanic membrane and is used to convert graph (B)into graph (c), which shows the
tuning curves in terms of stapcs displacement. Curve (a) is computed
from several other curves obtained in acoustic calibrations and stored in
the computer. Curve (b) is an average curve for several cats redrawn
after Guinan and Peake (967) and Peake and Guinan (1966) and is also
stored in the computer. Once the curves in () are obtained during the
experiment (1-2 min per curve), the curves in (B)and (c) can be immediately obtained. Each tuning curve in () was obtained by averaging two
separate curves. The vertical resolution is 2/3 dB; the horizontal scale
has 32 points distributed equally within each octave above 320 Hz.
There are 6 points in the octave between 60 and 32o Hz.
(From Kiang, Moxon & Levine, 1970).
.i 2
- 46 -
higher response threshold and a larger dynamic range, which is usually measured by the
difference in stimulus intensity between the threshold and saturation level. The ratio of the
number of fibers with high-SR (> 19 spikes/sec) over that with low- (< 1 spikes/sec) and
medium-SR (in between) common to most reports is about 2/1 (Kiang et al, 1970; Liberman,
1978; Evans & Palmer, 1980; Costalupes, 1985).
In the remaining sections of this chapter, the focus of discussion will be on some aspects
of the coding of acoustic signals, in particular high-frequency tones, in the auditory nerve in the
presence of background noise.
3.2 Noise Masking and Rate Coding of Signals in the Auditory Nerve
The mechanisms underlying the psychoacoustically observed masking of responses to
high-frequency signals by low-frequency noise can be traced back to (though not necessarily restricted to) the cochlea. It has been shown that in the auditory nerve the response of a high-CF
fiber to a tone at its CF can be powerfully masked by the presence of low-frequency noise*
(Here and in all later descriptions of physiological responses, "masking" refers to the reduction in
responsiveness of an auditory-nerve fiber to one sound by the presence of another). Kiang and
Moxon (1974) showed that the tip of the tuning curves (the part of the tuning curve around the
CF) of high-CF fibers, compared to other parts of the tuning curve, is most sensitive to masking
by low-frequency noise. A low-frequency noise which by itself barely elicits responses from a
high-CF fiber can produce a 10 dB elevation of the response threshold of the fiber at its CF
(while producing little change in the fiber's threshold for tones in the same frequency region as
the noise), and a 10 dB increase in the masking noise level can produce an additional 20 dB
elevation in the fiber's threshold at its CF (a masking growth-rate of 2 dB/dB). Geisler and Sinex
(1980) measured the effect of low-frequency masking noise on the rate-level functions of high-CF
auditory-nerve fibers. Their findings include the following: (1) The principal effect of low* Recall that it was mentioned in the last section that for most auditory-nerve fibers the average slope of the tuning
curve on the high-frequency side is steeper than that on the low-frequency side.
- 47 -
frequency masking noise on the rate-level function of a high-CF fiber was a shift of the function
towards higher stimulus levels, "thus acting to attenuate the effective intensity of the tone"; (2)
The masking effect of a low-frequency noise on a high-CF fiber's tonal response was strongest at
the fiber's CF; (3) The masking of tonal responses by low-frequency noise was found in every
high-CF fiber tested; (4) The relative masking strength of the low-frequency noise grew more
rapidly with intensity than the excitation produced by tones at fibers' best frequency (BF, the
equivalent of CF in the suprathreshold region), i.e., the growth rate of the masking was larger
than unity; and (5) In addition to the shift of the rate-level function, there was sometimes an
increase in the discharge rate in the low-intensity portion of the function, and a change in the
slope of the rate-level function.
The coding of high-frequency tones in the auditory nerve in the presence of low-frequency
noise will be discussed next in the context of the detectability of high-frequency tones in noise
from an information theoretic point of view. One reason for focusing on detection is that there
have been psychoacoustic studies on the effects of stapedius contractions on the detection of
high-frequency tones in noise, as was discussed earlier.
An obvious and easily characterizable change in the discharge pattern of a fiber in
response to an effective stimulus (e.g., a tone burst) is a change in its rate of discharge (Fig. 3.1).
There is evidence from both experimental and modelling studies suggesting that for a large class
of auditory tasks, including detection of high-frequency tones in noise and intensity discrimination with tonal stimuli, rate changes in auditory-nerve fibers can provide sufficient information to
the central nervous system to account for the observed psychophysical performance (Siebert,
1965, 1968; Viemeister, 1983; Costalupes, 1985; Young & Barta, 1986; Delgutte, 1987). For the
high-frequency ( 6 kHz) signals used in this thesis study, rate information can be particularly
pertinent because in this frequency region fine temporal information relating fiber discharges to
individual cycles of the stimulus waveform is hardly present in the discharge pattern of auditorynerve fibers (Johnson, 1980). Rate information from auditory-nerve fibers is therefore the primary
consideration in this thesis in discussing coding and detection of auditory signals.
- 48 -
Young and Barta (1986) studied the detectability of rate response to tone bursts at the BF
of auditory-nerve fibers in the presence of continuous broadband noise in the cat. The spikenumber distributions (SNDs) were assumed to be approximately normal (Gaussian)* and a detection characteristic, D, was used to measure the separation between the SNDs with noise only and
with noise+tone. This distinguishability measure, D, was defined as the difference between the
mean values of two unit-variance SNDs obtained by transforming the SNDs with noise only and
with noise+tone. In the case that the two original SNDs were of equal variance, D would simply
be the difference between the means divided by the standard deviation (square root of the variance) of the distribution. The rate-response threshold was defined as the tone level with which D
reached a value of unity, i.e., the difference between the means equaled one standard deviation.
Young and Barta obtained the D values for BF tones over a 32 dB range of levels centered at the
behavioral masked thresholds of cats, at three noise intensities with 20 dB separations. They
found that at all noise levels tested, the threshold associated with a unity D value in the rate
response of single auditory-nerve fibers was often very close to the behavioral threshold in cat for
detecting tones in noise. They also found empirically that the variance of the SND increased
with the mean and that spike-count statistics of auditory-nerve fibers differed from the behavior
of a Poisson process in that the mean and variance of the SND were significantly different.
Costalupes (1985) studied rate responses evoked by 8 kHz tone bursts in continuous
broadband noise as a function of the BF of auditory-nerve fibers in the cat. Instead of using a
sufficient statistic such as the D used by Young and Barta, however, he only considered the
difference in the mean discharge rate. The result is shown in Fig. 3.5, with the difference in the
mean discharge rate between "noise+tone" stimulation and "noise only" stimulation plotted
against the BF of the fibers. With the tone-to-noise ratio kept at 7 dB above the behavioral detection threshold, a profile that peaked near 8 kHz can be seen for both the low-and-medium and the
high SR fibers at low and moderate noise levels, and for the low-and-medium fibers at high noise
* As was mentioned at the beginning of this chapter, the SND is an estimate of the probability density function for the
number of spikes observed in a fixed time interval (see Fig. 3.2).
- 49 -
Figure 3.5
Rate-change profiles for a population of cat auditory-nerve fibers in response to
8 kHz test tone bursts in continuous broadband noise. S/N was 34 dB (the difference
between the tone level shown in the upper-left corner of each panel and the noise spectrum level shown at the upper-right comer of each panel). Arrows indicate the frequency
of the 8 kHz test tone. The solid line is a triangularly weighted moving average of the
data points. Symbols: square for low-SR fibers, triangle for medium-SR fibers, and cross
for high-SR fibers. (From Costalupes, 1985).
-50-
6/12/84
S/N 7 dB re DETECTION
HIGH SPONT.
LOW AND MEDIUM SPONT.
6
TONE LEVEL
NOSE SPECTRUM
LEVEL
I
TONE LEVEL
NOISE SPECTR
I
LVrFL
~A
,0u
A
sode
I
-448
.·%·. ofi l
0-
-2-
.
.
.......
.
.
.......
I
A
v,
n
30-
LJ
30-
B.
Ode
i6d8
E
E.
5d
50dB
o
16 e
.
0-
' Sa
_A.-
U-
-641f
II
n
-20- I
. I
I I I I'll
I
I 7
?0 d
30- C
I
wi
.
-20-
-
F.
30
36 de
\rrrl
.I
WW I.
.
o d70d
*
T~
-
6
I
I
-20
-20-
....
,
10
I
..
I
. ,,
I I'l .,
10.0
I
I
.
. . I
-20 -
-
100.0
BEST
. . .
1.0
FREQUENCY (kHz)
.
.,
10.0
.
100D
- 51 -
level. It can also be seen from Fig. 3.5 that the tone-evoked rate-change profile exhibited filter
properties in that the rate change was restricted to a region near 8 kHz. The average width of the
rate-change band was reported to be comparable to the "critical band" observed psychophysically,
which is also thought to be a property of peripheral filtering in the auditory system.*
Data such as those shown in Fig. 3.5 combined with the observation that the auditory nervous system is tonotopically organized from the cochlea to higher centers in the brain can be
taken as supportive of a coding scheme, known as "rate-place" coding, for representing acoustic
information in the auditory system (a review on this scheme is given in Colburn, 1981). In this
scheme, rate information (in its generalized sense, not limited to the mean rate) would be
represented as a function of the BF (or CF), which corresponds to some spatial "place", of a
population of auditory-nerve fibers in the form of a profile along the BF dimension (or its
equivalent). In the case of representing a high-frequency tone in background noise, a profile that
peaks near the tonal frequency (such as was shown in Fig. 3.5) would be required for the detection of the tone using rate information.
In summary, both Costalupes (1985) and Young and Barta (1986) concluded that rate
responses from auditory-nerve fibers can carry sufficient information to the central nervous system to account for the behaviorally observed levels of performance in detecting tones in noise,
one from a "critical band" point of view and the other from comparing the masked thresholds in
psychophysical and physiological "detection" tasks with a common information measure (D=I).
Furthermore, since the best-possible "detection performance" by a single auditory-nerve fiber
observed was close to the behaviorally observed detection performance, it was concluded by
Young and Barta that "There appears to be sufficient information in the rate response of a small
number of auditory nerve fibers to support behaviorally observed levels of detection performance".
* It is known from psychophysical studies that the noise power required to mask a tone is independent of noise
bandwidth within a restricted range of frequencies surrounding the tonal frequency. This constant power bandwidth is
known as the critical band.
- 52 -
3.3 Concluding Remarks
The previous section provided a brief review of the masking by background noise of
auditory-nerve fiber rate responses to tones. Four aspects were discussed: qualitative observation
of the masking effects (Kiang & Moxon, 1974; Geisler & Sinex, 1980), statistical measure for
threshold detection (Young & Barta, 1986), rate-place coding (Costalupes, 1985), and comparison
of physiological and psychophysical "detection" of tones in masking noise (Costalupes, 1985;
Young & Barta, 1986). From the point of view of this thesis, however, the kind of data that has
not been available in the literature is the integration of the above in a coherent way. Specifically,
what have been missing are systematic measurements of the masking, especially the growth rate
of masking, of auditory-nerve fiber rate responses to high-frequency tones by low-frequency
noise, using statistical measures for threshold detection. Such measurements will constitute the
first part of the results portion of this thesis, and the importance of the growth rate of masking
will become clear in the next chapter with a discussion of my working hypothesis.
_
____
___
- 53 -
Chapter IV
Outline of Thesis Research
4.1 Working Hypothesis
The part of the auditory system and the associated signal-flow pathways relevant to this
study are shown in Fig. 4.1. As pointed out in Chapters II and III, the effects of stapedius contractions on the masking of auditory-nerve responses to high-frequency tones by low-frequency
noise could not be quantitatively predicted because of the lack of knowledge on both the linearity
of the middle ear with stapedius contractions and the growth rate of neuronal masking. The primary goals of this study were (1) to make direct measurements of the effects of stapedius contractions on the masking of auditory-nerve fiber responses; (2) to measure the linearity of stapedius attenuation; (3) to measure the masking patterns of auditory-nerve fiber responses, particularly the growth rate of masking; and (4) to compare the measured stapedius effects on masking
with the effects predicted from the knowledge of (2) and (3). More specifically, since to integrate
the knowledge of (2) and (3) into a quantitative prediction requires some theoretical hypothesis
on the underlying mechanisms for the stapedius effects on the masking of auditory-nerve
responses, comparison of direct measurements of the stapedius effects on the masking of
auditory-nerve responses with predictions of such effects can provide a direct test for the
hypothesis. The results of such a test should either provide a mechanism that can explain the
effects or point out directions for further research or both.
Since contractions of the stapedius muscle attenuate low-frequency sounds more than
high-frequency sounds, one could hypothesize that such differential attenuation has an unmasking effect on auditory-nerve responses to high-frequency tones masked by low-frequency
noise. As the masking of high-frequency sounds by low-frequency sounds is intrinsic to the auditory periphery, it might be of great importance to the coding of high-frequency sounds in the
auditory nerve if stapedius contractions can provide an effective un-masking mechanism.
-
54 -
Figure 4.1
A block diagram of the auditory system illustrating the relationships of the signals
that were of direct concern to this study. "PT" is sound pressure at the tympanic membrane, "H(f,s)" is the input-output function of the middle ear, where "f' stands for frequency and "s" for stapedius contraction (the middle ear is known to be a linear processor when the stapedius is in its "resting" state), "UF" is the volume velocity of the stapes
footplate, "CM" is cochlear-microphonic potential, "N 1" stands for the N 1 waveform in
the auditory-nerve compound action potential, "A.N." is auditory nerve, and "RESP."
stands for "response". The olivocochlear efferents can be activated either by auditornerve inputs to the central nervous system or by other central nervous system signals.
w
0
ELECTRIC
STIMULATION
ANESTHESIA
I
I
TO
STAPEDIUS
I
-*.,-<-I
r
OLIVOCOCHLEAR
EFFERENTS
I
PT
MIDDLE EAR
SOUND
PRESSURE
H (f, s)
I
UF
-
COCHLEA
A.N.
RESP.
I
i
PSYCHOACOUSTIC
RESP.
S-
CENTRAL
v-----
NERVOUS
r-
SYSTEM
IF
I
CM
N1
0
-------^-----
- 56 -
Specifically, my working hypothesis is the following: When there is a masking of auditory-nerve
fiber representation of high-frequency tones by background low-frequency noise, contractions of
the stapedius muscle effect an enhancement of the representation by reducing the masking. Quantitatively, the hypothesis predicts that for each nerve fiber, the reduction of the masking (in dB)
AM - (AN)x(GR) - AS, where AN is the attenuation of the low-frequency noise in the effective
masking range (above masking threshold) by the contraction of the stapedius (in dB), GR is the
growth-rate of the neuronal masking function (measured in the absence of stapedius contraction)
in the range between the attenuated and un-attenuated noise level (in dB/dB), and AS is the
attenuation of the high-frequency tone by the stapedius contraction (see Fig. 4.2).
The implications of this hypothesis include: (1) While contractions of the stapedius muscle
only effect an attenuation of acoustic transmission through the middle ear and affect primarily
(and at moderate levels of muscle contractions, exclusively) the low-frequency region, stapedius
contractions can enhance responses of auditory-nerve fibers to high-frequency sounds when both
low- and high-frequency sounds are simultaneously present, as is frequently the case in the
natural acoustic environment; (2) The unmasking effect of the stapedius muscle is not restricted
to auditory-nerve responses to high-intensity sounds when the stapedius activation is not restricted
to high-intensity sounds; (3) Since noise masking of a fiber's response is most powerful when the
frequency of the signal is near the fiber's CF (Kiang & Moxon, 1974; Geisler & Sinex, 1980),
for signals of fixed frequency (e.g., 6 or 8 kHz), the unmasking effect of the stapedius is
expected to be largest for fibers whose CFs are near the signal frequency; (4) A sufficient condition for stapedius contractions to effect a reduction in the masking of auditory-nerve fiber
responses to high-frequency tones by low-frequency noise is that the growth-rate of the neuronal
masking is larger than unity, because stapedius contractions attenuate low-frequency sounds more
than high-frequency sounds. If the growth-rate of the neuronal masking function is larger than
two, then the unmasking will be powerful. For example, if a contraction of the stapedius effects a
20 dB attenuation of a low-frequency noise, and if the GR is 2 dB/dB and there is little attenuation of a high-frequency tone by the stapedius, then there can be an effective "unmasking" of a
___
- 57 -
Figure 4.2
Diagrams illustrating the working hypothesis.
(A) A hypothetical masking function of an auditory-nerve fiber's response to a highfrequency tone masked by a low-frequency noise, measured in the absence of stapediusmuscle contractions. "Th" stands for masking threshold. With noise level Na, the masking is Ma. If a stapedius contraction produced an attenuation of sound of AN in the
noise-frequency region, then the growth-rate (GR) of masking to be used in the prediction of the stapedius-produced unmasking effect by the working hypothesis should be
measured in the noise range between Na and Nb , where Na - Nb = AN. Note that this
measure. of GR only depends on the value of the end points Ma and Mb, not on the
exact shape of the masking function within this range. In other words, no straight-line
assumption for the shape of the masking function is necessary.
(B) Schematic illustration of the stapedius-induced attenuation of sound reaching the
inner ear. The attenuation in the low-frequency region, AN, should correspond to the
difference between Na and Nb of (A). The attenuation in the high-frequency region is
denoted as AS. Note that a constant attenuation in either the entire low-frequency or the
entire high-frequency region is also not necessary for the working hypothesis.
-58-
Ma
JR (dB/dB)
m
C,
Mb
C
I
I
I
I
I
I
I
I
ed
0
Th
Nb
(A)
Na
Noise Level (dB)
L
rn
A
'O
o
--: O
o
0
AS
ANN
(B)
Ca 10
._
mO._
C-<C
-
-
Frequency
- 59 -
fiber's response to the tone of 40 dB (near the maximum size of the unmasking seen psychophysically, see Fig. 2.2); and (5) The importance of the growth-rate of the neuronal masking function
to this study can be seen in that it is the only factor in the working hypothesis that is neuronal
and is unique to each nerve fiber.
4.2 General Experimental Approach
The general experimental approach involved the following: (1) Activation of cat stapedius
muscle by the central nervous system was pharmacologically suppressed so that there would be
no "uncontrolled" muscle contractions interfering with and complicating the study (details are
given in Section 5.7). (2) Contractions of the stapedius muscle were produced by directly stimulating the muscle with electric current (see Fig. 4.1).* The major advantages of this method of
producing stapedius contractions (as compared with other methods such as the acoustic reflex)
were: (A) Stable contractions of the muscle could be produced at desired times with desired
strengths; (B) Since it was not necessary to use intense sounds to evoke stapedius contractions,
the working hypothesis could be tested at any desired sound levels, and desensitizing and damaging of the auditory system due to the use of intense sounds could be avoided. (C) From a signalflow point of view, as shown in Fig. 4.1, a difference between activating the stapedius with
intense sounds and with electric current is that in the first case the activation is under closed-loop
conditions (the activation depends on the sound level reaching the cochlea and in turn affects that
level), whereas in the second case the muscle is activated in an open-loop condition (the muscle
activation is independent of sound level). Since closed-loop feedback was not the focus of this
study, the use of the open-loop approach could avoid complications which might be present in
the closed-loop situation. Specifically, one kind of complication could come from interactions
between two feedback loops: the stapedius system and the olivocochlear efferent system, as
shown in Fig. 4.1. It is known that the olivocochlear efferent system can be activated by sounds
* The electric stimulation could stimulate either the muscle fibers or the nerve fibers that innervate the muscle or both.
Which actually happened is of little consequence for this study.
-60-
at moderate levels (Liberman & Brown, 1986) and that activation of this efferent system can have
an effect on the response of auditory-nerve fibers (Wiederhold & Kiang, 1970; Gifford & Guinan,
1983). The exact nature of any possible interactions between the stapedius system and the olivocochlear efferent system is not known. Once the stapedius loop is opened, however, whatever the
olivocochlear efferents may do to the auditory-nerve fiber responses for a given input to the
cochlea can, for the purpose of this study, be treated as part of the cochlear processing of the
input, as long as the process of electrically stimulating the stapedius does not cause a change in
the activation of the olivocochlear efferents (Control experiments on this issue are discussed in
Section 5.7). Another important reason for adopting an open-loop approach has been mentioned
in Chapter II, namely that the stapedius is probably active more often in the open-loop condition.
(3) Responses of auditory-nerve fibers to selected acoustic stimuli were recorded with and
without contractions of the stapedius muscle. Statistical measures of the "difference" between
neuronal responses to identically generated acoustic stimuli with and without stapedius contractions were used to characterize the effect of stapedius contractions on auditory-nerve fiber
responses. (4) The attenuation of the stimulus tone and the masking noise by contractions of the
stapedius muscle was assessed with measurements of the cochlear microphonic potential.
4.3 Specific Experimental Approaches
The first issue investigated was whether the attenuation of an acoustic stimulus by a given
state of stapedius contraction depends on the level of the stimulus, in the range of 0-90 dB SPL
at 0.5 kHz (the center frequency for the masking noise used) and 6 and 8 kHz (the signal frequencies used). The cochlear microphonic (CM) potential near the round-window of the cochlea
was measured at these frequencies as a function of the stimulus level with and without stapedius
contractions. The CM is a non-neural "analog" signal closely related to basilar membrane and
hair cell motion, and is almost linearly related to middle-ear output, the volume velocity of the
stapes footplate, for a large range of sound frequencies and levels (e.g., Dallos, 1984). When the
- 61 -
stapedius muscle is in its "resting" state, the velocity of the stapes footplate (see Fig. 4.1) is"
linearly related to the stimulus level at the tympanic membrane (Guinan & Peake, 1967); so is the
CM for a large range of sound frequencies and levels (e.g., Wever & Lawrence, 1954). When the
stapedius contracts, however, it was not known whether the footplate velocity would still be
linearly related to stimulus level at the tympanic membrane. The reasons for using the CM measurements to assess this linearity issue were the following: (1) The CM measurement was much
easier to make than direct measurement of footplate velocity; (2) Since the dimension of the footplate (a couple of millimeters for cat) is much smaller than the wavelength in cochlear fluid of
virtually all sounds audible to the cat, the footplate motion can be viewed as a point-source of
sound to the cochlea and thus the linear relation between the footplate velocity and the CM
should not be affected by stapedius contractions (i.e., tilting of the footplate should not matter,
and the effect of stapedius contractions should be restricted to footplate volume velocity); (3)
From a signal-flow point of view, since the CM potential occurs later than the footplate motion in
the chain of events following stimulation of the tympanic membrane (see Fig. 4.1), if the CM is
still linearly related to the level of stimulus at the tympanic membrane when the stapedius contracts then it can be viewed as a "sufficient condition" for footplate velocity also being linearly
related to the level of stimulus at the tympanic membrane.* If, given a state of stapedius contraction, the CM is indeed linearly related to stimulus level at the tympanic membrane for the level
and frequency range of interest, then the stapedius attenuation of acoustic stimuli is levelindependent, and what is known about the effect of stapedius contractions on acoustic transmission through the middle ear measured at one sound level (e.g., Pang & Peake, 1985) can be
applied to the whole range of sound levels employed in this study. If, on the other hand, the CM
turns out to be not linearly related to tympanic stimulus level for a given state of stapedius contraction, then the characterization of the effect of stapedius contraction will have to be a function
* As pointed out to me by W.T. Peake (personal communication), the only way for CM to be linearly related but foot-
plate velocity not linearly related to the level of stimulus at the tympanic membrane is another nonlinearity between
footplate velocity and CM that exactly "corrects" the nonlinearity between tympanic stimulus level and footplate velocity, for all frequencies at all levels; but this is highly unlikely.
~____ 111
----------
- 62 -
of the level of the sound used in the measurements.
At the level of single auditory-nerve fibers, rate-level functions with CF tone bursts were
measured first with and without stapedius contractions for fibers with CFs near 0.5, 6 and 8 kHz;
and any stapedius-induced difference in the rate-level functions was compared with the
stapedius-induced difference in the CM level functions measured at the same frequencies. For the
main body of the experiments, tone bursts at 6 and 8 kHz (the "signals") and continuous lowfrequency (0.3 kHz bandwidth centered at 0.5 kHz) masking noise were used as the primary
acoustic stimuli for all auditory-nerve fibers that responded to the tonal stimuli, regardless of their
CFs. The choice for the tone and noise frequencies was based on three reasons. First, these were
the frequencies used in the psychophysical experiments on the "unmasking" effect of stapedius
contractions (Fig. 2.2), the effect with which the result of this study was to be compared. Second,
the frequency region of the noise is within the frequency range where the effect of stapedius contractions on transmission is largest (Fig. 2.1). Third, since for the frequency region of these
tones there is little fine temporal information relating fiber discharges to individual cycles of the
stimulus waveform available in the discharge pattern of nerve fibers (Johnson, 1980), the
interpretation of the coding in the auditory nerve as resulting from rate information could be free
of the complications that would be present if some temporal information was also involved.
Rate-level functions from auditory-nerve fibers were measured for tone-burst stimuli with a background of continuous noise at various signal to noise ratios (including signal only and noise only
for control and statistical characterizations), with and without stapedius contractions. The measurements obtained without stapedius contractions were used to construct noise-masking functions.
The difference between the measurements with and without stapedius contractions provided information on any "un-masking" effect of the stapedius contractions. Such measured effect was then
compared with the quantitative prediction of the working hypothesis as a test of the hypothesis.
For the prediction part, the growth-rate (GR) was provided by the noise-masking function in the
range between attenuated and un-attenuated noise levels (GR=AT/AN, where AT was the
difference in tone levels for maintaining a constant rate response) obtained from the same fiber as
- 63 -
that the stapedius unmasking effect was obtained from, and the noise and signal attenuation (AN
and AS) due to stapedius contractions were from CM measurements as described at the beginning
of this section.
4.4 Statistical Measures for Rate-Response Threshold Detection
Statistical measures derivable from the sample mean and sample variance of the neuronal
discharge rate were used for "detecting" threshold response. Specifically, if the discharge rate is
denoted as X, then X is a random variable with conditional probability density function (PDF)
P(X/Sc), mean Me and standard deviation a, when stimulus condition S is presented. The "noise
only" stimulus condition was denoted as condition "n", and the "noise+tone" stimulus condition
as condition "n+t". It was operationally assumed that the neuronal discharge rate in the auditory
nerve could be approximately characterized as a normal (Gaussian) random variable or a variable
that is monotonically convertible to normal. It is known from detection theory that for normal
PDFs, two statistical parameters
d=
Mn+t- Mn
(1)
(Yn+t
and
r-
(2)
an+t
can uniquely characterize the detectability of the tonal signal by determining the "iso-sensitivity
curve" or "receiver operating characteristic (ROC)", that is, the relationship between the probability of detection, Pd, and the probability of false-alarm, Pf (Green & Swets, 1966; Durlach, 1968).
Here "detection" means that the response indicates S+t when S+t is presented, and "false-alarm"
means that the response indicates Sn+t when Sn is presented. Specifically, with two parameters Zd
and Zf that are positive monotonic transformations of Pd and Pf, respectively,* the relation
* P. ..
zP
lexp(-x2/2)dx
42-i ,.~
_·___1_·1__1_
111(__·_
111_·__I·
_II_
·_
_I
_II_
I_
-64-
between the transformed Pd and Pf becomes a -simple linear relation:
(3)
Zd = (r)(Zf)+ a-
As a numerical example, for the case of equal variance (r-l), a d value of 1 corresponds to a Pf
of 0.16 for a Pd of 0.5. Other than the reason based on the central limit theorem,* the Gaussian
approximation to neuronal discharge rate is consistent with experimental observations of the
spike-number distributions from cat auditory-nerve fibers (Teich & Khanna, 1985; see Fig. 3.2),
and is supported by a recent study of statistical properties of auditory-nerve fiber discharges (Relkin & Pelli, 1987) in that empirical measurement of the relationship between Zd and Zf yielded
approximately straight lines. For each combination of "noise only" and "noise+tone" situations, a
(d, r) pair, and therefore a ROC, can be obtained for each nerve fiber studied. Intuitively, the
statistic d can be viewed as a measure of the "separability" of P(X/Sn+t) from P(X/Sn), and therefore the "distinguishability" of Sn+t from Sn, in the sense that if X is scaled by l/on so that
P( X /Sn) has unit variance and a mean of MIa/n, P( X /Sn+t) has a variance of (n+t/On)
OIn
2
and a
OIn
mean of Mn,/cn, then the separation of the mean divided by the non-unity standard deviation is
M n+t _Mn
an
n+t
an
On
Mn+t - Mn
-
(
an+t
In other words, P(X/Sn) can always be viewed as having unit variance and the "effective" separation of P(X/Sn+t) from P(X/Sn) can be assessed by d: if an+t is larger than unity, then the distance
(M+ t - Mn) alone would over-measure the true separability, and the division of (Mn+t - Mn) by
o,+t provides a corrective measure.
To compare the effects of stapedius contractions on the masking by low-frequency noise
of auditory-nerve responses to high-frequency tones with the effects of stapedius contractions on
the psychophysical detection of high-frequency tones in low-frequency noise (Borg & Zakrisson,
1974), a correspondence between the psychophysical detection "threshold" and the physiological
response "threshold" needs to be established. Since d is a measure of signal detectability and is
* The central limit theorem states that for a wide class of random processes, the distribution of the sample averages of
the random variables approaches normal as the sample size increases.
I
_
____ _
_
_
- 65 -
independent of response criterion, the comparison of physiological and psychophysical response
threshold can be based on measuring
in both cases. The average Pf in a behavioral task of
detecting tones in noise by the cat has been reported to be about 0.11 (Costalupes, 1983) and
0.15 (Pickles, 1979) for a Pd of 0.5, corresponding to a d of 1.2 and 1.0, respectively, for the
equal-variance case. Since, as concluded by Costalupes (1983), the detection performance for
tones in noise by cats is similar to that by humans, to a first order approximation, a
of unity
can be associated with the behavioral detection threshold for tones in noise for both cats and
humans. For the equal-variance case, a
of unity corresponds to a percentage correct (the area
under the ROC curve) of 75% in a two-alternative forced-choice detection task (Green & Swets,
1966). The auditory-nerve response threshold for a tone in noise was accordingly measured in
terms of the tone level required to produce a separation of the PDF with tone+noise from the
PDF with noise only (or whatever background) which corresponds to a
of unity. Masking of
auditory-nerve responses was then measured in terms of the elevation of this "detection" threshold, and the effect of stapedius "unmasking" on auditory-nerve responses was measured in terms
of the difference in tonal stimulus levels for maintaining a unity
value, with and without sta-
pedius contractions (for each given noise level).*
4.5 Concluding Remarks
The masking of auditory-nerve responses to high-frequency tones by low-frequency noise
was chosen as a "substrate" for studying the effects of stapedius contractions on auditory-nerve
fiber responses for the following reasons: (1) Masking is a general phenomenon in auditory-nerve
responses concerning almost all frequencies and intensities of acoustic stimuli. It is a complicated
nonlinear feature of the auditory response involving interactions of auditory stimuli in different
* Not knowing the number of auditory-nerve fibers involved in the psychophysical detection task and the way informa-
tion from these fibers is combined by the central nervous system, the above discussion is directed at the responses of individual nerve fibers. As will be seen in Chapter VII, however, the unmasking effects seen in individual nerve fibers
with several measurs of masking (and unmasking) are comparable in magnitude to those seen in the psychophysical
study.
1
11_ -1-111_.-111_·
1--_-----···-_··1^-·
.11^-1....._II-I--.-.^l-LII
_LI1
_C·__I____II_ 1_
II-^1----C.*-IIYI--L·1_III·IIIXIII-_lll·_--
·_ C_·l
I
- 66 -
frequency regions, and a better knowledge of masking is thus of fundamental importance to the
understanding of the auditory system. (2) With respect to frequency characteristics, auditory
masking and stapedius effect both have an asymmetry of the same polarity in that low-frequency
sounds mask high-frequency sounds more powerfully than the reverse and that stapedius contractions attenuate low-frequency sounds more than high-frequency sounds (possible consequences of
such similarity of asymmetry were discussed in Section 2.3.2). (3) There are data in the literature
on the effects of stapedius contractions on psychophysical responses to high-frequency tones
masked by low-frequency noise (Borg & Zakrisson, 1974).
One issue that requires a summary note is the cross-species comparison of the effects of
stapedius contractions on human psychophysical performance and on cat physiological responses.
Since there are no data existing on the effects of stapedius contractions on cat behavioral
responses and since physiological studies of the kind in this thesis can not currently be carried
out in humans, the cross-species comparison is presently the best that one can do. The validity of
such a comparison can be justified on the following basis: (1) The frequency dependence and
magnitude of middle-ear transmission changes produced by stapedius contractions are similar in
cats and humans (Mdller, 1965; Borg, 1968; Teig, 1973; Rabinowitz, 1977; Pang & Peake,
1985). (2) Psychophysical masking of tones by noise is similar for cats and humans with respect
to the growth-rate of masking (Watson, 1963) and the critical bandwidth (Costalupes, 1983). (3)
The effect of filtering human speech signals with cat stapedius contractions on the discrimination
scores for the speech by human listeners (Mahoney et al., 1979; see Section 2.3.2 for details) is
similar to the effect of filtering with human stapedius contractions on speech discrimination
scores (Borg & Zakrisson, 1975b; Dorman et al., 1986). (4) As mentioned at the end of the last
section, the information measure ()
chosen as an interface for comparing psychoacoustic and
physiological responses is independent of detection criteria and in that sense independent of the
subject's being cat or human for psychophysical experiments (if one assumes that the characteristics of the internal noise in the human and cat auditory system are similar).
m
0l
_
_______
______
- 67 -
Chapter V
Experimental Methods
The basic methods for animal preparation, generation of acoustic stimuli and recording of
responses from the cochlea and auditory-nerve fibers are similar to those described by
Kiang et al. (1965). The primary additions for the purpose of this study are the method for electric stimulation of the stapedius muscle and the use of statistical measures for characterization of
auditory-nerve fiber response threshold. The following sections give a general description of the
basic experimental methods, with particular attention to the methods developed for this study.
5.1 Animal Preparation
Thirty-two adult cats were used in the experiments for this thesis research. These animals
were without known pathological conditions and their external and middle ears were free from
apparent diseases. The anesthesia used for surgery and experiments was Dial-Urethane injected
intraperitoneally at an initial dosage of 0.75 milliliter per kilogram of body weight, with each milliliter containing 100 mg Dial (diallylbarbituric acid), 400 mg Urethane and 400 mg monoethylurea. Additional dosage of anesthesia (typically at one-tenth of the initial dosage) was given as
needed. A cannula was inserted into the trachea and a rectal thermometer was used to monitor the
animal's body temperature, which was maintained at 37 to 39 degrees centigrade via the control
of room temperature and/or the use of a heating pad. The external ear canal was cut near the
tympanic membrane for the insertion of an acoustic assembly. The bulla was opened and the
bony septum removed to make the round-window accessible and the incudo-stapedial joint visible. The tendon of the tensor tympani muscle was cut for every experiment so that contraction of
the tensor tympani muscle could have no effect on acoustic transmission through the middle ear.
The auditory nerve was exposed as it emerges from the internal auditory meatus by first opening
the posterior fossa of the skull and then gently retracting the cerebellum. In one experiment
__1_11_
- 68 -
(XDP89) the olivocochlear efferent nerve was cut near the Bundle of Oort after a part of the temporal bone dorsal to the auditory nerve in the interior auditory meatus was drilled and chipped
away.
5.2 Generation of Acoustic Stimuli
An acoustic assembly which housed an one-inch condenser earphone (Bruel & Kjaer
4144) as the sound source* and a quarter-inch condenser microphone (B & K 4136) for sound
monitoring was fitted into the central end of the cut ear canal. Calibration of the acoustic system
for generation of sinusoidal stimuli was essentially the same as that described by Weiss and
Peake (1972), resulting in a transformation between the voltage input to the earphone and the
sound pressure near the tympanic membrane as a function of frequency (an example was given
by curve(a) in Fig. 3.4). Unless otherwise stated, all sound levels indicated in the experimental
part of this thesis refer to the sound pressure near the tympanic membrane, calculated from the
voltage input to the earphone and the pre-measured transformation characteristic (re-measuring of
the characteristic during stapedius contractions was not done; for more details on the effects of
this see Appendix to the thesis). A computer-controlled system (Weiss et al., 1969) was used to
control stimulus presentation and response measurement (see Fig. 5.1), generating stimuli in
forms of clicks and sinusoidal bursts of controllable frequency, duration, level, repetition period
and rise-fall time. The noise stimulus used in this study was narrow-band low-frequency noise of
300 Hz bandwidth centered at 500 Hz, obtained by bandpass filtering (350-650 Hz, with an SKL
Model 302 Variable Electronic Filter) broadband noise (generated by a General Radio 1382 Random Noise Generator). The RMS value of the noise magnitude near the tympanic membrane was
measured with the quarter-inch microphone and a B & K 2608 Measuring Amplifier following
initial calibration of the acoustic system for tonal signals. The experiment was carried out with
the animal inside a low-noise chamber (VWr et al., 1975) which provided electric and magnetic
* The total harmonic distortion of sinusoidal sound generated by the sound source is less than 0.01% at 100 dB SPL
Therefore with the sound levels used in this study (no more than 100 dB SPL), distortion is considered negligible.
1___11
_
__
_
- 69 -
Figure 5.1
Block diagram of the experimental apparatus.
.
-70-
11
Programmable
Attenuators
- 71 -
shielding and at least 80 dB of acoustic isolation, and all control functions were performed from
outside the chamber.
5.3 Recording and Measurement of Responses
The cochlear microphonic potential and the auditory-nerve compound action potential
were recorded with a wire electrode on the cochlear bone near the round-window of the cochlea,
and a ground electrode connected to the head-holder. The microphonic response to a sinusoidal
stimulus was first amplified 2500x, then bandpass filtered at a quarter-octave bandwidth centered
at the stimulus frequency (with a Rockland Systems Model 751A Brickwall Filter with postfiltering gain set at 10 dB), and finally filtered by a tracking filter (Spectral Dynamics SD1O1B)
at 2 Hz bandwidth centered at the stimulus frequency. The signal was then sent through a log
converter and a phase meter before it was sampled by a 12 bit A/D converter. The quarter-octave
bandwidth filtering was introduced to suppress contamination of the microphonic signal by electric stimulation of the stapedius. The compound action potential response to short tone-pips (usually less than 3 ms in duration) or clicks was recorded and measured in the same way as that
described by Gifford and Guinan (1987).
Auditory-nerve fiber responses were recorded with glass micropipettes filled with 1 M
KCl solution. The resistance of the micropipet electrode was in the range of 40-80 megohm. The
microelectrode was inserted into the auditory nerve near the internal auditory meatus with a
micromanipulator that could be remotely controlled. The voltage between the microelectrode and
the grounded head-holder was pre-amplified with a unity-gain amplifier with high input
impedance and capacitance compensation (Instrumentation Laboratory PICO-metric amplifier),
and bandpass filtered at 300-3000 Hz to reduce low-frequency baseline fluctuations and suppress
contamination of the signal by electric stimulation of the stapedius. A reference wire electrode
was placed near the auditory nerve to record approximately the same artifact waveform as that
recorded by the microelectrode resulting from electric stimulation of the stapedius. The
- 72 -
waveforms from the microelectrode and the reference electrode were subtracted in a differential
amplifier with adjustable gain and phase for each input to reduce the electric artifact in the
auditory-nerve potentials. An aluminium-foil shield was often placed between the microelectrode
and the electrodes for stimulating the stapedius to further reduce contamination in the auditorynerve signals. With these precautions, the magnitude of the artifact from electric stimulation of
the stapedius sensed by the auditory-nerve recording electrode could be kept to less than 10% of
the magnitude of an action potential (neuronal spike), except at the highest electric stimulation
levels. With the highest levels of electric stimulation of the stapedius (those necessary to produce
the maximum muscle contraction), the shock artifact in the microelectrode recording was usually
too large to be satisfactorily cancelled (tried in seven preparations), and consequently only the
shock-induced attenuation of sound was measured (with cochlear microphonic potentials) at such
shock levels.
5.4 Electric Stimulation of the Stapedius Muscle
Two configurations of electrodes were used for stimulating the stapedius muscle. With the
first configuration, the dorso-lateral surface of the stapedius muscle was exposed by drilling a
small hole through the temporal bone posterior and dorsal to the external ear canal (Kobler, personal communication), and a pair of ball-tipped platinum-wire electrodes was placed on the surface of the muscle. With the second configuration, a pair of ball-tipped platinum-wire electrodes
were placed in the notch which abuts the stapedius and opens into the bulla just dorsal and lateral
to the round-window. The advantage of the first configuration was that a direct contact of the
electrodes and the muscle could always be maintained, therefore the current level required for
stimulating the muscle might be less than that with the second configuration where, because the
muscle could not be seen, direct contact of the electrodes with the muscle was not guaranteed.
The main disadvantage of the first approach was that drilling through the temporal bone, particularly through the external semicircular canal, often resulted in significant loss in the responsive-
- 73 -
ness of the cochlea (up to 20 dB at all frequencies), and such loss could be progressive if the
fluid leakage from the broken semicircular canal was not completely stopped. In practice, the
"notch approach" was usually tried first; if the current threshold for muscle contraction was too
high such that it produced untolerable artifact (in the form of either bodily animal motion or electric artifact too large to be cancelled in either microelectrode or round-window recording), then
the muscle-exposure approach was used. If exposure of the muscle resulted in hearing loss in
excess of 15 dB, the whole process would be re-started on the other ear. In no experiment did the
above efforts fail on both sides.
The use of ball-tipped platinum-wire electrodes was found to be important in that sharptipped electrodes produced damage to the muscle and/or its blood supply when there was relative
motion between the electrodes and the muscle (when the muscle contracted), resulting in progressive decrease in the responsiveness of the muscle. The use of platinum wire also helped to stabilize the effect of shock current by presumably stabilizing the junction-potential at the metal-tissue
interface. The use of ball-tipped electrodes was also superior to the use of cotton balls between
the sharp tips of the electrodes and the muscle, as was used in earlier experiments, in that the
cotton balls often presented a variable impedance in the circuitry when their moisture content
changed. With ball-tipped platinum electrodes a stable relationship between current intensity and
stapes-head displacement could be maintained for up to 24 hours or longer. The use of closely
spaced (1-2 mm separation) bipolar electrodes helped to restrict the spread of shock current and
therefore the shock artifact.
Two waveforms were used for muscle stimulation: sinusoid and pulse train. The general
consideration on the choice of stimulating waveform was that for a given effectiveness as a
stimulus, the waveform should have minimal energy content in the audio-frequency regions of
interest to this study, and in the frequency region of nerve-fiber discharge waveforms (for
minimal stimulus artifact). The "effectiveness" requirement means that the stimulating waveform
should be able to produce steady (fused) muscle contraction of desired strength reached with a
relatively short (e.g., 100 ms) rising time (a consideration important in the timing for recording
- 74 -
from erve fibers). In light of the general mammalian muscle dynamics (e.g., McMahon, 1984)
and the average maximum discharge rate of cat stapedius motoneurons (Kobler, personal communication), a periodic waveform with fundamental frequency above 60 Hz was required for both
fused contraction and maximum contraction strength. Of the two basic options of sinusoid and
pulse train, pulse stimuli had a lower (typically by a factor of 2) peak current threshold for contraction, presumably because of the sharp edges of the pulses. A low-frequency sinusoid had,
however, much less energy content in the frequency region of neuronal discharge waveforms,
therefore its artifact in that frequency region might be easier to deal with than that generated by
pulse shocks, even if the overall current level with sinusoid was higher. It was difficult to predict
which waveform would result in less artifact because the artifact varied with the contact between
the electrodes and the muscle and with the relative positions between the shock and recording
electrodes. In practice both waveforms were tried in most experiments and that producing smaller
artifact was chosen.
The shock parameters that produced the lowest current threshold for muscle contraction
with pulse stimulation were found to be 0.3 ms for the width of each monopolar square pulse,
and 5 ms for the interval between pulses. For sinusoidal stimulation, the current threshold
decreased as the frequency increased from 50 to 200 Hz (see Fig. 5.2). However, a sinusoidal
shock to the stapedius could, at high intensities, produce an undesirable sinusoidal sound which
could contaminate the microphonic response to a low-frequency tone (e.g., at 500 Hz) and/or
have a masking effect on neuronal responses. It was therefore important to keep a spectral
separation between the shock frequency and the frequency region of relatively low hearing threshold (the hearing threshold also decreases from 50 to 200 Hz). In practice, 100 Hz was usually
chosen for shock sinusoid as a compromise and it can be seen in the last section of this chapter
that the sound generated by sinusoidal shock at 100 Hz did not pose any significant problem.*
The equipment for generating shock waveforms was the same as that for generating
* With
good contact between the shock electrodes and the stapedius muscle, an even lower frequency, such as 67 Hz,
was sometimes used.
- 75 -
Figure 5.2
Threshold shock level versus shock frequency for stimulation of the stapedius muscle with sinusoidal waveforms to produce a visually just-detectable stapes-head displacement. Stimuli at each frequency were shock bursts of 200 ms with a repetition period of
3 seconds. The two upward arrows at 15 and 20 kHz indicate that at these intensities no
stapes-head displacement was detected. The arrow at 100 Hz shows the usual choice of
stimulus frequency (see text for details).
-76-
I
I
I
I
I
I
I
I
I
10XDP74-L
m
8-
.,
-'
C)
r
0
6-
4C
0
2-
0-
I
I
50
I
T
I
I
I
I
75 100 125 150 200
Shock Frequency (Hz)
"-
----
I
15K
I
I
20K
- 77 -
acoustic clicks and tone bursts. The shock waveforms were coupled to the shock electrodes
through an isolation transformer, which eliminated the DC component in the monopolar pulses
(the DC component could produce polarization of the electrodes at the electrode-muscle interface
and elevate shock current threshold). The "square" pulses were low-pass filtered first to reduce
the high-frequency components which would be most easily sensed by the auditory-nerve recording microelectrode as artifact and be difficult to cancel. The shock current was monitored with an
inductively-coupled calibrated current probe (Tektronix P6016) whose output was amplified and
displayed on an oscilloscope.
Shock current threshold was determined by delivering 200 ms shock trains at 3-second
intervals and observing a visually-just-detectable displacement of the stapes head (about 2 gm in
the direction of the stapedius tendon, see Pang & Peake, 1985) produced by contraction of the
stapedius. The displacement of the stapes head was observed and measured through a dissection
microscope with an eyepiece micrometer. A mirror was used so that observations of the stapeshead displacement could be made from a dorsal direction, which was important because the
recording of auditory-nerve fiber activity required a dorsal approach. When the stapedius muscle
was exposed, a visually-just-detectable twitch of the muscle corresponded to the same current
level (within 5%) that produced a just-detectable stapes-head displacement. A typical shock
current threshold was 0.1 mA (peak-to-peak) for a sinusoid waveform.
The stapedius muscle was stimulated with one of two timing modes: continuous or bursts
of shocks. After the onset of a suprathreshold shock train, the effect of the stapedius contraction
would quickly reach a plateau level (the time course of the onset of stapedius effect is described
at the beginning of Chapter VII), and be maintained at that level for a period of 4-40 seconds,
varying from animal to animal. After this period, the effect would decrease, even though the
shock current remained constant (see Fig. 5.3). The duration of the constant effect period did not
seem to be a strong function of the shock-current intensity (Fig. 5.4). After the termination of a
continuous shock train that lasted a few seconds or longer (e.g., 5 seconds), the stapedius would
remain in a "fatigued" state for a period of up to 4 minutes before it could be stimulated to
- 78 -
Figure 5.3
Stability and repeatability of stapedius contraction under continuous sinusoidal
stimulation at 100 Hz. The figure shows the RMS magnitude of cochlear-microphonic
response to a 70 dB SPL 562 Hz tone (the CM recording was first high-pass filtered at
460 Hz and then narrow-band filtered with a bandwidth of 2 Hz and center-frequency of
562 Hz). When a continuous electric stimulation to the stapedius was turned on ("First
shock on"), there was a reduction of about 18 dB in the cochlear-microphonic response
to the tone, and this reduction was stable for about 17 seconds before the muscle began
to "adapt" or "fatigue". All measurements of the effects of stapedius contractions were
made within the "stable" period whenever continuous muscle stimulation was used. A
second stimulation to the stapedius was delivered 23 seconds after the first stimulation
ended and produced little reduction in the CMP. In general, a "rest" period of up to 4
minutes was required after one continuous (lasting more than a few seconds) stimulation
before the same effect could be repeated with another continuous stimulation of the same
current intensity. (XDP74-L).
-79-
Tone on
ri
·_
Secon
h rst
shock on
e c n cl
I~
~
a
shock on
-
.
!
-
RMS
Cochlear
-
-
I
1ii
.4 = 1III
L_ L .a
I t
I
i
I
]:]- I;
;7FT]
]
} 3!/I
-_
i!
-i I
r ;-
Microphoni
Potential
*~~~~~
_
Measur!
i
IF
I
I I
-1
'
1
1,
11
I
I
.
.
r
.
I
i--
7-i
..
!- I.
m
i__i
m
I
------ r-
L__ L---
I
.W-
I
-I - -1
' -
. l.
!
_
a
_
i!
1__
11
m
ILU
.
i
It
i
.
in
interval
[
-9
!
4
:-= il[ ,~
n---B
7
.
m
i
)
I
I
I
I1
10 Isec
Time
p
U
- 80 -
Figure 5.4
The relationship between the stapedius shock current intensity and the stable contraction period of the stapedius muscle observed in one cat ear. The electric shock was a
continuous 67 Hz sinusoid. The "stable contraction period" is defined in the same way as
the "measuring interval" in Fig. 5.3, i.e., as the constant effect duration of the stapedius
contraction measured by recording the time course of the stapedius-produced attenuation
of the cochlear microphonic response to a low-frequency test tone (550 Hz at
65 dB SPL). The points were measured in random order and a sufficiently long muscle"recovering" period (longer than 5 minutes) was given after the measurement of each
point. In the figure labels, "int." stands for "intercept" and "corr." for "correlation". The
weak relationship between the stable contraction period and shock current intensity is
typical of all nine animals where such measurements were made.
0
-81-
I I I I I I I I II I
30-
II
I I I
II I
I I I I I I I
I I I
Linear regression: slope=0.51, int.=15.73, corr.=0.62
XDP6
o
0
20c
o
0
Q
Oo
-
C
-
-
10-
o
cU
0O
O
0
o0
0
O
0
0I
-30
I II II
iI
I II
-20
I I
I
I I I
-10
I I I
Stapedius Shock Current (dB re. 1mA p-p)
I
0
- 82 -
produce another effect f the same magnitude with the same current intensity (i.e., without such a
"rest" period the effect produced by the same stimulus current would be less than that in the previous stimulation, see Fig. 5.3). The duration of the "rest" period required for a full recovery of
muscle excitability increased in general with the duration of the previous stimulation, with 4
minutes being an upper limit. Such decrease in shock effect and muscle excitability was not
observed when the shock train was only left on for 300 ms or less and the shock repetition
period was 1 second or longer (i.e., with a repeated "burst" stimulation mode). In practice, burst
mode stimulation was used most of the time in. the measurement of auditory-nerve fiber responses
when acoustic stimulation was also presented in a repeated burst mode (as with the measurement
of auditory-nerve fiber rate-level functions), and the continuous mode of muscle stimulation was
used otherwise (as with the measurement of suprathreshold stapes-head displacement and the
measurement of auditory-nerve fiber tuning curves where the total number of stimulus presentations was not known a priori). When the continuous mode of muscle stimulation was used, all
measurements were made during the constant-effect period.
The effects of shocks to the stapedius (in the form of stapes-head displacement, such as
shown in Fig. 5.5, or attenuation of sound, such as shown in Fig. 5.6) usually increased monotonically with shock current level. A typical maximum stapes-head displacement produced with
electric stimulation of the stapedius was approximately one-tenth the diameter of the stapes head,
or approximately 50-55 gm. In no experiment did the stapedius muscle pull the stapes out of the
oval-window or pull apart the incudo-stapedial joint, even when the shock current was intense
enough to make the pinna and neck muscle twitch.
5.5 Experimental Procedures and Paradigms
At the beginning of each experiment, the cochlear response threshold was determined by
measuring the stimulus level required to elicit a criterion magnitude N 1 (usually 1.5 .v baselineto-negative peak) in the auditory-nerve compound action potential waveform in response to short
- 83 -
Figure 5.5
The relationship between the stapedius shock current intensity and the stapes-head
displacement (in gm) along the direction of the stapedius tendon, observed in five cats.
Each point is the average of six measurements. The electric shock was continuous
sinusoidal current of 67 Hz and all measurements were made during the "stable contraction" period (see Fig. 5.3 for definition). The current intensity was changed in 1 dB
steps. The curves were translated along the horizontal axis by arbitrary amount to avoid
overlap.
-84-
1
I
4
fr%#-1
I uU-
E
=
c
&}
L
1
i
i
I
A- XDP8
oo80- oo-
XDP10
XDP12
XDP13
XDP17
z~n
.5
40Ia-
a)
20t
,
n
U I
0
i
20
Stapedius Shock Current (dB re. arbitrary unit)
40
- 85 -
Figure 5.6
Cochlear-microphonic potential (CP) response to a continuous 3162 Hz tone of
about 90 dB SPL as a function of (stapedius) muscle-stimulation level (MSL) before and
after cutting of the stapedius tendon. The stapedius muscle was stimulated with
sinusoidal current at 100 Hz. When the stapedius tendon was intact, increasing MSL
reduced ICPI; with the tendon cut, MSL had no effect on ICPI. SHD stands for stapeshead displacement. The axis at the top corresponds to measurements made before the
tendon cutting. (XDP17-L).
-86-
SHD ( m)
25 38 47
01
&0:-605040m
0
013_
3 2 143
1
9 34145
26 42
TENDON CUT
30 20 10-
TENDON
INTACT
!~f
91.9dB
O- 3162Hz,
IIIl i1 1i
1 1 I I III
-25 -20 - -15 -10 -5
II Ii1 I I I
0
5
MSL (dB re 6.2 V)
- 87 -
tone-pips (less than 3 ms in duration) in the frequency region of 0.5-16 kHz. An ear was not
used if its cochlear response threshold was 20 dB or more above normal* in the frequency
regions of concern to this study. This "audiogram" measurement was made from time to time
throughout an experiment, especially following surgical manipulations (exposure of the stapedius,
etc.). All data included in this thesis are from the ears whose cochlear response threshold at the
end of data collection remained within 15 dB of the level at the beginning of data collection.
In early experiments, 1 kHz tone bursts of 200 ms duration repeated once per second were
delivered binaurally at 110 or 115 dB SPL, and the stapes head was watched to see whether there
was any residual acoustic reflex with Dial anesthesia, as a check on the pharmacological blockage
of stapedius activation by the central nervous system.** Since no stapes-head displacement was
observed after six consecutive experiments with observations made both at the beginning and
near the end of the experiment, it was concluded that with the dosage used, Dial anesthesia provided effective blockage of the stapedius activation by the central nervous system.
The auditory nerve was usually exposed after the stapedius shock electrodes had been
placed and shock current threshold determined. Acoustic clicks and sometimes broadband noise
bursts were used as search stimuli as the microelectrode was advanced inside the auditory nerve.
Once a fiber was isolated for recording, a tuning curve was measured with an automated procedure (Kiang et al., 1970; Liberman, 1978), which set the criterion for threshold response as an
increase of approximately 20 spikes/sec in mean discharge rate over the background rate (Rose,
1985). The tonal stimuli used for tuning-curve measurements were tone bursts of 50 ms duration
presented at 10 bursts/sec and the tuning curve was measured with a frequency resolution of 100
points per decade of frequency range and a sound-level step size of 0.75 dB. The tuning curve
provided a measure of the characteristic frequency (CF) and the response threshold at the CF in
terms of the tone level at the tympanic membrane. If the fiber responded to 6 or 8 kHz tones at a
level below 75 dB SPL, the fiber was "sampled". The sampling of a fiber typically included
* Here "normal" means an approximate average of the cochlear response threshold from over thirty ears of early experiments.
**One kilo-Hertz is the most effective frequency for activating stapedius motoneurons in the cat (Kobler et al., 1987).
-
88 -
measurements of rate-level functions in quiet and in several background noise levels, with and
without shocks to the stapedius muscle. All rate-level functions were, unless stated otherwise,
measured with 6 or 8 kHz tones, starting near the threshold for the tonal signal with the tonal
signal increasing in level by 5 or 10 dB steps. The maximum tone level used in an experiment
was 100 dB SPL. The rate-level functions were measured with tone bursts of 100 ms duration
(2.5 ms rise and fall time) presented once per second, and each datum point was the averaged
mean-rate over at least 10 presentations of the stimulus. The mean rate for each tone-burst
presentation was computed from the spike count in the window 80 ms long starting at 20 ms
after the onset of the tone burst so that an approximately "steady-state" rate response was sampled (Costalupes, 1985). The sample-variance of the rate response was also measured as an estimate of the variance of the rate distribution. The masking noise used was always narrow-band
low-frequency noise of 300 Hz bandwidth centered at 500 Hz, in the range from 50 to
100 dB SPL. The noise was turned on at least 15 seconds prior to presentation of tone signals
and was left on continuously through the measurement so that a steady-state of noise-evoked
activity was obtained (Costalupes et al., 1984). The need for measuring a noise masking function
was the reason to set the fiber-sampling criterion at 75 dB SPL tone level because if a fiber
barely responded to a 90 dB SPL tone of 8 kHz and the maximum tone level used was
100 dB SPL then the measuring of a masking function would be very difficult. When burst-mode
stimulation of the stapedius muscle was used, each shock burst was 200 ms in duration and
delivered 120 ms prior to the measurement of auditory-nerve fiber discharges so that the effect of
stapedius contraction would have reached a steady state (details in Fig. 7.1). The 1-second repetition period of stimulus presentation in rate-level function measurement was required for the
maintenance of stapedius excitability. The rate-level functions with shocks to the stapedius were
usually measured first among all rate-level functions measured from a fiber for best spike-toartifact ratio because the recorded neuronal spike size would usually decrease with time. The
sample-mean and variance of the discharge rate were also measured when no tonal signal was
presented, giving measures of the spontaneous discharge rate and/or rate information with
- 89 -
background noise with and without shocks to the stapedius. After contact with the fiber was lost,
the shock-induced attenuations of the noise and of the tone were measured with cochlear microphonic level functions for the same shock current level as that used in the fiber measurement
(With ball-tipped platinum shock electrodes the effect of a constant shock current would usually
remain constant over hours.). The search for the next fiber would then start and the experiment
continued.
Attempts were made in two animals to sever the olivocochlear efferent nerve fibers. As a
confirmation of the severing, after the electrophysiology part of the experiment the cochlea was
perfused with a 20% solution of horseradish peroxidase (HRP) in 0.9% NaCl. After the cochlear
perfusion with HRP, the animal was maintained under anesthesia at 36-38 degrees centigrade for
approximately 20 hours, and it was then ventilated with carbogen and perfused via the aorta with
a buffered solution of paraformaldehyde and glutaraldehyde. The brainstem segment was then
dissected free and stored in 30% sucrose in 0.1M phosphate buffer for 24 hours. Eighty-micron
sections were cut on a freezing microtome in the transverse plane. Histochemical reactions for
HRP were done on free-floating sections and the sections were examined by light microscopy for
any labelling of olivocochlear neurons (Warr, 1975). The olivocochlear efferents were considered
completely severed only when no olivocochlear-neuron labelling was found in the brain after
HRP perfusion of the cochlea; such was the case with one animal (XDP89) but not the other.*
5.6 Data-Processing Schemes
All rate-level functions, tuning curves, PST histograms and cochlear-microphonic (CM)
level functions were, unless noted otherwise, digitally smoothed once by a three-point triangular
moving average (weight: 1/4, 1/2, 1/4), performed after the experiment. The effect of a masking
noise (or stapedius contraction) on rate-level functions was measured in terms of the difference in
* In the other animal, the total number of olivocochlear neuron labelling was comparable to normal (Warr, 1975;
Guinan, personal communication) and there was no detectable difference between the physiological data obtained from
this animal and those from animals without attempted severing of the olivocochlear efferents.
- 90-
the tone levels required to elicit a constant mean rate response with and without the masking
noise (or stapedius contraction), and this constant rate was usually chosen as the mean of the
spontaneous and saturated rate from the rate-level function in quiet (the mid-rate). The effect of a
masking noise (or stapedius contraction) on tuning curves was measured in terms of the
difference between the curves (in dB tone level) with and without the masking noise (or stapedius
contraction), at the frequency of interest. The stapedius-induced attenuations of sound were usually measured in this study in terms of the difference in the level of a tonal stimulus that produced a constant magnitude of cochlear microphonic response with and without shocks to the stapedius, and this constant magnitude was chosen to be well above the noise floor of CM
response.*
From each rate-level function and corresponding variance measurement, a
constructed with
= (Mn t - Mn)/Cat , where "M" stands for mean rate, ""
function was
for standard devia-
tion, "n" for noise and "t" for tone. A tone was considered "detectable" when the d value of an
auditory-nerve fiber's rate response reached unity, and the level of the tone at d=1 was considered to be the threshold level. Since the unity-d measure of threshold is free of any specific
rate-response criterion, it was used in this study as the standard measure of threshold detection.
The standard way in this study for measuring the effect of a masking noise (or stapedius contraction) on rate responses of auditory-nerve fibers to tones was to measure the difference in the tone
levels required for a unity-d threshold detection with and without the masking noise (or stapedius
contraction, e.g., see Fig. 7.3B).
The construction of the receiver operating characteristic (ROC), which describes the relationship between the probability of detection, Pd, and the probability of false-alarm, Pf, was based
on the measurements of d and r=o/o.+, which determine the relationship between Zd and Zf as
Zd
=
(r)(Zf) + d, where Zd and Zf are monotonically related to Pd and Pf, respectively. The effect
of stapedius contractions on the "detection performance" of a primary auditory neuron for a tone
* It can seen in Chapter VII that since the CM level functions with shocks to the stapedius were translations of the level
function without shock, the magnitude of stapedius effect does not depend on any particular choice of CM level as
response criterion.
-
91 -
in noise was assessed by the difference in ROC (e.g., the area under ROC, which measures the
percentage correct in a two-alternative forced-choice detection task) with and without the stapedius contraction (Background information was given in Section 4.4).
5.7 Controls for Artifacts
The primary artifacts that might contaminate measurements of auditory-nerve fiber
responses in this study were from electric stimulation of the stapedius muscle. Other than spread
of electric field to the recording microelectrode in the auditory nerve, an issue that has been
covered in Section 5.3, two more types of artifacts had to be considered: (1) electric stimulation
of the cochlea, and (2) sound generated by stapedius-induced motion of the stapes which might
travel into the cochlea. While contamination of the spike waveforms could be visually detected,
the control for these two types of artifacts required other measurements.
Electric stimulation of the cochlea includes the possibility of stimulating the hair cells, the
auditory-nerve fibers, the olivocochlear efferent nerve fibers, and combinations of the above. In
any case, what concerned this study was whether elements in the cochlea were electrically stimulated which produced a change in the activity of the auditory-nerve fibers that were of interests to
this study.* Figures 5.7 and 5.8 show the "effect" of sinusoid electric stimulation to the stapedius
on the spontaneous and tone-driven activities in two auditory-nerve fibers, one with a low CF and
the other with a high CF, in the form of PST histograms, rate-level functions, and tuning curves.
Figure 5.9 shows, for pulse-shock stimulation of the stapedius, measurements similar to those in
Fig. 5.7C & D for two fibers in different classes of CF and SR. It seems clear from these three
figures that there was no detectable auditory-nerve fiber activity produced by the electric stimulation of the stapedius region. This was the case in the eight experiments where this issue was
investigated. Thus the cochlea did not seem to be electrically stimulated as far as this study was
concerned.
* These were the fibers that responded to 6 or 8 kHz tones.
- 92 -
Figure 5.7
(A) PST histogram of a low-CF auditory-nerve fiber's synchronized response to 100 Hz
tone bursts of 500 ms duration at 60 dB SPL (10 dB above the fiber's response threshold
at 100 Hz). Arrows indicate the turning-on and -off of the tone burst and there are 50
discernible "cycles" of firing within this portion of the histogram. This panel shows that
if there was an effective 100 Hz stimulus to this fiber then the response should be readily
detectable with this kind of PST histogram measurement.
(B) Same as (A) except that the tone-burst waveform was used to stimulate the stapedius
to produce a 42 m stapes-head displacement instead of being delivered to the tympanic
membrane.
(C) PST histogram of a high-CF auditory-nerve fiber's "response" to sinusoidal shock
bursts of 100 Hz delivered to the stapedius. The shock bursts were phase-locked to the
histogram windows and produced a stapes-head displacement of 37 lim. Arrows indicate
the turning-on and -off of the shock burst. The number of stimulus presentations and the
binwidth of the histogram are the same as those in (A).
(D) Same as (C) except that (1) a continuous 6 kHz tone was presented to the tympanic
membrane to increase the background firing of the fiber for the detection of any "modulation" effect by the 100 Hz stimulus (i.e., even though the shock might not excite the
fiber directly), and (2) Flaxedil (a neuromuscular paralyzing agent) was applied to the
stapedius to prevent contraction (the same current level was used in (C) and (D)), so that
shock-related effects other than that of stapes-head displacement, if any, could be isolated.
._
-93-
600-
CF=0.27kHz; SR=106sp/sec
llll
1111111l l
1111111111111
I
1
IIIII111111111
(B)
XDP81-12
(A)
o t IIll
0IIlI
750
III 11
300~
Illl
I I
I II I I I I 1111111
llllll
(C)
CF=5.07kHz; SR=61sp/sec
XDP82-34
750
1
II
(D)
-
i
_
_
-E
3
o
IIll
IIII I ll lllllll
Time (ms)
Time (ms)
a)
o
I
-
.
AAId-A.I
I
Ilillllllllllllllllllll
o
t
Ij
ll 1
l11l111l
-
-
_=
UA,
E -
LO AiDI ju
(n
I
0
I I I I11l
A
0
I 1 1 I I"I I I
1
Time (ms)
II
500
rI I I I I I I
o
I Il Ill1 1 1 1
t
1
Time (ms)
500
-
94 -
Figure 5.8
Rate-level functions (A) and tuning curves (B) of a high-CF auditory-nerve fiber
with and without 100 Hz continuous sinusoidal shocks to the stapedius muscle. The
shock current had an intensity at which it could produce a stapes-head displacement of
37 rnm, but the stapedius muscle was paralyzed with Flaxedil to prevent contraction, so
that shock-related effects other than that of stapes-head displacement, if any, could be
isolated.
-95-
.0
XOP82-34; CF = 5.07 kHz; TH = -0.9 dB SPL; SP = 61 sp/sec
I
I
I
I
I
250a-
6 kHz Tone Only
x- Tone With Shocks
0
Q)
200 -
a,
CY
(A)
150-
a,
.-
rC
100-
50 I
I
10
20
t
30
I
I
40
50
I
60
Tone Level (dB SPL)
I
80-
m
_0
,
- I
III
I I
I
I I II
Thin curve: No shocks
Thick curve: With shocks
60 -
0
40-
-o
ED
U0
(B)
20-
o
0-
I
0.2
I
'
'
I I'
' I 'I I
2.0
Acoustic Frequency (kHz)
I
20
- 96-
Figure 5.9
PST histograms recorded from two auditory-nerve fibers with bursts of pulse shocks
to the stapedius muscle.
(A) PST histogram of a high-CF (7.59 kHz), high-SR (82 spikes/sec) fiber in response to
repeated bursts of pulse shocks to the stapedius. Each shock pulses was 0.3 ms in duration and the inter-pulse interval was 5 ms. Each burst of shock train was turned on
50 ms after the beginning of the counting window of the PST histogram and was of
200 ms duration, as indicated by the arrows. The shock produced a 33
m stapes-head
displacement. The histogram is the average of 25 measurements.
(B) Same as (A) except (1) that a continuous 8 kHz tone was presented to raise the
background discharge level for the detection of any "modulation" effect by the shock
bursts (i.e., even though the shocks might not excite the fiber directly); and (2) that Flaxedil (a neuromuscular paralyzing agent) was applied to the stapedius to prevent contraction with the same current stimulation as in (A), so that shock-related effects other than
that of stapes-head displacement, if any, could be isolated.
(C) PST histogram of a low-CF (0.55 kHz), medium-SR (4.6 spikes/sec) fiber in
response to pulse-shock bursts delivered to the stapedius region. Arrows indicate the
turning-on and -off of the shock bursts. The shocks were such that they produced a
stapes-head displacement of 41
m previously (e.g., see Fig. 7.1). With this recording,
however, the stapedius muscle was paralyzed with Flaxedil and a continuous 0.5 kHz
tone at 10 dB above the fiber's response threshold was presented to raise the background
discharge level for the detection of "modulation" effect of the shock bursts.
(D) Same as (C) except that the tone level waf..increased by 10 dB.
-97-
C,
a,
8kHz Tone
dT(8kHz)=O
Pulse Shocks;
25 Repetitions;
SR=82sp/sec
CF=7.59kHz;
XDP83-182
400
o
4O (A)
_
_(B)
c0
C._
5l
I I I
I I I I
I
I II I
I I
Time (s)
ot 1
I
I
300
au
Q
00
c a
=
I
I
I
I
I
W
E0
I
I
I
II
Time (ms
Time (s)
II
I
I
I
I
I
- (D)
_
_
rhLe
0C
III
I
32dB SPL 0.5kHz Tone
dT(O.5kHz)=O
25 Repetitions;
(C)^
b)
300
Time (ms)
TH=12dB SPL
CF=0.55kHz;
XDP84-117
SR=4.6sp/sec;
22dB SPL 0.5kHz Tone
L
ciO
I
I I
I I1
II
(ms)
Tie
Time (ms)
Q'
I I I
I I I I I
I
50
I
.
0
.
0 A
I
.
.
I
.
.
I I
,
.
.
.
r I I
I
Time (ms)
.
.
I1
500
- 98 -
There could be two kinds of sound detectable at the tympanic membrane associated with
the stapes motion produced by sinusoid electric stimulation of the stapedius muscle (Fig. 5.10A).
One was a predominantly low-frequency transient sound lasting typically less than 100 ms,
thought to be generated by the step displacement of the stapes head following the onset and
offset of muscle stimulation, respectively. This transient sound had a magnitude typically less
than 45 dB SPL measured in the ear canal and had not been observed to evoke activity in nerve
fibers with CFs above 0.5 kHz (it did occasionally evoke transient activity in nerve fibers with
CFs less than 0.2 kHz). Recordings of auditory-nerve discharges were always made with a delay
of at least 100 or 120 ms relative to the onset of a shock train to avoid this transient sound (no
post-effect had been observed after this not-so-intense transient sound). The duration of such transient sound with pulse stimulation was comparable or less than that with sinusoid stimulation
(Fig. 5.10B). The other sound was a sustained sound at the frequency of the sinusoid shock
current (0.1 kHz), and had a magnitude typically less than 20 dB SPL measured in the ear canal
(the second and third harmonic of this sustained sound was at least 14 and 20 dB smaller in manitude than the fundamental, respectively, and were therefore considered negligible). As shown in
Fig. 5.7C, such a sound did not evoke activity by itself in high-CF fibers that were of concern to
this study. In addition, Figure 5.11 shows for two high-CF fibers of high- and medium-SR,
respectively, that their activities were not affected by 0.1 kHz tones of 70 dB SPL. The nervefiber activities measured include the spontaneous and that driven by 6 and 8 kHz tones, as well
as that driven by the noise used in this study (0.3 kHz bandwidth centered at 0.5 kHz). The effect
of the less than 20 dB SPL sustained sound produced by sinusoid stimulation of the stapedius
was therefore considered negligible in this study. (Even though with respect to the sound pressure reaching the inner ear, a stapedius-produced sound that measures 20 dB SPL in the ear canal
may be more intense than a 20 dB SPL sound delivered to the tympanic membrane from an
external source, there is no known reason to expect that the difference can be on the order of
70-20=50 dB. In fact a study by Matthews (1983) indicates that the difference at 0.1 kHz is less
than 10 dB.) With pulse-shock stimulation of the stapedius muscle, no sustained sound was
- 99 -
Figure 5.10
Sound waveforms measured near the tympanic membrane following 200 ms bursts
of electric shocks to the stapedius muscle.
(A) Sound produced by 100 Hz sinusoidal electric stimulation of the stapedius. The
lower trace with arrows indicates the timing of shock bursts. The horizontal dashed line
indicates the base line of the output of the measuring microphone in the absence of any
stimulation. The waveform was high-pass filtered at 22 Hz. A peak-to-peak magnitude of
one vertical division corresponds to a sound pressure of 283 pPascals, that is 200 gIPascals RMS or 20 dB SPL (therefore the waveform shown after 100 ms had a magnitude
less than 20 dB SPL).
There were 20 discernible cycles in the sound waveform
corresponding to the 200 ms duration of the 100 Hz electric stimulation. The waveform
shown was the average of eight repeated stimulations and was sampled at an equal interval of 0.4 ms.
(B) Similar to (A) except that the sound was produced by pulse shocks to the stapedius
(in a different ear). The stapes-head displacement was comparable to that in (A) (approximately 50 gm). Notice the absence of "sustained sound" with pulse shocks (5 ms interpulse interval, 0.3 ms pulse width).
-100-
I
",
L.
20dB SPL
(A)
o
c
.-
I
XDP69-R
(-
0-
C
n
-
|~~~
A
I
-u
0
I
00-
1
I
100
i
200
300
400
I
1
Time (msec)
I
I
i
I 2OdS SPL
XDP88-L
o-
(n
(B)
c
00
L
U,
0
a;
C
I
I
I
0)
0
100
200
400
300
Time (msec)
-
-
-
--
-------
- 101 -
Figure 5.11
(A) PST histogram of a high-CF auditory-nerve fiber's "response" to 100 Hz tone bursts
of 70 dB SPL. Arrows indicate the turning-on and -off of the tone burst. Histogram
parameters are the same as those in Fig. 5.7C.
(B) Same as (A) except that a continuous 6 kHz tone was added to raise the background
firing rate for the detection of any possible "modulation" effect by the 100 Hz tone burst.
(C) Rate-level functions of a high-CF fiber at 8 kHz with and without a continuous background tone of 100 Hz at 70 dB SPL.
(D) Same as (C) except that the 8 kHz tone bursts were replaced by the noise bursts.
-102-
0
-J1
a
liii
(A)
400
j
0
©
II
liii!1111111111 II
CF=6.68kHz; SR=96sp/sec
XDP82-32
I I
II1111
II
(B)
O -_-
01
a
-
0
o
F-)
I11II
III
05__
O.
II
0
o
500
t
III
liii
I
I
I
500
t
Time (ms)
Time (ms)
XDP81-16; CF = 7.16 kHz; TH = 23.1 dB SPL; SP = 1.4 sp/sec
I
300 C
i
(C)
I
I I
I
I
I
8 kHz Tone
I
I
I
I
(D) 350-650Hz bcndpossed No:se
7
o-Withouft
o-Without 100Hz Tone
x -With 1OCHz Tone
of=
lf)OH
-
1C
Tone
+x-W
Q
U
0
CD
V
==
,I
cn
-
G
=
-
--
7
I
-0C
O=
I
20
I
40
Tone
I
I
60
I
I
80
evel (dB SPL)
i
I
60
I
I
I
SC
Noise Level (dB SPL)
i
1CO
- 103 -
detected near the tympanic membrane (see Fig. 5.10B). For shock intensities below the threshold
for displacing the stapes head, no shock-generated sound was detected near the tympanic membrane.
The artifact by electric stimulation of the stapedius muscle in the recording of cochlear
microphonic (CM) potential could be practically eliminated by the use of bandpass and tracking
filters, as was mentioned in Section 5.3. In addition, when the stapedius tendon was cut, electric
stimulation of the muscle from near the round-window of the cochlea produced no change in the
CM response (after filtering) to acoustic stimulus (see Fig. 5.6). A different kind of artifact in
the measurement of CM, however, was observed, and that was the variation in the slope of CMlevel functions with the recording site. It was found that although generally the CM magnitude
grew linearly with the magnitude of a tone, i.e., the CM-level functions had a unity slope on a
log-log plot, the slope could vary as the recording site was moved around the round window of
the cochlea in the same ear (Fig. 5.12). Since one could spend a lot of time repositioning the CM
recording electrode without obtaining an exactly unity slope, and since for all purposes of this
study only the amount of shift of the CM-level function with stapedius contractions and any
change in the slope, rather than the exact value of the slope, were of importance, CM-level measurements were usually accepted without particular attention to the exact value of the slope.
The issue of whether the olivocochlear efferents were electrically stimulated by the shocks
to the stapedius region was directly addressed by an experiment carried out in the same way as
the others but with the olivocochlear efferents severed (XDP89). The result was consistent with
other control measurements such as shown in Figs. 5.7, 5.8 and 5.9, indicating an absence of
electric excitation of the olivocochlear efferents (detailed data are described in Chapter VII).
_I
II
CI-Il
I
·_
_lyl_^·lllll--.II-
I CIII·l-(lll^-l--l---·-
-104-
Figure 5.12
Three cochlear-microphonic potential (CMP) level functions recorded consecutively from slightly different sites near the round-window of the cochlear. The dashed
line serves as a reference with a slope of one.
_
_
____ ____
_
-105-
I
OU-
I
I
P9
CI-
I
, ,~~~~~~~~~~~~~~~~~/
%
I
r
I
40-
30m
C)
20I
10Hz
-- L
I
n-
I
40
I
50
I
60
I
I
70
80
I
90
Tone Level (dB SPL)
____11-___11_·-
_.1_11111-·_1111_1.
--( -
--·
- 106-
Chapter VI
Results (I): Masking Patterns of Auditory-Nerve Fiber Responses
The main body of the experimental results are presented in this and the next chapter. In
this chapter, the masking of tone responses of high-CF auditory-nerve fibers by narrow-band
low-frequency background noise will be presented using three measures of masking: elevation of
tuning curves, level shift of tonal rate-level functions using constant-response criterion, and the
difference in tone levels required to maintain a constant detectability of a tone (shift of a functions). A primary focus of the presentation will be on the growth-rate of masking since this will
be a key element in the prediction of the effect of stapedius contractions on the masking in
auditory-nerve fibers. Masking at 6 and 8 kHz will be particularly important because the masking
at these frequencies will be used for a direct comparison with the psychophysical data.
6.1 Masking Measured with Elevation of Tuning Curves
The tuning curve of an auditory-nerve fiber provides an estimate of the tone level required
to evoke a "threshold" rate response at the frequencies tested. Tuning curves in this study were
measured by adjusting the tone level at each frequency until a criterion increase in discharge rate
over the background level was just reached (more details were given in the Methods chapter).
Figure 6.1A shows an example of the effects of continuous low-frequency background
noise on the tuning curve of a high-CF auditory-nerve fiber for six noise levels. The overall
effect of the masking noise was a monotonic increase with noise level of the fiber's threshold for
frequencies near the CF, an effect typical of all fibers observed. The relationship between the
increase in threshold and the masking noise is shown in Fig. 6.1B as the "masking function". It
can be seen that for all noise levels the masking at the CF was larger than at the selected frequencies below and above the CF. This is also representative of all fibers. It can also be seen that
the amount of masking at CF was over 60 dB with 95 dB SPL noise. The growth-rate of
- 107-
Figure 6.1
Masking pattern observed from tuning curves of a high-CF auditory-nerve fiber.
(A) The effect of the continuous narrow-band low-frequency masking noise on the tuning
curves (TCs) of a high-CF (6.31 kHz) auditory-nerve fiber. The tonal stimuli were tone
bursts of 50 ms duration at 10 bursts/sec. The tuning curves were measured with a frequency resolution of 100 points per decade and sound-level steps of 0.75 dB. The heading has the following convention (for this and all figures illustrating data from a single
fiber): the number pair following "XDP" indicates cat number (87 in this case) and fiber
or "unit" number (73 in this case); "CF" stands for "characteristic frequency", "TH" for
"threshold" at the CF, and "SR" for "spontaneous rate". The data values in the heading
are all from measurements made in the absence of masking noise. The use of different
line thickness in an alternating order is meant to facilitate the identification of curves. All
tuning curves throughout the rest of this thesis were measured with an automated procedure (see Methods) and were digitally filtered once with three-point standard triangular
(weight: 1/4, 1/2, 1/4) moving average.
(B) Masking functions obtained from the measurements in (A). Masking (M) was defined
as the elevation of the tuning curve at the frequency of interest. In particular, masking
was measured at the fiber CF (6.31 kHz), at 6 kHz, and at 8 kHz. The average growthrate (GR) of masking in the noise range from N 1 to N 2 is defined as the ratio of the
difference in masking (M 2 - M 1) over the difference in noise levels (N 2 - N 1). For the
noise range used in the measurement of masking in this fiber, the GR at CF is
1.99 dB/dB, the GR at 6 kHz is 1.90 dB/dB, and the GR at 8 kHz is 2.03 dB/dB.
-108-
XDP87-73; CF = 6.31 kHz; TH = 1.8 dB SPL; SR = 51 sp/sec
I
I
I
I
I
From top down:
I
v,
TC In 95dB SPL Noise
In 90dB SPL Noise
In 85dB SPL Noise
In 80dB SPL Noise
60 In 75dB SPL Noise
In 70dB SPL Noise
TC In Quiet
40 -
o0
20 -
.
I
!
I
80-
QDn
n
03
V)
!_
0.
t-)
0-
I
I
Dashed lines mark 6 & 8kHz
·
_·_
·_
1.5
(A)
·
r
·
I I
6
_ _
1
15
Acoustic Frequency (kHz)
Masking Noise: 350-650 Hz bandpcssed noise
Masking measured from the elevation of Tuning Curves
I
I
I
I
I
crn
a- Masking at CF
o- Masking at 6kHz
o- Masking at 8kHz
Dashed line h
60 -
40._
C,
iR
20-
00-
(B)
·
·
60
70
-
-
80
--
·
90
RMS Noise Level (dB SPL)
I
100
- 109-
masking measured at all three frequencies had a value close to 2 dB/dB. Again this is typical, as
will be seen from Fig. 6.2.
Figure 6.2 shows all masking functions at fiber CF and at 6 and/or 8 kHz obtained from
tuning curves of twenty-five high-CF auditory-nerve fibers. The maximum masking was almost
80 dB. The growth-rate (GR) of masking averaged over all noise levels and all fibers at the
above three frequencies had a value of 2.07 dB/dB. The average GR at CF was higher than that
at either 6 or 8 kHz, a point to be further illustrated in Figs. 6.3 and 6.4.
Figure 6.3A shows the growth-rate of masking as a function of fiber CF for all fibers with
masking functions measured at 6 kHz. The maximum GR was from fibers whose CFs were near
or slightly below 6 kHz. Figure 6.3B provides a similar picture for GR measured at 8 kHz.
Besides focusing on 6 and 8 kHz, similar analysis can be made for all frequencies for which
there were tuning-curve measurements. The relationship between GR measured at fiber CF and at
frequencies away from the CF is shown in Fig. 6.4. Again the GR was, on the average, maximum when the test frequency was slightly higher than the fiber CF, which is consistent with
Fig. 6.3A.
Since typically the amount of masking was largest and the GR of masking was highest
near the fiber CF, it can be expected that the masking noise also reduced the sharpness of tuning.
The sharpness of tuning of a bandpass filter is generally characterized by a tuning (resonance)
quality factor, Q, which is defined as the center frequency of the filter, the CF in the case of an
auditory-nerve fiber, divided by some measure of the bandwidth of the filter. By this definition, a
larger Q corresponds to a sharper filter. In this study, the bandwidth of tuning curves was measured at the tone level 10 dB above the threshold level at the CF, and the Q calculated with this
measure of bandwidth is denoted as Qlo. Figure 6.5 shows the relationship between noiseproduced elevation of tuning curves at the fiber CF and the corresponding percent change in the
Qlo. It can be seen that in general Q1o decreased with increasing elevation in tuning-curve threshold, reaching 60% decrease at about 75 dB elevation of the threshold.
It was also noted that with noise masking there was sometimes a shift of the "CF' in the
- 110-
Figure 6.2
Masking functions at CF, 6 kHz and 8 kHz obtained from tuning curves. Shown
are data from 25 auditory-nerve fibers with CFs ranging from 4.5 to 11 kHz from seven
animals (all available data; not all fibers had measurements available at all three frequencies). The statistics for the average growth-rate (GR) of masking are (in dB/dB):
GR at all frequencies: minimum = 0.313, maximum = 3.44, mean = 2.070, standard deviation = 0.592.
GR at CF: minimum - 1.547, maximum = 3.44, mean = 2.295, standard deviation
=
0.432.
GR at 6 kHz: minimum = 1.092, maximum = 2.735, mean = 2.020, standard deviation =
0.489.
GR at 8 kHz: minimum = 0.313, maximum = 2.74, mean = 1.682, standard deviation =
0.744.
If "masking" less than 5 dB is ignored and the GRs are computed from the masking functions above the 5 dB level, the mean of the average GR for all frequencies
becomes 2.074, the mean of the average GR at CF 2.290, the mean of the average GR at
6 kHz 2.033, and the mean of the average GR at 8 kHz 1.689. The difference is mostly
small.
-111-
Masking Noise: 350-650 Hz bandpassed noise
·
on
OU-
·
Mnskinna: Rlevaticn of Tunina Curves
o-
M
A- M
+-
60-
M
Dashe
Dottee
40C
12
20-
- -0
nnr
vv
60
60
70
70
80
RMS Noise Level (dB SPL)
90
- 112-
Figure 6.3
All available data from tuning-curve measurements on the relationship between the
average growth-rate of masking at 6 and 8 kHz and fiber CF.
(A) The average growth-rate of masking obtained from tuning-curve measurements at
6 kHz versus fiber CF. Each point corresponds to a fiber and the GR was averaged over
all noise levels used. The continuous line was obtained through a three-point standard triangular moving average of the data points (13 total), except for the two end ("anchoring") points at which there is no averaging.
(B) Same as (A) except that the masking was measured at 8 kHz and that the number of
fibers is 12 (the first and second point above 6 kHz in the plot superimposed).
-113-
Masking by 350-650 Hz bandpassed noise
Masking measured from the elevation of Tuning Curves
IL
5-
,
I,
II
I
I
Growth-Rate of masking measured at 6kHz
m
Continuous line from 3-point moving average
'a
m
C
V)
0
0
::Q)
0
-Y
O
CD
tI
0I
I
2
I
I
(A)
'I
I
6
I
,
I
8
20
Characteristic Frequency (kHz)
.
5-
I
i
I
,
l
I
Growth-Rate of masking measured at 8kHz
m
Continuous line from 3-point moving avercge
_
4-
a
0
I
t0
t
.
0I
2
I
I
I
6
I
°
I
8
Characteristic Frequency (kHz)
(B)
I
20
- 114 -
Figure 6.4
The average growth-rate of masking obtained from all available tuning-curve measurements as a function of the distance between the test frequency and fiber CF. The
growth-rate was measured between the most and the least intense noise levels used for
each fiber above the masking threshold, which was defined as the noise level at which an
elevation of 5 dB of the tip of the tuning curve was produced. Measurements were
made in 0.2 dB steps along the frequency axis. The thick dashed line is the mean of all
data points at each frequency in the range from -3 to +3 dB with respect to fiber CF.
Data are from 25 auditory-nerve fibers (CF range: 4.5-11 kHz) from seven animals.
0.
-115-
Masking by 350-650 Hz bandpassed noise
Masking measured from the elevation of Tuning Curves
I
I
I
I
I
I
I
II 1
I
I
I
I
I
I
I
I
I
i
I
Each thin solid line corresponds to one fiber
Thick dashed line: Mean at each frequency
Cashed vertical line: fiber CF
m
m
73
0
I
o
3
0o
I
hv.
I I
-10
I I
I I I i
-3
I I
I
0
I
I
3
I
Test Frequency (dB re. fiber CF)
I
I
10
- 116-
Figure 6.5
The effect of low-frequency masking noise (300 Hz bandwidth centered at 500 Hz)
on the sharpness of frequency tuning (Q 10 of tuning curves) of high-CF fibers versus its
effect on the threshold of the tuning curve at the fiber CF. All available data are shown.
The percentage change in Q 10 is defined as: 100 times [the difference between the Q 10
with noise and the Q 10 without noise] normalized (i.e., divided) by the Qlo without
noise. The solid linear-regression line has a slope of -0.7 and an intercept of -7.45. The
correlation coefficient of the data points is -0.67. The characteristic frequency of the
fibers ranged from 4.47 to 11.09 kHz. There are a total of 88 data points from 25 fibers
from six animals in the plot. ("Q10" in the figure labelling is the same as Q 10 here and
in the text.)
-117-
Change in Q10 vs. Change in Threshold Due to Noise Masking
I
40-
I
I
I
I
I
I
I
I
Dashed line marks no change of Q10 from TC in quiet
.4,
0
V
U
o
0o
O
O
_____-------t'3 0
r
L.
w
cv
0~
0-
-----
0
---
---
C
0
00
I8O8
oo
C
40-
--
C
0
0
c
-
0
)O
CIr-
e
0
O
o0
o
C
0
-o
0
o
_vj
0
C
rU
C
0
0
o
ooo
0
0
-
0
0
0
U
.T- -F3O
I
30
I
I
60
Elevation in TC Threshold (dB)
U
90
- 118 -
sense that the frequency with the lowest threshold with noise masking differed from the frequency with the lowest threshold without noise (the CF). The typical value of such shift was,
however, rather small (0.03 octave up or down), and no systematic trend was observed with
increasing noise level either in the direction of the shift or in the magnitude.
The primary advantage of measuring masking with tuning curves is that one can obtain
masking functions at many frequencies from one set of measurements. One disadvantage associated with measuring masking with tuning curves is that it does not provide a means for determining the effect of a given masking noise on a fiber's response as a function of tone level, which is
the issue to be addressed in the next section. Another disadvantage is that since both the number
and the level of the tone bursts required for the determination of each point of a tuning curve are
random variables, it is difficult to estimate the variability of the response, an estimate important
for characterizing the detectability of a signal and the information content in the response. In a
later section (Section 6.3), masking will be discussed in the context of signal detectability, which
will offer the advantage of comparability of the physiological results with psychoacoustic studies
on both masking and stapedius-produced unmasking.
6.2 Masking Patterns Observed from Rate-Level Functions
The rate-level function of an auditory-nerve fiber describes a relationship between the
fiber's average discharge rate and the level of a stimulus (usually a tone in this study). Rate-level
functions were measured by obtaining the number of spikes in the time intervals of the stimulus
duration or a fixed portion of the duration and averaging over a set number of stimulus presentations at each stimulus level.
Examples from a low-SR auditory-nerve fiber of the effects of continuous low-frequency
noise on the rate-level function for a high-frequency tone are shown in Figure 6.6A. Two features
typical of all fibers can be seen: one is a monotonic* shift of the rate-level function towards
* Here "monotonic" is for the tested noise range above the noise level where masking becomes detectable.
- 119 -
Figure 6.6
Masking patterns observed from rate-level functions from a low-SR, high-CF
auditory-nerve fiber.
(A) The effects of continuous narrow-band low-frequency masking noise on the rate
response to 8 kHz tones of a low-SR (0.4 spikes/sec), high-CF (8.04 kHz) auditory-nerve
fiber. The method of measuring rate-level functions was given in Section 5.5. The leftmost curve corresponds to no noise, and then from left to right each curve corresponds
to a noise level in increasing order from 65 to 90 dB SPL in 5 dB steps.
(B) Masking function obtained from the measurements in (A). Masking was defined as
the difference in tone levels between the rate-level functions with and without noise for a
constant rate response. Unless stated otherwise, this constant rate was the mid-rate (the
arithmetic mean of the maximum and minimum rate of the rate-level function in quiet).
The average growth-rate of masking was 1.72 dB/dB.
-120-
.
XDP80-71; CF = 8.04 kHz; TH = 14.2 dB SPL; SR = 0.4 sp/sec
I
400-
N,
Co
300-
to0
o
-c
a
o
v9
·I
_
vo-
I
III
PEn9
Wl)Aie
I
I
o- 8kHz Tone In
a- In 65dB SPL
+- In 70dB SPL
x- In 75dB SPL
v-
)
·I
Quiet
Noise
Noise
Noise
I
I
I
-I
I
Dashed Line Marks Mid-Rate
CI
In
In
200-
100-
C)
(A)
oI
I
10
0
I
20
I
I
30
40
I
I
50
I
60
I
70
I
80
90
Tone Level (dB SPL)
Masking Noise: 350-650 Hz bandpassed noise
Masking: Shift of rate-level functions at mid-rate
I
0FN
I
I
I
I
I
ov-
Dashed Line Has a Slope of 2
/1.
/_
/1
/1
/1
60-
//l
,/
J.
-.
m
40-
0
20-
nnVV
I
/
r
r-%\
~t0)
I
60
70
80
RMS Noise Level (dB SPL)
90
- 121-
higher tone levels with increasing noise intensity, and the other is a monotonic increase in the
background discharge rate as the noise was increased in intensity and started to excite the fiber,
as can be seen from the discharge rate at 10 dB SPL tone level (below the fiber's response threshold to the tone; also observed in the absence of the tone). There was probably also some masking by the tone of the fiber's response to high-intensity noise, as is visible from the curve with
90 dB SPL noise when the tone level increased from 20 to 60 dB SPL.*
One way to measure the masking by a noise of a fiber's mean-rate response to a tone is to
use a constant-rate response criterion and measure the difference in tone levels required to produce this constant rate with and without the noise.** Since the noise-produced shift of the tonal
rate-level function is rarely a strictly parallel shift of the function obtained in quiet, different criterion rates can result in different measures of the amount of masking. A criterion near the spontaneous rate would not be appropriate because noise excitation of the fiber could elevate the background discharge rate to above the criterion value. A criterion near the plateau rate would also be
inappropriate because of the rapid slope change and because the noise often lowered the plateau
rates of high-SR fibers, as will be shown later. With the above considerations, the "mid-rate" was
selected as the constant-response criterion level, with the mid-rate defined as the arithmetic mean
of the spontaneous rate and the plateau rate measured with no masking noise. One advantage of
using the mid-rate criterion is the relative robustness of the measurement against small variations
in the determination of the criterion value, because he slope near the mid-rate region of most
rate-level functions measured in quiet background is relatively large and constant and tends to
retain the same constant value in the presence of the masking noise, as is shown in Fig. 6.6A. In
other words, the noise-produced shift of the tonal rate-level function is mostly likely to be close
to a parallel shift near the mid-rate region. Figure 6.6B shows the masking function measured
* Such masking of a high-CF fiber's response to the low-frequency noise by the presence of a high-frequency tone was
observed from time to time, but it was much smaller than the masking of the tone response by the noise and is not the
focus of the present study.
** As the rate-level function is nonlinear with both "threshold" and "saturation" C'plateau") features, a constant-rate criterion is superior to a measurement of the rate-difference with a constant tone level in describing the effect of noise on
a fiber's rate response to a tone.
- 122 -
from Fig. 6.6A with the mid-rate criterion. With 90 dB SPL background noise, there was a masking of the tone response of about 50 dB. The average growth-rate of masking was 1.72 dB/dB for
this fiber at 8 kHz.
Figure 6.7A shows the effect of continuous low-frequency noise on the rate-level function
for a high-frequency tone of a high-SR auditory-nerve fiber. Besides the features common with
those shown in Fig. 6.6A, an additional feature, a monotonically lowered plateau rate with
increasing noise, can be seen. This effect of the masking noise on the plateau rate was more frequently observed in high-SR fibers than in low-SR fibers, but was not always seen in high-SR
fibers. A decrease of the plateau rate has been reported in the literature for the masking of ratelevel functions to CF tones by broadband noise. It has been suggested that the decreased plateau
rate is a result of adaptation to the noise-produced increase in background discharge rate, with
low-SR fibers being more resistant to adaptation than high-SR fibers (e.g., Costalupes et al.,
1984; Winslow & Sachs, 1987). Figure 6.7B shows that a 90 dB SPL noise also had a masking
effect of more than 50 dB using the mid-rate criterion and that the average GR of masking was
2.1 dB/dB.
A comparison of the masking pattern obtained from rate-level functions with that obtained
from tuning-curve measurements, in the same fiber at the same frequency and the same noise levels is provided in Figure 6.8. As shown in Fig. 6.8C, there was slightly more masking and a
slightly higher GR of masking measured from tuning curves. This was typically the case.
Figure 6.9 shows all of the masking functions obtained from rate-level functions at either
6 or 8 kHz. There was a maximum masking of over 70 dB, and the mean of the average GR of
masking was 1.9 dB/dB.* Both the average amount of masking and the average GR of masking
were slightly smaller than those from tuning-curve measurements.**
In one experiment, the olivocochlear efferents were completely severed to provide a direct
control for the possible artifact of stimulating the olivocochlear efferents with electric stimulation
* Here "mean" is across fibers and "average" is across noise levels.
** Such comparison is, however, not strict in that the samples for the two kinds of masking measurement do not completely overlap.
- 123 -
Figure 6.7
Masking patterns observed from rate-level functions of a high-SR, high-CF
auditory-nerve fiber.
(A) The effects of continuous low-frequency masking noise on the rate-level function of
a high-SR (79 spikes/sec), high-CF (8.13 kHz) fiber measured at 8 kHz.
(B) Masking function obtained from the measurements in (A). The average growth-rate
of masking was 2.1 dB/dB.
-124-
XDP76-105; CF = 8.13 kHz; TH = -5.9 d
I
. r%^
9tU
I
I
I
I
I
-
I
SPL; SR = 79 sp/sec
I
I
I
I
I
Dashed Line Marks Mid-Rate
8kHz Tone In Quiet
In 70dB SPL Noise
+- In 75dB SPL Noise
x- I
oa-
o
0
co
0.
o-
I,
v-
II
.__
C"
0
V)
0
_
(A)
0I I
-20
-
.
I
I
.
- -
.
-
T
-.
.
-
I
I
I
I
90
Tone Level (dB SPL)
Masking Noise: 350-650 Hz bandpassed noise
Masking: Shift of rate-level functions at mid-rate
On_
OVU
I
I
I
I
I
Dashed line has a slope of 2
0
C
o
U)
0O
/..
,'-
(B)
-1
6
I
1 00
60
RMS Noise Level (dB SPL) .
I_-.1--·.·_
1111
- 125 -
Figure 6.8
A comparison of masking measured from rate-level functions with masking measured from tuning curves in the same fiber.
(A) Same as Fig. 6.7A, replotted here for convenience of comparison.
(B) Tuning curves measured with the same masking noise levels as those used in the
rate-level function measurements.
(C) The masking function obtained from rate-level functions at 8 kHz had an average
growth-rate of 2.1 dB/dB, and the masking function obtained from tuning-curve measurements at 8 kHz had an average growth-rate of 2.2 dB/dB.
_
____
-126-
XDP76-105; CF=8.13kHz; TH=-5.9dB SPL; SR=79sp/sec
I
400-
x-
U-)
I
I
I I
I
I
I
I
I
I
_
0-
.5
OL
In
%_
-
o
(A
0
i
I
I
.
From top down:
TC In 90dB SPL Noise
' 80- In 85dB SPL Noise
_ In 80dB SPL Noise
U)
In 75dB SPL Noise
m
v 60- In 70dB SPL Noise
TC In Quiet
Dashed Line Marks 8kH
o 40-
_
, -
,- an4D-
I
100-
a- 8kHz Tone In Quiet
·- In 70dB SPL Noise
+- In 75dB SPL Noise
.
I I I
I
j
(B)
El
'!20-
0
-
U,
0-
Dashed Line Marks Mid-Rate
O-
-
20
I
I
I
I
I
I
I
I
I
I
i
.
4
90
,
4
90
Masking Noise: 350-650 Hz bandpossed noise
I
a-
I
I
I
Masking at Mid-Rate
Masking at 8kHz from TC
Dashed Line Has a Slope of 2
m
1'V
Cn
(C)
Cl
M8
I.,
/
¢1m
v
-
6
60
,I
Acoustic Frequency (kHz)
Tone Level (dB SPL)
80-
8
8
·
·
I
RMS Noise Level (dB SPL)
100
16
-
127 -
Figure 6.9
All masking functions obtained from rate-level functions at either 6 or 8 kHz (82
fibers with a CF range of 4.5-12.5 kHz from ten animals all with intact olivocochlear
efferents). The statistics for the average growth-rate (GR) of masking are (in dB/dB):
Minimum = 0.43, maximum = 3.8, mean = 1.9, standard deviation = 0.72.
If "masking" less than 5 dB is ignored and the GRs are computed from the masking
functions above the 5 dB level, the mean of the average GR remains 1.9.
__ ____
-128-
Masking Noise: 350-650 Hz bandpassed noise
Masking: Shift of Rate-level Functions at Mid-Rate
Dashed heavy diagonal line has a slope of 2
80-
Tested at 6 kHz
--*-A
A-*
Teste
Each line is fr
60- Dotted line: 5(
,I
-0
*_
40-
v
0
20-
00I
I
60
70
80
I
80
RMS Noise Level (dB SPL)
.
90
90
- 129-
of the stapedius muscle (see Section 5.7); it was also of interest to see how such a manipulation
would affect masking patterns, since it was suggested (Bonfils et al., 1986, 1987) that masking
might be influenced by sound-evoked activity of olivocochlear efferents. Figure 6.10 shows all
of the masking functions obtained from rate-level functions in the animal with completely severed
olivocochlear efferent fibers. The masking functions had a mean GR almost identical to the
overall mean GR from all animals with intact olivocochlear efferents. The average masking for a
given noise level, however, seems to be less than the average from other animals (compare
Fig. 6.9 with 6.10). This might be due to an increase in the masking threshold (i.e., the noise
level at which masking begins) on the order of 5 dB noise level, but the sample size with the
severed olivocochlear efferents is not large enough to warrant a definitive conclusion or detailed
analysis. Since it is the growth-rate, rather than the threshold or the absolute amount of masking
that is of primary interest to this study, the issue of a possible difference in the noise-masking
with and without olivocochlear efferents is mentioned here as a suggestion for future investigation and will not be pursued further in the present study.
It was pointed out earlier that as the masking noise increased in level, it not only shifted
the tonal rate-level function but also raised the background discharge rate, reducing the range for
differential rate response. Therefore, rather than completely characterizing the masking effect,
measuring masking only along the dimension of a shift at the mid-rate may tend to underestimate
the growth-rate of the overall masking effect. In the next section, a third measure of masking will
be introduced that not only takes into account the noise-induced level shift of rate-level functions
and the noise-produced elevation of background discharge rate, but also takes into account the
variability in the rate response. This measure is thus (at least theoretically) more "complete" than
measures of masking provided by either the tuning curves or the rate-level functions.
6.3 Masking Measured with Elevation of Detection Threshold
One problem with describing auditory-nerve fiber responses using tuning-curves or rate-
- 130 -
Figure 6.10
All masking function measurements (9 fibers with CFs ranging from 5.7 to 7.5 kHz)
from an animal with severed olivocochlear efferents. The masking functions were
obtained from rate-level functions. The statistics for the average growth-rate (GR) of
masking are (in dB/dB):
Minimum = 1.24, maximum = 2.78, mean = 1.9, standard deviation = 0.48.
If "masking" less than 5 dB is ignored and the GRs are computed from the masking
functions above the 5 dB level, the mean of the average GR remains 1.9.
-131-
Masking Noise: 350-650 Hz bandpassed noise
Masking: Shift of rate-level functions at mid-rate
Dashed heavy diagonal line has a slope of 2
on
ou-
i
_
Tested at 6 kHz
Tested at 8 kHz
-A*---R*
-
Each line is from one fiber
60-
#
Dotted line: 5dB masking
/
XDP89(R)
40-
J.
U)
cn
20/~~-l9
-f
/
Oe~~
le
r
-,
nn
_ 10
u-
I
-
_
60
70
¢%
V~~~~~~~W
¢-
X
80
RMS Noise Level (dB SPL)
90
/
- 132-
level functions is that, due to the stochastic nature of neuronal discharges, the average discharge
rate or a change in the average discharge rate is not an adequate measure of the information contained in the rate response. Specifically, with two spike-number distributions,* one from the
"noise only" situation and the other from "noise+tone", the true separability or detectability of the
tone from noise is determined by both the separation of the means and the variances of the
spike-number distributions: for a fixed separation of the means, the larger the variances, the less
the certainty in tone detection. Having this in mind, an effort was made in this study to construct
a detection characteristic, d, that describes the separation of the spike-number distribution with
"noise+tone" from the distribution with "noise only" (details were given in Sections 4.4 and 5.6).
Briefly, for each noise and tone level, the value of d equals the mean discharge rate with
"noise+tone" minus the mean discharge rate with "noise only", divided by the standard deviation
of the discharge distribution with "noise+tone". An estimate of the mean discharge rate with
"noise+tone" was obtained from the rate-level functions described in the last section, and an estimate of the mean discharge rate with "noise only" (including with the "internal noise" alone or
the mean spontaneous discharge rate) was obtained with the tone turned off. With the rate-level
functions measured with a known number of tonal stimulus presentations at known sound levels,
the sample variance of the spike-number distribution with each noise and tone level was rather
easily measured on-line to provide an estimate of the distribution variance. A tone was considered
detectable when the difference between the means of the spike-number distributions with
"noise+tone" and that with "noise only" became equal to or larger than one standard deviation,
i.e., a d value of one or larger was reached (details regarding assumptions, rationals and modifiers
for the use of d were given in Section 4.4). Such characterization of the tone responses made it
possible to measure the masking of tone responses by noise in terms of the noise-produced elevation of tonal detection threshold, or more specifically in terms of the difference in tone levels
which are required to produce a
of unity with and without the masking noise, respectively.
* As was mentioned in Chapters III and IV, a spike-number (or pulse-number) distribution is a description of the pro-
portion of trials in which N spikes occurred, as a function of N; and is an estimate of the conditional probability density
function.
- 133-
Figure 6.11 shows the masking of a high-CF (near 8 kHz), high-SR fiber's highl-frequecy
tone responses by low-frequency
noise, both in the forms of d-level functions and the
corresponding rate-level functions. The -level functions and the rate-level functions are similar
in overall shape, but the different background discharge rates in the rate-level functions due to the
different noise intensities were all "normalized" according to their effect on tone detectability in
the
functions. Since the d function takes into account the noise-produced elevation of back-
ground discharge rate in describing the noise-masking effect, it is not surprising that the growthrate of masking measured from d functions was somewhat larger than the corresponding GR from
the average-rate functions (Fig. 6.11C). This was generally the case. The negative values of the d
functions in Fig. 6.11B were probably due to masking by the tone of the fiber's response to the
noise.
Figure 6.12 shows the same kind of measurements as shown in Fig. 6.11, but obtained
from a low-SR fiber with CF near 6 kHz. Again the GR of masking from d functions was larger
than that from rate-level functions.
Figure 6.13 shows all masking functions obtained from d functions measured from 91
auditory-nerve fibers from eleven animals. The mean of the average GR of masking for all fibers
was 2.09 dB/dB. Using the statistics given in the caption of Fig. 6.13, hypothesis tests were performed to determine the significance level of any difference between the mean of the average GR
of masking among the sub-groups of the total samples, assuming independent random samples
from normally-distributed populations. The results of the tests are as follows:* (1) The (null)
hypothesis that the means of the average GR of masking are equal for animals with intact and
with severed olivocochlear efferents (against the alternative hypothesis that they are not equal,
i.e., two-sided t-test) cannot be rejected at a significance level of a--=0.7. Here the significance
level a is the probability of rejecting the null hypothesis when it is true (type I error). In other
* The specifics regarding the construction of the test statistic and its distributions and the determination of the critical
regions for rejecting the null hypothesis for hypothesis tests concerning the difference between the mean of two normal
populations with unknown variances can be found from standard sources on statistics, such as the "CRC Standard
Mathematical Tables".
- 134-
Figure 6.11
Comparison of masking measured from rate-level functions with the masking measured from
functions (the use of d' in the plot was due to the unavailability of "d" in
the font) in a high-SR fiber with CF near 8 kHz.
(A) Same as Fig. 6.7A, replotted here for convenience of comparison.
(B) These d functions were constructed using the sample mean of the discharge rate with
the tonal signal presented as shown in (A), the sample mean of the discharge rate
without the tonal signal (either in quiet or with background noise), and the sample variance of the discharge rate with noise+tone (see Section 5.6 for detail).
(C) Masking measured from
between the
functions was defined as the difference in tone levels
functions with and without masking noise at a d level of unity. The mask-
ing function obtained from rate-level functions had an average growth-rate of 2.1 dB/dB,
and the masking function obtained from
2.4 dB/dB.
functions had an average growth-rate of
-135-
XDP76-105; CF = 8.13 kHz; TH = -5.9 dB SPL; SR = 79 sp/sec
0
I
.,
4(jU
O
0
0.
V)
%--
I
t't
I
I
I
a-
8kHz Tone In Quiet
a+-
In 70dB SPL Noise
II
x-
II
I
II
I
I
I
I
Dashed Line Marks Mid-Rate
o-
0
a
v-
a
C,
II
(A)
0
U,
n
I
nv
·I
-20
·I
1I
I
·I
I
·I
I
I
rI
!
!
·I
90
Tone Level (dB SPL)
I
I
I
I
I
I
I
I
I
r-
o- 8kHz Tone In Quiet
9]
a+-
In 70dB SPL Noise
In 75dB SPL Noise
x-
In 80dB SPL Noise
i
O-
V-
(E
i-
1-
0L
I
I
I
I
I
i
-20
I
I
I
90
Tone Level (dB SPL)
Masking Noise: 350-650 Hz bandpassed noise
I
OA
I
I
I
I
OU-
o-- Masking at d'=1
"-
Masking at mid-rate
Dashed line h
m
0'
cr
(C)
o
06
60
I
1
100
RMS Noise Level (dB SPL)
- 136 -
Figure 6.12
Comparison of masking measured from rate-level functions with the masking measured from a functions in a low-SR fiber with CF near 6 kHz.
(A) Tonal rate-level functions with different background masking-noise intensities.
(B) The a functions corresponding to the rate functions in (A).
(C) Masking functions obtained from measurements in (A) and (B). The masking function obtained from rate-level functions had an average growth-rate of 1.85 dB/dB, and
the masking function obtained from
2.48 dB/dB.
functions at d=l had an average growth-rate of
-137-
XDP87-28; CF = 5.96 kHz; TH = 22.6 dB SPL; SR = 0.1 sp/sec
·I
o
ao
400-
_
I
I·
·I
I·
·I
I
·
I_
v- In 90dB SPL Noise
6kHz Tone In Quiet
In 70dB SPL Noise
In 75dB SPL Noise
Aoo-
.-
i
I_
L
Uj
In 95dB SPL Noise
+-
Dashed Line Marks Mid-Rate
o- In 80dB SPL Noise
o- In 85dB SPL Noise
0
a,
-t
(A
n
._
0I
I
5
I
I
I
i
I
I
I
I
I
I I
105
Tone Level (dB SPL)
I
14-
I
I
I
I
I
a-
6kHz Tone In Quiet
ooo-
In 70dB SPL Noise
In 75dB SPL Noise
In 8dB SPL Noise
I
E
o- In 85dB SPL Noise
v- In 9CdB SPL Noise
+- In 95dB SPL Noise
r
-
i
10I
5
I
I
I
1
I
I
I
I
105
Tone Level (dB SPL)
Masking Noise: 350-650 Hz bandpassed noise
onl
ou-
I
o-
Masking at d'=1
"-
Masking al
m
Dashed line h
1o
(C)
:em
o-
6
I
100
60
RMS Noise Level (dB SPL)
- 138 -
Figure 6.13
All masking functions obtained from a functions at d=l (91 fibers with a CF range
of 4.5-12.5 kHz from eleven animals). The statistics for the average growth-rate (GR) of
masking are (in dB/dB):
For all fibers: minimum = 0.723, maximum = 3.52, mean = 2.09, standard deviation =
0.647.
For animals with intact olivocochlear efferents: n = 82, minimum = 0.723, maximum =
3.52, mean = 2.10, standard deviation = 0.658.
For the animal with severed olivocochlear efferents: n = 9, minimum = 1.138, maximum
= 3.097, mean = 2.02, standard deviation = 0.620.
For fibers with low- and medium-SR (40% of the total): n = 36, minimum = 0.723, maximum = 3.27, mean = 2.05, standard deviation = 0.626.
For fibers with high-SR: n = 55, minimum = 0.99, maximum = 3.52, mean = 2.15, standard deviation = 0.632.
For fibers tested with 6 kHz tones: n = 40, minimum = 0.931, maximum = 3.316,
mean = 2.10, standard deviation = 0.583.
For fibers tested with 8 kHz tones: n = 51, minimum = 0.723, maximum = 3.52,
mean = 2.04, standard deviation = 0.686.
If "masking" less than 5 dB is ignored and the GRs are computed from the masking
functions above the 5 dB level, the mean of the average GR for all fibers becomes 2.10.
-139-
Masking noise: 350-650 Hz bandpassed noise
Masking: Shift of d' functions along tone-level axis at d'=l
Dashed heavy diagonal line has a slope of 2
l
I
80- e---·
I
I
I
i
I nw- & Med--SR
60-
C)
0_
40-
Cn
zL
_2
20-
nr) _
-'.U
-
6I
I
I
i
60
70
80
90
RMS Noise Level (dB SPL)
1.
- 140 -
words, if one is to reject this-null hypothesis then the probability that one i wrong is larger than
0.7. (2) The (null) hypothesis that the mean of the average GR of masking of high-SR fibers
equals that of low- and medium-SR fibers (against the alternative hypothesis that the means are
not equal) cannot be rejected at a significance level of 0.4. (3) The (null) hypothesis that the
mean of the average GR of masking of fibers tested with 6 kHz tones equals that tested with
8 kHz tones (against the alternative hypothesis that they are not equal) cannot be rejected at a
significance level of 0.6. The tests were performed with and without assuming equal-variance for
the sub-populations and the results of the tests were the same in all cases with and without such
assumption. In the case of assuming equal-variance, a hypothesis test of this assumption was also
performed and the hypothesis could not be rejected at a significance level higher than 0.2. In
summary, it is considered that the difference in the means of the average GR of masking among
the sub-groups discussed is not of adequate statistical significance for these groups to be treated
separately in future analysis.
A closer examination of the masking functions in Fig. 6.13 indicates that the growth-rate
of masking varies with the level of the masking for most fibers. In general, as masking increases
the GR of masking increases. A "mean masking function" in this case could therefore provide a
more specific description of the masking pattern than a single-valued "mean of the average GR"
would. Such an "averaged" version of all the masking functions in Fig. 6.13 is shown in
Fig. 6.14. The growth-rate of masking increased monotonically with the masking level from 1.65
to 2.37 dB/dB, with the average GR being 2.08 dB/dB. The point of Fig. 6.14 is that while a
2 dB/dB value provides a reasonable first-order approximation of the GR of masking in general,
any quantitative treatment of the GR, such as in the prediction of stapedius-produced unmasking,
should take into account the dependence of the GR on the masking level. This is also the reason
that it was emphasized in Section 4.1 that the GR used in the working hypothesis of stapediusunmasking is to be measured for each fiber in the range between the stapedius-attenuated and the
un-attenuated noise level.
The relationship between the average growth-rate of masking measured from
I__l__lII___
_1_111
functions
- 141 -
Figure 6.14
The average masking function from d measurements in 91 fibers (all data available).
The averaging was first performed for each level of masking in 1 dB steps, resulting in a
relationship between masking and noise level. This relation was then averaged for each
level of the noise in 5 dB steps (all original masking functions were measured in 5 dB
steps of noise level). The growth-rate of masking, GR, increases with noise level:
Between 70 and 75 dB SPL noise level, GR = 1.65 dB/dB;
Between 75 and 80 dB SPL noise level, GR = 1.92 dB/dB;
Between 80 and 85 dB SPL noise level, GR = 2.17 dB/dB;
Between 85 and 90 dB SPL noise level, GR = 2.29 dB/dB;
Between 90 and 95 dB SPL noise level, GR = 2.37 dB/dB;
The overall GR across all noise levels is 2.08 dB/dB.
0
_
-142-
Masking Noise: 350-650 Hz bandpassed noise
Masking: Shift of d' functions toward higher tone levels
i
L--
80-
·
·
Dashed line has a slope of 2
J1
J,f
60e-Q
C
40-
U)
0
20-
00I
60
I
1
80
RMS Noise Level (dB SPL)
1
100
- 143 -
and the fiber CF is shown in Fig. 6.15. With masking measured from
functions, the maximum
GR was again typically from fibers whose CFs were near and slightly below the test frequency.
A possible explanation for the maximum GR's being from fibers whose CFs are slightly below
instead of at or above the test frequency is that due to the asymmetry in the slope of the tuning
curve of high-CF fibers, a test tone is more likely to mask the fiber's response to the noise when
its frequency is at or below the fiber CF than above the fiber CF.
While the use of
functions is conceptually helpful for characterizing the information
content of neuronal rate responses, one may wonder about the associated computational requirement and how it might affect the likelihood that the brain employs it in performing detection
tasks. As noted by Young and Barta (1986), the variance in the rate response is an increasing
(although not in a Poisson fashion) function of the mean rate and rate-response variance in lowSR fibers is generally smaller than that in high-SR fibers. Since the brain can have a priori the
knowledge of the spontaneous rate of a fiber and the (statistical) relationship between the mean
and variance of the discharge rate, it can incorporate the variability of rate response by registering
the SR of the fiber and applying appropriate weight to rate changes from fibers of different SRs,
all with relatively simple circuitry realizations, in its "judgement" of the information content of
rate responses. In other words, with current knowledge of the possibilities of synaptic organizations and auditory-nerve fiber properties, the computational requirement for using measures similar to
in an auditory task is likely to be a small part of the overall computational requirement
for the task and therefore unlikely to reduce the likelihood of the brain's using such measures.
One issue that was of interest to this study was the comparison of the masking patterns of
auditory-nerve fibers with similar tuning properties, for such information would be important for
assessing the "population properties" of auditory-nerve fibers in terms of the tonotopic organization and "rate-place" coding in the auditory periphery. For this purpose, two fibers most similar
in CF, SR, threshold and Q10o were selected from all fibers encountered in one penetration of a
micro-electrode, and comparisons of the masking patterns are shown in Figures 6.16-6.20 in
measures of tuning curves, rate-level functions and
_ _
-level functions. It can be seen from
-144-
Figure 6.15
The average growth-rate of masking versus the fiber CF from all data available.
Masking was obtained from
functions at d=l. As the relationship between change in
GR and the distance from fiber CF to signal frequency is of primary interest, GRs measured with 6 kHz and 8 kHz tones are unified with respect to a single signal frequency by
shifting the horizontal axis for GRs measured with 6 kHz tones such that 6 kHz on this
axis coincides with 8 kHz on the horizontal axis for GRs measured with 8 kHz tones.
Each point corresponds to a fiber and the GR for each fiber was averaged over all noise
levels used. Data are from 91 fibers from eleven animals. The continuous line was
obtained through a five-point rectangular moving average of all data points.
___ __
___-^.
_IP-.----_-^·--L-LI--_I
I_ _11 __I __
I_ ·---..----·I-_------·I -- -
---- 1_111----
-145-
Masking by 350-650 Hz bandpassed noise
Masking measured from d'-Level functions at d'=l
I
-
I
,
,I
I
I
I
I
I
o- GR measured with 6kHz tones (upper X-axis)
m
a-
mx
-
C)M
c
o0
o
GR measured with 8kHz tones (lower X-axis)
Continuous line: moving average of all points
Arrow indicates signal frecuency
-
oCD
V
'
V)
0
I
a
6o a
I
3.1
_0 C_
I
I
v
I
I
I
I
1 .5
I
2
I
I
I
I
I
l
I
6
I
I
8
15
t
20
Characteristic Frequency (kHz)
A*
---
--
- 146 -
Figure 6.16
Tuning curves from two auditory-nerve fibers recorded in one penetration of an
electrode. The difference in penetration depth between the encounter of these two fibers
was 215 gm, and the difference between the times of initial encounter was 40 minutes.
-147-
XDP87-65: CF=6.24kHz; TH=1.3dB SPL; SR=46sp/sec; Q10=4.34
XDP87-73: CF=6.31 kHz; TH=1.8dB SPL; SR=51sp/sec; Q10=4.78
I
I
1~
~
l
l
l
l
F
Thin line: XDP87-65
80-
Thick line: XDP87-73
.J
L
m
60-
a)
L
-n
Cn
o
F
200-
1
1.5
6
Acoustic Frequency (kHz)
___
_I
1
15
- 148-
Figure 6.17
Comparison of the effects of masking noise on tuning curves from the two fibers
described in Fig. 6.16.
-149-
XDP87-65: CF = 6.24 kHz; TH = 1.3'dB SPL; SR = 46 sp/sec
I
I
I
I
From top down:
-
I
TC In 8UOd 5PL Noise
In 75dB SPL Noise
In 70dB SPL Noise
TC In Quiet
8C
-J
CL
6CO-
-
I
I I
I
I
r
P~~
',
Cn
cn
4,
40 -
C
200
C,)
0-
(A))
Dashed lines mark 6 & 8kHz
T
I
1.5
15
I
6
15
Acoustic Frequency (kHz)
XDF87-73: CF = 6.31 kHz; TH = 1.8 dB SPL; SR = 51 sp/sec
I
8C
0
C,
u,
4n
1L
m
-J
1Du1
I
I
.
I
I-
From top down:
-
In ouc.
3r-L
I
I
Noise
In 75dB SPL Noise
In 70dB SPL Noise
TC In Quiet
IB
I
I
40 -
20 C
-0
C,
0-
(B)
Dashed lines mark 6 & 8kHz
I
I
1.5
I
I
I
I I I I
6
Acoustic Frequency (kHz)
l
5
115
.v
- 150 -
Figure 6.18
Comparison of the effects of the masking noise on rate-level functions measured
with 6 kHz tones from the two fibers described in Fig. 6.16.
-151
XDP87-65: CF = 6.24 kHz; TH = 1.3 dB SPL; SR = 46 sp/sec
II
I
I
I
I
I
I
I
I
I
I
II
A e* %
--
1tuV
Aooo=-
0
o
C,
0
v- In 90dB SPL Noise
+- In 95dB SPL Noise
6kHz Tone In Quiet
In 70dB SPL Noise
In 75dB SPL Noise
In 80dB SPL Noise
In 85dB SPL Noise
Dashed line marks mid-rate
Co
o
r%.O
C)
O
C,
0
(A)
0-
-
I
-15
·
I
i
iI
I
I
I
I
·
-
I I
105
Tone Level (dB SPL)
XDP87-73: CF = 6.31 kHz; Tnr = 1.8 dB SPL; SR = 5
! I
I
I
I
I
I
I
I
i
sp/sec
!I
400v- In 9CdB SPL Noise
+- In 95dB SPL Noise
6kHz Tone In Quiet
In 70dB SPL Noise
"o-
o- In 75dB SPL Noise
o- In 80dB SPL Noise
a- In 85dB SPL Noise
0
to
Cr)
0.
Dashed ine marks mid-rate
a,
-C
O
r~
.,-
O
(B)
0I
-15
I
I
I
I
I
I
I
I
Tone Level (dB SPL)
I
I
I
105
- 152 -
Figure 6.19
Comparison of the effects of masking noise on d functions measured with 6 kHz
tones from the two fibers described in Fig. 6.16.
-153-
XDP87-65: CF = 6.24 kHz; TH = 1.3 d
I I
I
aoooo-
In 65dB
In 70dB
In 75dB
In 80dB
In 85dB
I
I
SPL
SPL
SPL
SPL
SPL
Noise
Noise
Noise
Noise
Noise
I
I
I
SPL; SR = 46 sp/sec
I
I
I
I
4 A
1-
I
In 90dB SPL Noise
+- In 95dB SPL Noise
v-
(A)
1OI
I 1
-15
II
I
I
I
I
I
I
I
i
I
I
I
I
i
I
105
Tone Level (dB SPL)
XDPF87-73: CF = 6.31 kHz; TH = 1.8 dB SPL; SR = 5
!
I
I
I
1
14"-
6kHz Tone In Quiet
o-
In 7dB SPL Noise
oo-
In 75dB SPL Noise
a-
I
!
i
I
sp/sec
I
!
7
v- In 90dB SFL Noise
+- In 95dB SPL Noise
KL
In 80dB SPL Noise
In 85dB SPL Noise
L
L
(B)
i
m
10I I
-15
I
I
I
I
T
I
Tone Level (dB SPL)
I
I
I
I
105
-154 -
Figure 6.20
(A) Comparison of masking functions obtained from tuning-curve measurements at fiber
CF, at 6 kHz, and at 8 kHz for the two fibers described in Fig. 6.16. Average growthrate of masking (in dB/dB):
XDP87-65: 1.87 (at CF); 1.65 (at 6 kHz); 1.65 (at 8 kHz).
XDP87-73: 1.88 (at CF); 2.0 (at 6 kHz); 1.66 (at 8 kHz).
(B) Comparison of masking functions obtained from rate-level functions and from d
functions for the two fibers described in Fig. 6.12. Average growth-rate of masking (in
dB/dB):
XDP87-65: 1.41 (from rate functions); 2.14 (from d functions).
XDP87-73: 1.45 (from rate functions); 1.99 (from d functions).
.
-155-
Masking Measured from Elevation of Tuning Curves
_I
I
I
80-
m
I
_I
_I
I
.-e
Filled symbols & thick lines: XDP87-65
Open symbols & thin lines: XDP87-73
Dashed line has a slope of 2
o- Masking at CF
60- A- Masking at 6kHz
o- Masking at 8kHz
-
.11
.-I
1-1
'a
C
40-
E3
20-
00-
(A)
I
I
,I
I
I
60
I
I
I
10CC
11
RMS Noise Level (dB SPL)
Masking Noise: 350-650 Hz
I
80-
1
I
I
Filled symbols & thick lines: XDP87-65
Open symbols & thin lines: XDP87-73
-A -
I:
_^
LOuskNf
a60- o-
C
'R
cn
a
andpcssed noise
l
-
h-
_-
Ilre n
/
,-I
ot_
Masking a
Masking a
40-
ME
20/'
I,
"I
"I-
00I
60
I
I
%
(B)
-i,~~~~
I
I
100
RMS Noise Level (dB SPL)
- 156-
Fig. 6.20 that the masking functions of these two fibers were quite similar for each measure of'
the masking at all tone and noise levels. The masking patterns of these two fibers were also typical of others in that there was more masking at the fiber CF than at either lower or higher frequencies, and that the GRs of masking measured from
functions were larger than the
corresponding ones from the average-rate functions.
In the next section of this chapter, the masking data presented so far will be summarized
and discussed in the direction leading to the next stage in this study to be described in
Chapter VII.
6.4 Summary and Conclusions
In this chapter, patterns of the masking of high-frequency tone responses of high-CF
auditory-nerve fibers by narrow-band low-frequency noise were described with data from 144
auditory-nerve fibers from fourteen cats. The main points are: (1) With each of the three measures of the masking, the masking effect observed could be larger than 70 dB. (2) For each noise
level tested, the largest noise-induced elevation of threshold occurred near the tip of the tuning
curve. (3) The effects of the noise on the tonal rate-level function was to shift the function
towards higher tone levels, to elevate the background discharge rate, and sometimes to reduce the
plateau rate. All of these increased monotonically with noise intensity once the noise reached the
threshold for the effect. (4) The growth-rate of masking generally increased with the level of the
masking. The average GR of masking over all noise levels and fibers was approximately
2 dB/dB. (5) The masking patterns in two "randomly" sampled fibers which had similar tuning
properties were very similar in every aspect of the masking measured in this study, including the
growth-rate of masking. (6) There was no significant difference between the means of the average
growth-rate of masking for fibers from animals with intact versus with severed olivocochlear
efferents, for high SR fibers versus low-and-medium SR fibers, and for fibers tested with 6 kHz
versus with 8 kHz tones. (7) The average growth-rate of masking was typically largest in fibers
- 157-
whose 1tFs were near or slightly below the test frequency. Therefore, it is predicted by the working hypothesis of stapedius unmasking that the maximum unmasking provided by contractions of
the stapedius muscle should typically occur in these fibers, a prediction to be directly tested in the
next chapter.
The next chapter will discuss the testing of the working hypothesis of the effect of stapedius contractions on the masking described in this chapter. The significance and implications,
in the context of the overall goal of this study, of those above-mentioned points pertaining to the
growth-rate of masking should then become clearer.
-
158 -
Chapter VII
Results (II): Effects of Stapedius Contractions on Auditory-Nerve Fiber Responses
Two main topics will be addressed in this chapter: the effects of stapedius contractions on
auditory-nerve fiber responses, and the test of the working hypothesis for the effects of stapedius
contractions on the masking of auditory-nerve fiber responses. The first topic includes the effects
of stapedius contractions on auditory-nerve fiber responses to both single tones and tones in background noise, measured in the form of tuning curves, rate-level functions and
functions. The
effects of stapedius contractions on auditory-nerve fiber responses to single tones are to be compared with the effects of the same stapedius contractions on Cochlear-Microphonic level functions
at the same frequencies in the same ear, with emphasis on whether the stapedius-produced
attenuation of sound depends on the level of the sound. The second topic, the test of the working
hypothesis, will be addressed at the levels of both individual and populations of auditory-nerve
fibers.
7.1 Stapedius Effects on Auditory-Nerve Fiber Responses to Single Tones
Before any measurement of the effect of stapedius contractions on auditory-nerve fiber
responses is discussed, the time course of the effect of stapedius contractions produced by electric
stimulation needs to be described in the context of the timing of the measurement. With continuous electric stimulation of the stapedius the measurement is relatively straight-forward, as covered
in the "Methods" chapter; with short bursts of electric stimulation, on the other hand, rather precise timing is required for the measurement. The results of such measurement also enable one to
inspect the time course of the effects with a fine scale. The PST histogram in Figure 7.1 shows
the time course of the effect of stapedius contraction produced by bursts of electric stimulation of
200 ms duration on a low-CF auditory-nerve fiber's rate response to a continuous low-frequency
tone. The rate response started to decrease at approximately 25 ms after the onset of the burst of
- 159 -
Figure 7.1
Time course of the effects of bursts of electric stimulation to the stapedius muscle
on a low-CF auditory-nerve fiber's rate response to a low-frequency tone. A 0.5 kHz
tone, which had an excitatory effect on the fiber's discharge rate, was continuously
present. Electric pulse trains of 200 ms duration (5 ms inter-pulse interval) were
delivered to the stapedius 50 ms after the beginning of the PST sampling window. The
shock-induced stapes-head displacement was approximately 40 m. The two dashed vertical lines indicate a "steady-state" window of 100 ms duration. Unless otherwise stated,
all auditory-nerve fiber rate-level responses to tone bursts with bursts of shocks to the
stapedius were measured within the last 80 ms of this window (i.e., 120-200 ms after the
onset of the shock burst); and the timing and duration of the tone bursts were the same
as this 100 ms "steady-state" window.
-160-
XDP84-19; CF = 0.47 kHz; SR = 31 sp/sec
I
II
a)
_4--
m
U
Q)
U
-
0
_
I
'I)
*
,
..m,
L.
,
I
I
I
I
I
I
0.5kHz Continuous Tone;
Pulse-Shock Bursts
20 Repetitions at 1 per sec.;
5ms Binwidth
14UU-
I
I
I
i
I
I
Axa
I-
0
I I I
t
I
iI
Shocks
Time (ms)
I
I
I
500
0
- 161 -
electric stimulation to the stapedius muscle, and this reduction of the rate response reached a
maximum less than 50 ms after the onset of the shocks. The effect changed relatively little over
the next 175 ms or so and can be considered to be in a "steady-state". Such a time course was
typical of other observations. The choice of the "counting window" marked by the two dashed
vertical lines in Fig. 7.1 was based on the desire to measure the "steady-state" effect and the need
to avoid any possible "transient sound" following the onset of electric stimulation to the stapedius
muscle (see Section 5.7). Unless continuous electric stimulation of the stapedius was used, in all
other measurements of the effects of stapedius contractions on auditory-nerve fiber responses, the
timing of the tone bursts was the same as this window and the rate response was measured in the
last 80 ms of this window.
Figure 7.2 compares the effect of electric stimulation of the stapedius muscle on the tuning curve from a low-CF auditory-nerve fiber with the effect of the same electric stimulation on
the cochlear-microphonic (CM) level function in the same ear. The shock-induced stapedius contraction resulted in an elevation of the tuning curve (Fig. 7.2A) of 22.6 dB at 0.5 kHz. The effect
of the same electric stimulation of the stapedius on the CM level function at 0.5 kHz (Fig. 7.2B)
was approximately a parallel shift of the function towards higher tone levels, and the amount of
this shift (measured with a constant-response (mid-range) criterion) was 21.5 dB. These measures
are very close in magnitude, presumably because both are measures of the attenuation of the
acoustic stimulus reaching the inner ear produced by the stapedius-induced decrease in middle-ear
transmission. This point will be strengthened as it will be shown shortly that a close match as
seen in Fig. 7.2 between the shift in auditory-nerve fiber responses and the shift in CM level
functions for the same level of electric shocks to the stapedius muscle is not coincidental.
Figure 7.3 compares the effects of electric stimulation of the stapedius on the CF-tone
rate-level and
functions from a low-CF, high-SR auditory-nerve fiber with the effects on the
CM-level functions measured at the same frequency. Figure 7.3A & B show that at both intensities of the electric stimulation to the stapedius the effect of stapedius contractions on the ratelevel and
functions was to produce an approximately parallel shift of the functions towards
- 162 -
Figure 7.2
Comparison of the effects of a constant level of stapedius contraction on auditorynerve fiber tuning curve and on CM-level function.
(A) Tuning curves of a low-CF (0.5 kHz) auditory-nerve fiber with and without electric
stimulation of the stapedius muscle. The stapedius was stimulated continuously with
100 Hz sinusoidal current during the tuning-curve measurement and the resulting stapeshead displacement was 34 gm. The tuning-curve threshold was elevated at the CF by
22.6 dB with electric shock to the stapedius.
(B) Measurements of the cochlear-microphonic potential (CM) level functions at 0.5 kHz
with and without electric shocks to the stapedius.
These measurements were made
within a few minutes following the measurements in (A). The electric stimulation to the
stapedius was the same as that employed in (A). The CM level function with shocks to
the stapedius was approximately a translation towards higher tone levels of the level
function without shocks to the stapedius (the noise floor for the CM measurements was
approximately 5 dB re. 1 gv). The amount of translation near the mid-range of the CM
response (the arithmetic mean of the maximum and minimum CM response measured in
the dB scale), or the attenuation of sound produced by the contraction of the stapedius
muscle, was 21.5 dB. The dashed curve with open circles is a replot of the solid curve
with open circles translated by 21.5 dB towards higher tone levels. Unless otherwise
stated, for all CM-level functions presented in this chapter, each point is the average of
four measurements under identical conditions.
~~~
~~
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~-~~ ~~1·1·~
1~1~~~~~~~~~~~~~~~~~~~~~~~__._
~~~~~~~_
---------_.____'I
-163-
SPL; SR = 0.1 sp/sec
XDP74-36; CF = 0.5 kHz; TH = 24.4 d
lJ
CL
(,
90-
,
,
,
I
110-
,
,
, I,
i
I
Lower Curve: Normal TC (TH=24.4dB SPL)
Upper Curve: TC with 8dB Shocks to Stapedius
Upper Curve: Elevated by 22.6dB at CF
Dashed line marks CF
m
a,
70 -
(A)
Cn
50Co
u,
30-
2
inv
I
0.1
_
2
I
2
0.5
Acoustic Frequency (kHz)
I
I
60 -
=3
>
40 -
¥
&_
03
o-
e
I
!
0.5kHz Tone-Only
-
Tone with Bd3 Shocks (21.5dB shift)
- -
Tone-Only Translated by 21.5dB
Dashed horizontal line marks mid-range
XDP74-L(36)
(B)
%v
M
:E
(J
20-
---------
0
4
40
60
80
8O
I
100
Tone Level (dB SPL)
-·
-164-
Figure 7.3
Comparison of the effects of stapedius contractions on rate-level and d-level functions of a low-CF, high-SR auditory-nerve fiber, with effects on CM-level functions at
the same low-frequency.
(A) The effects of electric shocks to the stapedius muscle on the rate-level function of a
low-CF (0.49 kHz), high-SR auditory-nerve fiber. The stapedius was stimulated with
electric pulse bursts of 200 ms duration (5 ms inter-pulse interval, 1 second repetition
period). The onset of each shock burst preceded the beginning of the spike-count window by 120 ms. The stapedius-produced shifts of the rate-level functions towards higher
tone levels were 6 and 14.1 dB (measured at mid-rate) for the 2 dB and 4 dB shock
intensities, respectively. The three curves are superimposed on the right with the curves
with filled triangles and filled squares translated by 6 and 14.1 dB, respectively, relative
to the curve with open squares. The value of the horizontal axis is only meaningful for
the non-superimposed curves (same for (B) and (C)). In all figure captions, only when
referring to the same ear does a larger number in the amplitude of the electric shock
indicate a stronger shock.
(B) Same as (A) except that the d-level functions are plotted. The stapedius-induced
shift of d functions (at d = 1) were 6.3 and 14.1 dB, respectively, for the 2 dB and 4 dB
shock intensities. The three curves are superimposed on the right with the curves with
filled triangles and filled squares translated by 6.3 and 14.1 dB, respectively, relative to
the curve with open squares.
(C) The effects of electric shocks to the stapedius the same as those employed in (A) on
CM-level functions measured at the same frequency as in (A). These measurements were
made within a few minutes after the measurement of (A). The stapedius-induced shifts
(at mid-range) were 6 and 13.5 dB, respectively, for the 2 dB and 4 dB shock intensities.
The three curves are superimposed on the right with the curves with filled triangles and
filled squares translated by 6 and 13.5 dB, respectively, relative to the curve with open
squares. Each division of the horizontal axis is the same in (A), (B) and (C).
-165-
XDP86-98; CF = 0.49 kHz; TH = 11.9 dB SPL; SR = 44 sp/sec
I
I
0
300=
I
I
I
I
I
I
I
I
Dashed Line Marks Mid-Rate
0.5kHz Tone Only
a-
I
=
- *- Tone and 2dB Shocks to Stapedius (6dB shift)
0co
*- Tone and 4dB Shocks to Stapedius (14.1dB shift)
(/
0
0
C
I
I
I
0
I
I
I
I
I
I
70
Tone Level (dB SPL)
I
I
I
I
I
I
I1
I
I
I
I
14-
0.5kHz Tone Only
3-
*- Tone and 2dB Shccks to Staoedius (6.3dB shift)
a- Tone and 4dB Shocks to Staedius (14.1dB shift)
/ r-,\
F
E
CI
I
I
0
I
I
I
I
I
I
70
i
I
Tone Level (dB SPL)
]I
I
0-;2'
L.
I
I
I
I
I
o- 0.5kHz Tone Only
A- 2dB Shocks to Stapedius (6dB shift)
m- 4dB Shocks to Stapedius (13.5dB shift)
XDP86-L(98)
I
I
i
I
Dashed Line Marks Mid-Rcnge
(C)
V4;
- - - - - - -
m
a
- 9;
V
I
40
1
I
I
90
Tone Level (dB SPL)
I
I
I
F
- 166 -
higher tone levels. For both intensities of the electric stimulation to the stapedius muscle, the
stapedius-induced shift of the rate-level function corresponds well with the shift of the d-level
function, which also corresponds well with the shift of the CM-level function.
Figure 7.4 shows the same kind of measurements as shown in Fig. 7.3, but for a high-CF,
medium-SR auditory-nerve fiber at a high frequency. Again the effect of the stapedius contractions on the level functions was approximately a parallel shift of the functions and the amount of
shift of the single-fiber level functions corresponds well to that of the CM-level functions.
More complete measurements of the effects of stapedius contractions on CM-level functions are shown in Fig. 7.5 and again the effects were approximately parallel shifts of the level
functions towards higher tone levels for all intensities of the electric stimulation to the stapedius.
The amount of the effect (shift) increased monotonically with the shock intensity, reaching about
30 and 14 dB at the low- and high-frequency, respectively, with the highest shock intensities
used.
Figure 7.6 compares measurements from single auditory-nerve fibers with those from CM
of the attenuation of sound reaching the inner ear produced by contractions of the stapedius muscle. The single-fiber measurements included tuning curves and rate-level functions from fibers of
either low- or high-CF at either low or high frequencies (The large attenuations of sound were
measured at low frequencies and most of the small attenuations were measured at high frequencies.). The corresponding CM measurements were all made from the same ear at the same frequencies and with the same shocks to the stapedius as for the single-fiber measurements. Statistics for the differences between the CM and the single-fiber measurements are given in the figure
caption. The (null) hypothesis that the mean of the difference is zero (against the alternative
hypothesis that it is not zero, i.e., two-sided t-test) cannot be rejected at a significance level of
ca=0.2.
As discussed in Sections 2.4 and 4.1, a key assumption involved in quantitatively predicting the unmasking effect of stapedius contractions is that for a given status of stapeds contraction, the attenuation of a sound does not depend on the level of the sound. Measurements of 84
- 167 -
Figure 7.4
Comparison of the effects of stapedius contractions on rate-level and d-level functions of a high-CF, medium-SR auditory-nerve fiber, with effects on CM-level functions
at the same high-frequency.
(A) The effects of electric shocks to the stapedius muscle on the rate-level function of a
high-CF (5.07 kHz), medium-SR auditory-nerve fiber. Stimulation of the stapedius had
the same temporal structure as that described in the caption for Fig. 7.3, but at higher
levels. The stapedius-induced shifts of the rate-level function towards higher tone levels
were 6.2 and 11.9 dB (measured at mid-rate), respectively, for the 5 dB and 7 dB shock
intensities.
The three curves are superimposed on the right in a similar way as in
Fig. 7.3 (same for (B) and (C)). The value of the horizontal axis is only meaningful for
non-superimposed curves.
(B) Same as (A) except that the -level functions are plotted. The stapedius-induced
shifts of the
function were 6.0 and 11.1 dB, respectively, for the 5 dB and 7 dB shock
intensities.
(C) The effects of electric shocks to the stapedius the same as those employed in (A) on
CM-level functions measured at the same frequency as (A). These measurements were
made within a few minutes following the measurement of (A). The stapedius-induced
shifts were 6.1 and 11.3 dB, respectively, for the 5 dB and 7 dB shock intensities.
._l
q______
__
-168-
XDP86-85; CF = 5.07 kHz; TH = 22.2 dB SPL; SR = 4.7 sp/sec
I
I
X
J
o
400-
I
I
I
I
I...
I
-
I
I
I
I
I
I
I
1
I
I
I
Dashed Line Marks Mid-Rate
6kHz Tone Only
Tone and 5dB Shocks to Stapedius (6.2dB shift)
Tone and 7dB Shocks to Stapedius (11.9dB shift)
aA-
·Co
V
U)
0.
a,
0
C.)
-C
U)
0I
I
30
I
I
I
I
i
I
I
90
I
I
I
Tone Level (dB SPL)
I
I
I
I
I
I
I
I
I
146kHz Tone Only
*- Tone and 5dB Shocks to Stapedius (6.0dB shift)
*- Tone and 7dB Shocks to Stapedius (11.1dB shift)
a-
(I
1-
0I
30
I
I
I
I
I
I
90
I
I
I
I
I
I
I
I
I I
Tone Level (dB SPL)
I
65,J
I
I
I
I
I
I
I
I
Dashed Line Marks Mid-Range
o- 6kHz Tone Only
*- Tone and 5dB Shocks to Stapedius (6.1dB shift)
*- Tone and 7dB Shocks to Stapedius (11.3dB shift)
XDP86-L(85)
L.
(C)
m
______.-
C
-
-5I
I
30
I
I
I
I
90
Tone Level (dB SPL)
I
II
II
I
- 169 -
Figure 7.5
The effects of electric shocks to the stapedius muscle on cochlear-microphonic
(CM) level functions.
(A) CM-level functions measured at a low frequency (562 Hz). The stapedius was
stimulated with continuous sinusoidal current at 100 Hz. The attenuation of sound reaching the inner ear, as seen from the shift of the CM level function along the tone-level
axis with a constant-response criterion (mid-range), ranges from 3 to 30 dB. The "No
Stimulus" and "8 dB Shock Only" measurements provide an estimate of the noise floor
of the measurement and a control for shock artifact. The five level functions are superimposed on the right (excluding the two control measurements shown in the left half of the
panel) by translating each curve with shock to the stapedius by the amount shown in the
corresponding parentheses, respectively, relative to the "Tone Only" curve. The numbers
on the horizontal axis are only meaningful for the non-superimposed curves.
(B) Similar to (A) except that the CM-level functions were measured at a high frequency
(5623 Hz) from a different ear. The stapedius was stimulated with continuous pulse
shocks (5 ms inter-pulse interval). To provide a control for muscle "fatigue" during the
measurement, the level function with 7 dB shocks was measured twice, one with tone
level changing from low level to high, and the other from high level to low (as indicated
by "(L-H)" and "(H-L)" in the figure labels, respectively).
-170-
I
50-
I
aao-
I
I
I
II
I
I
I
I
I
Tone & 2dB Shocks
+- Tone & ldB Shocks
x- Tone & 4dB Shocks
T¶nI
-
562 Hz Tone Only
I,
hr
QA
.
I
^
br i '
.1.
cJ._,
L
I
0-
XC
(
VL
(
m
0Dashed line: Mid-range
I
40
I I
I
I
I
90
I
I
Tone Level (dB SPL)
I
I
50o-
I
I
I
I
I
I
I
I
I
I
I
I
I
I
5623 Hz Tone Only
+- Tone (L-H) and 7dB Shocks (3.7dB shift)
d-
Tone (H-L) and 7dB Shocks (3.7dB shift)
x- Tone and 8dB Shocks (13.7dB shift)
XDP85-L
(B)
m
'2
--- --- --
0
0Dashed line: Mid-range
I
I
40
I
I
1
1
1_
90
1
1
I I
I
1I
Tone Level (dB SPL)
--1---^11--11------
- 171 -
Figure 7.6
Stapedius-induced attenuation of sound assessed from cochlear-microphonic potential measurements versus that assessed from single auditory-nerve fiber measurements, at
the same frequency and with shocks of the same intensity, both using constant response
criteria. All test sounds were single tones. Single-fiber measurements include both tuning curves and rate-level functions, at either low- or high-frequencies. There are 24 data
points from 20 auditory-nerve fibers from eight animals (all data available) in the plot.
The statistics for the difference between CM and single-fiber (SF) measurements are (in
dB):
For CM-SF, minimum = -4.2, maximum = 1.8, mean = -0.25, standard deviation = 1.02.
For ICM-SFI (the absolute value of the difference), minimum = 0.0, maximum = 4.2,
mean = 0.58, standard deviation = 0.88.
The result of a linear-regression analysis is given in the figure labelling, with "int."
stands for "intercept" and "corr." for "correlation". (The dashed line is not the regression
line, but rather a reference line.)
-172-
Stapedius-lnduced Attenuation of Sound
I
I
I
I
Al f
I_
Linear regression: slope=1.03, int.=-0.17, corr.=0.99
/
Dashed line has unit slope
m
/
/
E)
I
/
o
IV)
cv
a)
(/
0/
E
0
._
C
8/
0
o/
/,
C
4-a
/o
0
t'3_
/'
0
I
I
I
I40
40
Sound-Attenuation from CM Measurement (dB)
_._ ._.III___I_^IL- 1. _·11__11__11_____1^_1_11_^_____1_1_.__.
_I
-
173 -
CM-level functions similar to those 'shown in Fig. 7.5 (other examples can be found in
Figs. 7.2-7.4, 7.8, 7.10 and 7.12) show that when the level functions with and without shocks to
stapedius are superimposed in the way as shown in Fig. 7.5, the maximum difference between the
level function with shocks and the level function without shocks is about 2 dB in tone level over
all CM levels in both the low and high frequency regions.* These measurements directly support
the linearity assumption with stapedius contractions, thus clearing the path for quantitatively
predicting the stapedius-unmasking effect.
7.2 Stapedius Effects on Auditory-Nerve Fiber Responses to Tones in Noise
The effects of contractions of the stapedius muscle on masking of the responses of highCF auditory-nerve fibers to high-frequency tones by continuous narrow-band low-frequency noise
will be described in this section, along with the test of the working hypothesis which attempts to
quantitatively explain such effects.
Figure 7.7A shows the effects of a moderate-strength stapedius contraction on the masking
of the tone threshold of a high-CF auditory-nerve fiber by a low-frequency noise. The masking
effect of a 55 dB SPL noise, which is 7.7 dB above the noise level for masking to occur, on the
tone threshold of the fiber at the CF is a 17.8 dB elevation. This effect was completely removed
by the stapedius contraction, which produced a 7.9 dB attenuation of the noise, in that the "residual" difference of 2.5 dB in the threshold at the CF between the tuning-curve measurement with
55 dB SPL noise and the stapedius contraction, and the tuning curve measured in quiet approximately equals the stapedius-produced attenuation of sound (2.6 dB) in the CF region, as shown in
Fig. 7.7B. The relevant growth-rate (GR) of masking at the fiber CF can be calculated from the
* At the turning corner from the CM noise floor to the "suprathreshold" region of the level function, there is sometimes
a technical problem in measuring the difference between two level functions in that since the data points were measured
in discrete steps and connected by straight line segments, when for one level function there is a datum point at the
corner and for the other level function the closest data points to the corner are on the two sides of the corner and are
connected by a "chord" which "bypasses" the corner, then a difference between the two level functions is "created" near
the corner due to sampling. The same problem exists with rate-level functions at both the corner near the background
discharge rate and the corner near the "plateau" rate. Such "chord-produced" difference between level functions is not
counted in measuring the maximum deviation from "parallel shifts" of the level functions.
- 174 -
Figure 7.7
The effects of stapedius contractions on the masking by continuous low-frequency
noise of tone responses from a high-CF auditory-nerve fiber observed from tuning-curve
(TC) measurements, and the effect on middle-ear transmission in the tone and noise frequency regions.
(A) Four "tuning curves" from a high-CF auditory-nerve fiber measured under the following conditions: (1) Tonal stimuli only ("Normal" TC, CF=8.04 kHz,
TH=7.8 dB SPL); (2) Tonal stimuli with a masking noise of 55 dB SPL (solid line,
elevated near the "tip" of the curve from the "Normal" TC by M=17.8 dB); (3) Tonal
stimuli with a masking noise of 55 dB SPL and with stapedius contraction (heavy dashed
line, with "threshold" = 10.3 dB SPL at CF); and (4) Tonal stimuli with a masking noise
of 50 dB SPL (solid line, elevated near the "tip" of the curve from the "Normal" TC by
m=6.3 dB). The difference between (2) and (3) in "threshold" at CF (the amount of
"unmasking" by the stapedius contraction) was 15.3 dB. From "M" and "m", which
denote the amount of masking corresponding to the two noise levels, respectively, the
growth-rate of the masking function can be calculated as: GR = (M-m)/(NM-Nm)
(17.8-6.3)/(55-50) = 2.3 dB/dB, and the masking threshold can be inferred by extrapola-
tion as 55-(GR/M) = 47.3 dB SPL noise. The masking noise was continuous noise
bandpassed between 350-650Hz. At an intensity of 55 dB SPL, the noise was just intense
enough to excite the fiber by itself. The stapedius-muscle contraction was elicited with a
continuous 100 Hz sinusoid electric stimulation of the muscle and produced a stapeshead displacement of 37 m.
(B) Magnitude of the stapedius-induced attenuation of sound in the frequency regions of
the noise and of the fiber CF, respectively, with the shocks to the stapedius the same as
in (A). These measurements were made by taking the difference in CM levels with and
without shocks to the stapedius. The CM-level functions had approximately unity slopes
in the sound-level region (60-70'dB SPL) involved in the measurements. The horizontal
dashed line marks 0 dB and the vertical dashed line is placed at 8 kHz to facilitate the
reading of AITI. The attenuation of sound at 0.5 kHz and 8 kHz was 7.9 and 2.6 dB,
respectively. Measurements were made in equal intervals of 1/6 octave in log frequency
and each point was the average of four measurements. All curves were processed with
one three-point triangular moving average.
-175-
XDP65-3; CF = 8.04 kHz; TH = 7.8 dB SPL; SR = 65 sp/sec
I
I
I
IIII
I
I
I
I
I
I
I
J
I
m=6.3dB (50dB Noise)
M=17.8dB (55dB Noise);
80-
I
GR = (M-m)/(55-50) = 2.3dB/dB
Dashed TC: 15.3dB unmasking
(A)I
-j
CV)
M
60-
L.
cn
C,,
4)
t
40-
Normal
L.
C-0
C
0
U,
20m
V I1
I
0-
_
0
I
I
T
,
i
I
I
I
t i
I
1
20
8
2
0.2
.
Acoustic Frequency (kHz)
I
-0
I
I
,
I
,
II
I
I
I
I
I
I
I
,
0.5kHz: 7.9dB;
8kHz: 2.6dB
Predicted Unmcsking: 7.9xGR-2.6 = 15.5 dBr
0._
-
(B)
.I- -E
I
,_...
00
-
:
.aj :
-20-
I
I
0.2
I
I
I
I
I
I
I__
III·
11111
I II
III
2
Acoustic Frequency (kHz)
II
8
20
- 176-
elevation of thresh6ld at CF produced by 55 and 50 dB SPL noise, respectively, as shown in
Fig. 7.7A. The measurement of the stapedius-produced attenuation of sound in the frequency
regions of the noise and the CF tone is shown in Fig. 7.7B. From these, the working hypothesis
predicts a stapedius-unmasking effect of 15.5 dB, which is close to the measured stapedius
unmasking of 15.3 dB. Note that in this chapter for the purpose of predicting the stapediusproduced unmasking, the GR of masking is measured in the noise intensity range (with the
closest possible approximation) which corresponds to the stapedius-induced attenuation of the
noise. Since the masking function was measured in 5 dB steps of noise level, if the stapediusinduced noise attenuation was not an integer multiple of 5 dB, then an extrapolation or interpolation was used to determine the noise range for GR measurement. In the case of an extrapolation,
however, since the resultant GR will be the same as the GR used for the extrapolation, no explicit extrapolation is needed (as is the case with Fig. 7.7).
Figure 7.8 shows the effects of stapedius contractions on the masking by a low-frequency
noise of the average-rate response to a high-frequency tone of a high-CF auditory-nerve fiber,
measured with a constant-response (mid-rate) criterion. The 85 dB SPL noise produced a 43.6 dB
shift of the tone rate-level function. The addition of shock-induced stapedius contraction reduced
this to a 11.9 dB shift (i.e., the stapedius contraction produced a 31.7 dB "unmasking"). The
same level of stapedius contraction produced 13.1 dB and 3.3 dB attenuations of sound in the
frequency region of the noise and the tone, respectively. In this case the working hypothesis
predicts an unmasking effect of 31.3 dB by the contraction of the stapedius muscle, which is very
close to the measured unmasking of 31.7 dB.
Figure 7.9 shows the effects of stapedius contraction on masking by low-frequency noise
measured with
a functions
in a high-CF auditory-nerve fiber at both 6 and 8 kHz. The masking
effect of a 90 dB SPL noise on the
function at 8 kHz (a 36.2 dB masking) and at 6 kHz (a
33.1 dB masking) was reduced by 30.8 dB and 27.1 dB, respectively, by the stapedius contraction. Figure 7.10- shows the corresponding stapedius-produced attenuations of sound at 8 and
6 kHz and at the center frequency of the narrow-band masking noise. Combining Figs. 7.9 and
- 177 -
Figure 7.8
The effects of stapedius contractions on the masking by continuous low-frequency
noise of tone responses of a high-CF auditory-nerve fiber observed from rate-level functions, and the stapedius-induced attenuation of sound in the tone and noise frequency
regions.
(A) The right-most curve is the rate-level response to 8 kHz tone bursts in 85 dB SPL
masking noise. The curve third from the right with filled symbols was measured under
conditions identical to that of the right-most curve except that the stapedius muscle was
shocked at the same time. Electric stimulation of the stapedius had the same time structure as that in Fig. 7.3; when not explicitly stated this will be the default case throughout
the rest of this chapter for auditory-nerve fiber level function measurements. The
difference between these two curves at mid-rate along the tone-level axis (the amount of
"unmasking" by the stapedius contraction) is 31.7 dB. The growth-rate of masking GR
can be computed from the difference between the two curves measured with 85 (right
most) and 75 dB SPL (second from the right) masking noise, respectively, as
2.64 dB/dB. The left-most curve is rate-level function measured in quiet and is plotted to
serve as a reference for mid-rate and masking measurement.
(B) Measurements of CM-level functions at 0.5 kHz, the center frequency of the masking
noise, with and without shocks to the stapedius. These measurements were made within a
few minutes following the measurements in (A). The electric stimulation to the stapedius was at the same level as that in (A). The CM level function with shocks to the
stapedius was approximately a translation along the tone-level axis of the level function
without shocks to the stapedius (the CM noise floor was approximately 1 Lv). The
amount of translation near the mid-range of the CM response (i.e., the stapedius-induced
attenuation of sound at 0.5 kHz) was 13.1 dB.
(C) Same as (B) except that the CM level functions were measured at 8 kHz, the frequency of the tonal signal used in (A). The stapedius-induced attenuation of sound was
3.3 dB.
-178-
XDP86-79; CF = 7.5 kHz; TH = 7.3 dB SPL; SR = 90 sp/sec
I
400-
I
o*o-
U
V)
I
I
I
I
I
I
I
I
I
kHz Tone In Quiet
In 85dB SPL Noise (43.6dB masking)
85dB SPL Noise & 4dB Shocks (11.9dB masking)
In 75dB SPL Noise (17.2dB masking)
a-
Predicted Unmasking:
13.1 xGR-3.3=31.3dB
Unmasking: (43.6-11.9)=31.7dB
r,
-
Measured Unmasking:
31.7dB
(A
Q
I..
C
Dashed Line Marks Mid-Rate
GR=(43.6-17.2)/(85-75)=2.64
nI5
-5
I
I
I
I
I
I
I
I
I
85
Tone Level (dB SPL)
I
45-
0-
I
I
I
I
I
I
F
*- 0.5kHz Tone with 4dB Shocks
Das
Shif
XDP
IJ
I
I
0.5kHz Tone Only
45-
V_-13
I
Y
I.
I
I
I
I
I
I
8kHz Tone Only
*- 8kHz Tone with 4dB Shocks
Dashed Line Marks Mid-Range
Shift: 3.3dB
XDP86-L(79)
(B
m
I
0-
(C)
-
-
-
-
-
-
-
_
0
-5I I
15
I
I
I
r
90
Tone Level (dB SPL)
I
15
.
I
.
I
.
I
.
I
.
s
r
I
I
I
Tone Level (dB SPL)
-
I
90
- 179-
Figure 7.9
The effects of stapedius contractions on the masking by continuous low-frequency
noise of tone responses of a high-CF auditory-nerve fiber observed from d-level functions at 8 and 6 kHz.
(A) Measurements with 8 kHz tone bursts. The right-most curve is the d-level function
in 90 dB SPL masking noise. The curve third from the right with filled symbols was
obtained under conditions same as that for the right-most curve plus shock to the stapedius muscle. The difference between the two curves at d=l along the tone-level axis
(the amount of "unmasking" by the stapedius contraction) is 30.8 dB. The growth-rate of
masking GR can be computed from the difference between the two curves measured with
90 (right most) and 80 dB SPL (second from the right) masking noise, respectively, as
2.8 dB/dB. The left-most curve is d-level function obtained in quiet and is plotted to
serve as a reference for masking measurement.
(B) Similar to (A) except that data were obtained with 6 kHz tone bursts from the same
fiber. The amount of stapedius unmasking was 27.1 dB and the GR of masking was
2.7 dB/dB.
-180-
XDP86-104; CF = 7.0 kHz; TH = 10.3 dB SPL; SR = 75 sp/sec
I
I
a-
8kHz Tone In Quiet
In 80dB SPL Noise (8dB masking)
In 90dB SPL Noise (36.2dB masking)
90dB SPL Noise & 3dB Shocks (5.4dB masking)
I
I
I
I
I
I
I
I
14oo*-
Unmasking: (36.2-5.4) = 30.8dB
GR8=(36.2-8)/(90-80)=2.8dB/dB
(A:
10I
15
I
I
I
I
I
I
I
95
Tone Level (dB SPL)
4
1
I
1
oo-
6kHz Tone In Quiet
In 80dB SPL Noise (6dB masking)
I
)
I
I
I
I
-
o- In 90dB SPL Noise (33.1dB masking)
*- 90dB SPL Noise & 3dB Shocks (6dB masking)
Unmasking: (33.1-6) = 27.1dB
GR6=(33. 1-6)/(90-80)=2.7dB/dB
(B\
(E
- - -
1O-
I
15
I
I
I
I
I
95
Tone Level (dB SPL)
.
I
- 181 -
Figure 7.10
Measurements of the stapedius-induced attenuation of sound at 8, 6 and 0.5 kHz
produced by electric shocks same as those in Fig. 7.9. These measurements were made
within a few minutes following the measurements in Fig. 7.9.
(A) Measurements of CM-level functions at 8 kHz with and without shocks to the stapedius. The stapedius-induced attenuation of sound (measured at mid-range) was 1.6 dB.
(B) Measurements of CM-level functions at 6 kHz with and without shocks to the stapedius. The stapedius-induced attenuation of sound (measured at mid-range) was 5.2 dB.
(C) Measurements of CM-level functions at 0.5 kHz, the center frequency of the masking
noise, with and without shocks to the stapedius. The stapedius-induced attenuation of
sound (measured at mid-range) was 12.2 dB.
0
s
-182-
I
I
I
I
I
I
I
A C_
_
o*-
I
I
I
J
6kHz Tone Only
6kHz Tone with 3dB Shocks
nnarhpd Line Mnrks Mid--Rnnoe
Shift: 5.:
XDP86-L
(eB)
I;
-
I
_AC_
okHz Tone Only
*- 8kHz Tone with 3dB Shocks
Dashed Line Marks Mid-Range
Shift: 1.6dB
XDP86L.
(A)
m
_-V
0
0
-_
I
40
I
I
I
I
I
90
Tone Level (dB SPL)
I
45-
I
I
I
I
40
I
I
Tone Level (dB SPL)
I
t
Predicted Unmasking:
1 o- 0.5kHz Tone Only
Ow
Sh
XD
At 8kHz:
At 6kHz:
(¢
Measured Unmasking:
9L.
At 8kHz:
At 6kHz:
-5o
;e
-5I
40
I
i
I
I
90
Tone Level (dB SPL)
12.2xGR8-1.6=32.5dB
12.2xGR6 -5.2=27.8dB
30.8dB
27.1dB
I
90
- 183 -
7.10, it can be seen that the stapedius contraction almost completely removed the masking effect
of the 90 dB SPL noise at both 6 and 8 kHz, and that the stapedius unmasking predicted by the
working hypothesis matched well the measured stapedius unmasking at both 6 and 8 kHz.
It was mentioned in Section 6.3 that the growth-rate of masking measured from
func-
tions was typically larger than the GR measured from the corresponding rate-level functions.
Consequently, it is expected from the working hypothesis that the unmasking effect of a stapedius
contraction would also be larger with
function measurement than with the corresponding rate-
level function measurement. Experimental measurements showed, with an example given in
Fig. 7.11, that such was the case. Figure 7.11 compares the growth-rate of masking and the
unmasking effect of a stapedius contraction measured from rate-level functions with that from the
corresponding d functions for the auditory-nerve fiber that had been described in Fig. 7.8.
Although the absolute amount of masking and unmasking was different with the two measures,
for a given measure of masking (and unmasking) the predicted stapedius unmasking matched well
with the measured unmasking.
Figure 7.12 shows the same kind of measurements as shown in Figs. 7.9 and 7.10, but
from the animal with severed olivocochlear efferents. The effects of stapedius contractions on the
CM-level functions (Fig. 7.12B & C) was still a parallel shift of the function.
Again the
stapedius-unmasking effect predicted from the working hypothesis matched well with the measured unmasking.
The examples presented so far of the effects of stapedius contractions on the masking by
low-frequency noise of tone responses of high-CF auditory-nerve fibers all showed the effects
with one level of noise. Figure 7.13 shows examples of the effects of stapedius contractions on
the masking of the response to a high-frequency tone at two noise levels, measured with
func-
tions or with tonal rate-level functions in auditory-nerve fibers of high- and medium-SR, respectively. If the masking function in the absence of stapedius contraction is a straight line (as in
Fig. 7.13A), then the working hypothesis predicts that the effects of a stapedius contractions on
the masking function would be a parallel shift of the function towards higher noise levels, and
- 184 -
Figure 7.11
Comparison of the stapedius-unmasking measured from rate functions with that
from d functions in an auditory-nerve fiber.
(A) Same as Fig. 7.8A, replotted for convenience of comparison.
(B) The a functions corresponding to the relevant curves in (A). The effect of the stapedius unmasking was larger than that seen in (A). The growth-rate of masking, GR
(denoted in the figure as GRd for clearity), however, was also larger than that seen in
(A), and the close match between the predicted unmasking and measured unmasking is
maintained. The curve corresponds to "8kHz Tone in Quiet" is left out here because it
makes no point.
-185-
XDP86-79; CF = 7.5 kHz; TH = 7.3 dB SPL; SR = 90 sp/set
I
400-
I
I
I
I
I
I
I
I
I
8kHz Tone In Quiet
=-
o- In 85dB SPL Noise (43.6dB masking)
*- 85dB SPL Noise & 4dB Shocks (11.9dB masking)
o- In 75dB SPL Noise (17.2dB masking)
Unmasking: (43.6-1 1.9)=31.7dB
U)
0
Predicted Unmasking:
13.1xGR-3.3=31.3dB
Measured Unmasking:
31.7dB
D,
V)
q)
(L
.0
0
n
Dashed Line Marks Mid-Rate
GR (43.6-17.2)/(85-75)=2.64
0I
I
I
I
I
I
I
I
I
i
85
-5
Tone Level (d8 SPL)
I
I
9-
I
I
I
I
I
I
I
o-
8kHz Tone In 85dB SPL Ncise
*-
8kHz Tone with 85dB SFL Noise & 4B Shocks
o-
8kHz Tone In 75dB SPL Noise
I
i
-
Predicted Unmasking:
13.1 xGR.-3.3=34.8d8
Unmasking: 35.1 dB
.
,, _
fl;
.-
Measured Unmasking:
. .-
-
(E
-o
!
0I
-5
I
·
I
I
I
·
·
·
I
I
I
Tone Level (dB SPL)
·
I
I
I
85
35.1dB
- 186 -
Figure 7.12
The effects of stapedius contractions on the masking of tone responses of a high-CF
auditory-nerve fiber by continuous low-frequency noise observed from d-level function
measurements in a cat with severed olivocochlear efferents, and the effects on middle-ear
transmission at the tone and noise frequency regions.
(A) The right-most curve is a d-level function obtained with 6 kHz tone bursts in
95 dB SPL masking noise. The curve second from the right with filled symbols was
measured under conditions identical to that of the right-most curve plus shock to the stapedius muscle. The difference between these two curves at d=l along the tone-level axis
(the amount of "unmasking" by the stapedius contraction) is 24.3 dB. The growth-rate of
masking GR can be computed from the difference between the two curves measured with
95 and 75 dB SPL masking noise (first and third from the right, respectively), as
1.64 dB/dB. The left-most curve is -level function measured in quiet and is plotted to
serve as a reference for the masking measurement.
(B) Measurements of CM-level functions at 0.5 kHz, the center frequency of the masking
noise, with and without shocks to the stapedius. These measurements were made within a
few minutes following the measurements in (A). The electric stimulation to the stapedius was at the same level as that in (A). The CM level function with shocks to the
stapedius was approximately a translation along the tone-level axis of the level function
without shocks to the stapedius (the CM noise floor was near -3 dB re. 1 pv). The translation near the mid-range of the CM response (the stapedius-induced attenuation of sound
at 0.5 kHz) was 21.3 dB.
(C) Same as (B) except that the CM level functions were measured at 6 kHz, the frequency of the tonal signal used in (A). The stapedius-induced attenuation of sound was
10.9 dB.
_
-
_C.__._
.-..-.
--I^__
-·----^I__
-187-
21.2 dB SPL; SR - 1.6 sp/: sec
XDP89-22; CF = 6.92 kHz; TH
I
1C
I
o-
I
I
I
I
I
I
I
I
6kHz Tone In Quiet
A- In 75dB SPL Noise (9.2d8 masking)
o- In 95dB SPL Noise (41.9dB masking)
*- 95dB SPL Noise & dB Shocks (17.6dB masking)
Unmasking: (41.9-17.6) = 24.3dB
Predicted Unmasking:
21.3xGR-1 0.9=24dB
Measured Unmasking:
24.3dB
GR = (41.9-9.2)/(95-75) = 1.64dB/dB
(A)
PP
1I 0
I
L :
I
I
0
I
I
I
I
I
i
I
100
Tone Level (dB SPL)
I
45-
I
I
I
I
c- 0.5kHz Tone Only
a- 0.5kH7 Tone witn ldR Shocks
Sh;f
4.5-
-
o-
I
n
Onl
6Hz Tone Only
)
)
Shi
XDF
2
I
XOF
-23
-
-
(e
(C
m
_-
ao
a,,
rrl1
<J
0
r
Uasrea Line MarKs Ma-Range
__C,_
--
I
40
I
i
I
I
Tone Level (dB SPL)
neC.
91
c30
I
40
I
I
I
Tone Level (dB SPQ
I
1
c)O
- 188-
Figure 7.13
The effects of stapedius contractions on the masking functions of two high-CF
auditory-nerve fibers.
(A) The effects of a fixed level stapedius contractions on the masking of tone responses
at two noise levels in a high-SR fiber observed from d-level function measurements. The
growth-rate of masking with and without shocks to the stapedius was 1.706 and
1.710 dB/dB, respectively.
(B) The effects of a fixed level stapedius contractions on the masking of tone responses
at two noise levels in a medium-SR fiber observed from rate-level functions. The
growth-rate of masking with shocks to the stapedius was 1.642 dB/dB, and in the
corresponding masking range the GR without shocks to the stapedius was 1.619 dB/dB.
__
I-·--··----LCIII- Il-L_--lll-
··11_1
1.-·.-·1-------_^·-·1111111.
IIYIII·-----ll Ill-·--C 1...
----11_1 ·- 1-- -·
I
-189-
Masking Noise: 350-650 Hz bandpassed noise
6.4 dB SPL; SR = 116 sp/sec
XDP83-123; CF = 8.04 kHz; TH -
80- Tested at 8kHz; Dashed line has a slope of 2
o-Masking at d'=l, no shocks
a-Masking at d'=1, with shocks
V
C
_ (A) /
Cm
_
X~~~~~~
'
b
O--
'
,
I
I
I
60
100
RMS Noise Level (dB SPL)
XDP83-117; CF = 8.61
80-
Hz;
TH = 19.1 cB SPL; SR = 9.2 sc/sec
Tested a-t 8kHz; Dashed line hcs c slcpe of 2
o-Masking ct mid-rate, no shocks
*-Masking ct mid-rote, with shocks
V
,
Cn
,/
0E
101
Ji
I
60
I
O0
I
RMS Noise Level (dB SPL)
II_
L
~
1
IL
- 190 -
such was the case in Fig. 7.13A. The data in Fig. 7.13B are consistent with the same idea,
although the numerical prediction of the effect of the stapedius contraction on the masking function is less precise because the masking function in the absence of stapedius contraction was not
exactly a straight line.
Besides measuring the effects of stapedius contractions on the masking of tone responses
in terms of the gain in the signal (tone) to noise ratio (the difference in the tone level for a given
noise level) for a constant (e.g., threshold) detectability of the tone, the effect can also be measured in terms of the difference in the detectability of the tone for a given tone and noise level.
This can be done by measuring the difference in the a values and the corresponding variability in
the responses with and without contractions of the stapedius (background information for this was
given in Section 4.4). Figure 7.14 gives an example showing the effects of stapedius contraction
on the d value of a high-CF auditory-nerve fiber's response to an 8 kHz tone masked by an
85 dB SPL low-frequency noise (Fig. 7.14A), and the effects on the detectability of the tonal signal (Fig. 7.14B). For the seemingly small difference in the
(2.0-0.2=1.8) produced by the
moderate stapedius contraction, the difference in the "best possible detection performance" of this
auditory neuron is quite remarkable; for example, for a probability of false-alarm of 0.1, the probability of detection increased from 0.15 to 0.82 as a result of the stapedius contraction.
The working hypothesis for the stapedius-unmasking effect will now be tested using all
available data from populations of auditory-nerve fibers. Comparisons of the measured stapediusunmasking with the stapedius-unmasking predicted by the working hypothesis are given in Figures 7.15-7.17, using all the available data from tuning curves, rate-level functions and d-level
functions, respectively. In each case, the mean of the differences between the measured and
predicted unmasking was less than 0.5 dB, the mean of the absolute values of the differences was
less than 2 dB, the correlation coefficient between the measured and predicted masking was
above 0.97, and the absolute value of the difference between the slope of the linear-regression
line and unity was less than 0.07. Furthermore, when all data from Figs. 7.15-7.17 are combined
(Fig. 7.18), there is still no statistically significant systematic difference between the measured
- 191 -
Figure 7.14
Stapedius-unmasking in terms of the difference in a's and ROCs with and without
shocks to the stapedius muscle.
(A) The a functions from a high-CF fiber measured with 8 kHz tones in 85 dB SPL
masking noise with (left curve with filled symbols) and without electric shocks to the
stapedius muscle. The a value with shocks to the stapedius is larger than the a without
shocks at all tone levels above 50 dB SPL, and the difference at 60 dB SPL tone level is
2.0 - 0.2 = 1.8.
(B) The difference in the "best possible detection performance" of this auditory neuron
for the 60 dB SPL 8 kHz tone in 85 dB SPL masking noise, as seen from the difference
in receiver-operating-characteristics (ROCs), with and without shocks to the stapedius.
Each ROC shows the relationship between the probability of detection (of the tone), Pd,
and the probability of false-alarm, Pf. The ROCs were derived from the
values at the
60 dB SPL tone level shown in (A) by the vertical dashed line, and the sample variances
of the fiber's discharge rates with noise alone and with noise+tone, with and without
shocks to the stapedius. The ratio, r, of the sample variance with noise alone over that
with noise+tone was 0.987 without shocks to the stapedius and 0.838 with shocks to the
stapedius. The area under each ROC curve equals the percentage correct in a twoalternative forced-choice detection task. The fiber's discharge rate was assumed to have a
normal distribution in all cases.
-192-
XDP86-93; CF = 7.08 kHz; TH = 4.3 dB SPL; SR = 80 sp/sec
I
I
I
I
I
7o*-
8kHz Tone In 85dB SPL Noise
85dB SPL Noise & 3dB Shocks to Stapedius
(A)
1-
0I
30
I
I
I
60
I
80
Tone Level (dB SPL)
60dB SPL 8kHz tone with 85dB SPL noise
Upper curve: ROC with shocks to stopedius (d'=2, r=0.838)
Lower curve: ROC without shocks to stopediu s (d'=0.2, r=0.987)
I
I
I
I
I
I
I
I
I
I
I
I-
C
0
o
.4-i
U
O-,
)
0lb.
._
CL
Dashed line:
ce
0-
[
I
I
-
I
I
I
I
,Ir
v--
-
.
I
o
Probability of False-Alarm
s
I
1
- 193 -
Figure 7.15
Comparison of measured versus predicted stapedius unmasking using all data from
tuning curves from high-CF auditory-nerve fibers. In the plot there are 15 data points
from three animals. The statistics for the differences between prediction and measurement are (in dB):
For (p-m), minimum = -1.2, maximum = 2.5, mean = 0.0, standard deviation = 1.05.
For Ip-ml (the absolute value of the differences), minimum = 0.3, maximum = 2.5, mean
= 0.8, standard deviation = 0.62.
-194-
Stapedius Unmasking Measured from Tuning Curves
40-
Linear regression: slope=0.93, int.=1.1, corr.=0.99
30o/
/
C
/o
0
EC
0
20;/
/O
3
10-
//
/
/
00
I
10
I20
I30
30
Predicted Unmasking (dB)
4
40
- 195 -
Figure 7.16
Comparison of measured versus predicted stapedius unmasking using all data from
rate-level functions from high-CF auditory-nerve fibers. The unmasking effects were all
measured at the mid-rate. In the plot there are 32 data points from five animals. The
statistics for the differences between prediction and measurement are (in dB):
For (p-m), minimum = -2.6, maximum = 8.3, mean = 0.5, standard deviation = 2.02.
For Ip-ml (the absolute value of the differences), minimum = 0.0, maximum = 8.3, mean
= 1.3, standard deviation = 1.56.
-196-
Stapedius Unmasking Mecsured from Rate-Level Functions
c-r
DU -
Linear regression: slope=0.97, int.=0.03, corr.=0.98
/
Dashed line has unit slope
/
/
/
/ /
C
/
/
/
E0
9r O
NV)
0/
o/01-
E)
0-
I
I
-I
-
I
r
-
0
Predicted Unmasking (dB)
1
5
50
- 197 -
Figure 7.17
Comparison of measured versus predicted stapedius unmasking using all data from d
functions. In the plot there are 33 data points from six animals. The statistics for the
differences between prediction and measurement are (in dB):
For (p-m), minimum = -5.4, maximum = 4.2, mean = -0.1, standard deviation = 2.25.
For Ip-ml (the absolute value of the differences), minimum = 0.2, maximum = 5.4, mean
= 1.7, standard deviation = 1.42.
-198-
Stapedius Unmasking Measured from d' Functions
50
-
Linear regression: slope=1.02, int.=-O.3, corr.=0.97
/
Dashed line has unit slope
/O
/
8//
/
E
o
o
-o
00
v
o/
C)
0
o
/
d
o/ O
o /
o
/
o
/
/
0
/
I
I
I
U
Predicted Unmasking (dB)
5
50
- 199 -
Figure 7.18
Comparison of stapedius-produced unmasking in the tone responses of high-CF
auditory-nerve fibers measured directly with that predicted by the working hypothesis,
using all data from tuning curves, rate-level functions and d-level functions. The amount
of unmasking ranges from 5 to 40 dB. In the plot there are 80 data points from 58
auditory-nerve fibers from eight animals. The statistics for the differences between prediction and measurement are (in dB):
For (p-m), minimum = -5.4, maximum = 8.3, mean = 0.2, standard deviation = 1.96.
For Ip-ml (the absolute value of the differences), minimum = 0.0, maximum = 8.3, mean
= 1.4, standard deviation = 1.38.
-200-
Unmasking Produced by Stopedius Contractions
I
I
I
I
I
I'"
3u-
Linear regression: slope=0.99, int.=-0.03, corr.=0.97
Dashed line has unit slope
/
/
/
/
0
t2
Co
-n
o /
/8
Cn
E
¢0 oP
c*,d 0©0
0.
C
0
0I
0
I
Predicted Unmasking (d B)
5
50
- 201
-
and predicted stapedius-unmaSking effect. The (null) hypothesis that the mean of the difference
between the measured and predicted unmasking is zero (versus the alternative hypothesis that it is
not zero) cannot be rejected at a significance level of a-0.3. The largest difference between the
prediced and measured stapedius-unmnasking effect was 8.3 dB as shown in Fig. 7.16. As that
point was from an early experiment where sharp-tipped shock electrodes were used, one possibility for the deviation is that the relationship between shock current and stapes-head displacement
changed between the measurements of single-fiber responses and of CM responses (there was a
19 minutes separation in measurement time). Another possibility is that since that fiber's masking
function was measured with only two noise levels 5 dB apart, the extrapolated growth-rate of
masking deviated from (over-estimated) the true GR of masking. The sample variance of rate
responses was not accurately measured in that early experiment due to the use of integer arithmetic in the computer program, so no d functions were computed.
Two issues should be pointed out here in connection with Fig. 7.18: (1) Figure 7.18 only
includes all measurements sufficiently complete to provide comparison of the measured stapedius
unmasking with the predicted. For example, in the case of rate-level function measurement, at
least four rate-level functions had to be measured (before the contact with the fiber was lost) to
make such a comparison: level function in quiet background (to provide a reference for determining whether a particular intensity of noise produced any masking), level function with a strong
masking noise, level function with the strong noise and shock to the stapedius (including the
adjustment for shock artifact control), and level function with a weaker masking noise. If this
weaker masking noise was below or too close to the masking threshold (which was not known
a priori) or too far from the level that corresponded to the stapedius-attenuation of the strong
masking noise (which was also not precisely known beforehand), then more tone level functions
with different noise intensities had to be measured for determining the growth-rate of masking.
(2) Since with shock current that produced maximum stapedius contractions (corresponding to
30 dB attenuation of low-frequency sound) the shock artifact in microelectrode recording became
overwhelming (see Section 5.3), the stapedius-unmasking effect could usually only be reliably
- 202 -
measured with shock current up to the level that produced about 20 dB attenuation of lowfrequency sound. Fortunately, however, such amount of low-frequency attenuation is adequate for
comparing stapedius-unmasking effects at the neuronal level with the effects measured
psychoacoustically, which were all measured with 20 dB or less of stapedius-attenuation of lowfrequency sound (Borg & Zakrisson, 1974). Given about 20 dB stapedius-attenuation of lowfrequency sound, the maximum stapedius unmasking observed was in the range of 40-45 dB
(from five fibers with CFs near either 6 or 8 kHz); only for one of these fibers (shown in
Fig. 7.18), however, were all the necessary measurements for constructing the prediction of the
working hypothesis completed before lossing contact with the fiber.
Besides general tests of the working hypothesis as shown in Figs, 7.15-18, a test that can
be incorporated into the framework of coding in the auditory nerve will now be described for
populations of auditory-nerve fibers. As was mentioned in the last chapter, the growth-rate of
masking was a function of the difference between fiber CF and the frequency at which the GR
was measured. The working hypothesis predicts that stapedius unmasking will have the same
dependence on the difference between fiber CF and the test frequency. In fact, in the working
hypothesis, the GR is the only factor unique to each auditory-nerve fiber. The test of working
hypothesis along the dimension of fiber CF, however, requires that variables along other dimensions, in particular, the stapedius-induced attenuation of sound, be held constant for all fibers in
the population, which can pose a severe constraint on the sample size. For a given attenuation of
sound in the noise and tone frequency regions (13 dB at around 0.5 kHz, 5 dB at 6 kHz, and
3 dB at 8 kHz) produced by stapedius contraction, Figure 7.19 shows all available data of stapedius unmasking measured with 6 and 8 kHz tones as a function of the fiber CF. The maximum
stapedius unmasking occurred in both cases in fibers whose CFs were near the test frequency.
The corresponding measurements of the GR of masking in these same fibers are shown in
Fig. 7.20. It is not difficult to see that the overall shapes of the profiles in the corresponding
panels of Fig. 7.19 and Fig. 7.20 match rather well, again supporting the working hypothesis of
the stapedius-unmasking effect. One modifier needed to be added to Figs. 7.19 and 7.20,
- 203 -
Figure 7.19
Stapedius-unmasking as a function of the fiber CF from all available measurements
with common stapedius-induced attenuation of sound. The masking noise level was
either 85 or 90 or 95 dB SPL, and in each case was above the masking threshold by at
least the amount of the stapedius-induced attenuation of sound in the low-frequency
region.
(A) Stapedius-unmasking measured with 6 kHz tones from 10 fibers from two animals
(CF range: 4.3-7.0 kHz). The stapedius-induced attenuation of sound was approximately
13 dB in the frequency region of the masking noise and 5 dB near 6 kHz.
(B) Stapedius-unmasking measured with 8 kHz tones from 14 fibers from three animals
(CF range: 7.0-9.9 kHz). The stapedius-induced attenuation of sound was approximately
13 dB in the frequency region of the masking noise and 3 dB near 8 kHz.
-204-
Unmasking: Difference in Tone Levels at d'=1 dut to Shock
I
I
I
50-
I
I
I
I
I
I
Continuous line from 3-point triangular moving average
Tested at 6kHz (shown by arrow)
0
(A)
D
¢n
TI
ia
o
I.
_
I
i
I
i
2
I
I
6
I
I
I
8
20
Characteristic Frequency (kHz)
I
DU-
I
I
I
II
I
i
I
I
Continuous line from 3-point triangular moving average
Tested at 8kHz (shown by arrow)
-11
M
C
C
O
E
(B)
C
CO
a0
a,
0-
2
II
6
2
I
I
8
2
20
Characteristic Frequency (kHz)
-···----
---
---
.~~~~~~~.__
.
.-
1
- 205 -
Figure 7.20
The average growth-rate of masking as a function of the fiber CF from the same
fibers from which the stapedius-unmasking data in Fig. 7.19 were obtained. The GRs
were from masking functions measured in the absence of stapedius contractions in the
noise ranges corresponding to the stapedius-induced attenuation of the noise.
(A) Growth-rate of masking measured at 6 kHz from the same fibers sampled in
Fig. 7.19A.
(B) Growth-rate of masking measured at 8 kHz from the same fibers sampled in
Fig. 7.19B.
-206-
GrowthRate from Masking Functioles Measured at d'=1
GR in the noise range corresponding to stcpedius attenuation
I
5-
I
I
I
I
i
I
I
I
-
0-
Continuous line from 3-point triangular moving average
Tested at 6kHz (shown by arrow)
0
Cr
I
(A)
O0
Q)
I
nv -
I
I
2
I
I
t
I'
6
I'
I
I
8
I
20
Characteristic Frequency (kHz)
cO
a
I
-
I
I
I
I
I
I
I
I
Continuous line from 3-point triangular moving average
Tested at 8kHz (shown by arrow)
L.
o
0
(B)
I
CL
C
nv -
I
2
-I
I
I
I
6
I
I
I,1_
20
8
Characteristic Frequency (kHz)
--- ----i-----I-'
- 207 -
however, is that the noise levels involved were not the same for all the fibers. While this has no
effect on the test of the working hypothesis and is unlikely to make a first-order difference in the
overall shape of the profiles, it may have an effect on the ("second-order") difference between
Figs. 7.20 and 6.15 in the exact location of the maximum GR. Other factors such as the
difference in the samples, and the difference in the measuring of GR in Chapter VI and VII, may
also be the reasons for the slight mismatch between Figs. 7.20 and 6.15.*
7.3 Summary and Conclusions
Effects of stapedius contractions on auditory-nerve fiber responses were described in this
chapter with measurements from 103 auditory-nerve fibers from nine cats. The main points are:
(1) Contraction of the stapedius muscle acts as a linear filter in that the stapedius-produced
attenuation of sound does not depend on the level of the test sound, at least for the frequency and
level ranges that were tested and were of interest to this study. (2) Measurements of the
stapedius-produced attenuation of sound with cochlear-microphonic potentials and with responses
of single auditory-nerve fibers (to single tones) yielded the same result for attenuations up to
30 dB. (3) Even though a contraction of the stapedius muscle only effects an attenuation of
acoustic transmission through the middle ear and affects primarily the low-frequency region, it
can strongly enhance responses of auditory-nerve fibers to high-frequency sounds when both lowand high-frequency sounds are present, by reducing up to at least 40 dB the masking by the lowfrequency sounds of an auditory-nerve fiber's response to the high-frequency sounds. (4) The
reduction of the masking (AM) produced by stapedius contractions can be much larger than (e.g.,
twice or larger) the stapedius-produced attenuation of the low-frequency sounds and this reduction can be fully explained and predicted by the masking properties of the auditory-nerve fibers
and the stapedius-produced attenuation of sound; quantitatively, AM = ANxGR - AS, where AN is
* When the growth-rate of masking for the fibers in Fig. 7.20 is measured in the same way as in Chapter VI, i.e., measured across all noise levels used instead of in the noise region corresponding to the stapedius-induced attenuation of
sound, the match in the dependence of GR on CF between the fibers in Fig. 7.20 and those in Fig. 6.15 becomes closer.
- 208 -
the stapedius-produced attenuation of the low-frequency sound (a noise here) in the suprathreshold region of the masking (in dB), GR is the growth-rate of the neuronal masking function of
the high-frequency sound response in the range between the attenuated and un-attenuated noise
(in dB/dB), and AS is the stapedius-produced attenuation of the high-frequency sound (the "signal"). (5) The validity of this mechanistic explanation of the stapedius-unmasking effect does not
depend on any particular measure of the masking effect. (6) For a given status of stapedius contraction, maximum stapedius unmasking occurs in fibers whose CFs are near the signal frequency.
Combining the positive result of the testing of the working hypothesis on stapedius.
unmasking in this chapter with the features of the neuronal masking patterns described in the last
chapter, the following additional points can be made: (1) Since, on the average, the growth-rate
of neuronal masking increases with masking noise level, the unmasking effect of a given stapedius contraction should also, on the average, increase with noise level. (2) For two auditorynerve fibers with similar tuning and masking properties the effect of stapedius unmasking should
also be similar. (3) Since on the average there is no significant difference between the growth-rate
of masking in high-SR and that in low-SR auditory-nerve fibers, with a given stapedius contraction there should typically be no significant difference between the stapedius-unmasking effect for
high-SR and that for low-SR auditory-nerve fibers.
In the next and last chapter of this thesis, the results described so far will be discussed in
a broader framework, including comparison with data in the literature relevant to various aspects
of the results, possible applications of the results, and some inferences and speculations.
-
I
--------- II
~I x"-
------
I
Is
A-·
- 209 -
Chapter VIII
Summary, Discussion and Conclusions
In this chapter, the main line of results of this study will be first summarized. The
significance of the results of this study will then be explored in two directions: (1) relationship
with previous knowledge of the auditory system and (2) possible applications in improvement of
acoustic communications.
8.1 Summary of Major Results
Figure 8.1 provides a schematic integration of the main line of results in the form of an
example of the stapedius unmasking with realistic numerical values. More generally, the major
results of this study are the following. (1) For a given level of stapedius contraction, the attenuation of sound does not depend on sound level. (2) The stapedius-induced attenuation of lowfrequency sound reduces masking by low-frequency noise of auditory-nerve fiber responses to
high-frequency tones, in the amount up to at least 40 dB. (3) The observed unmasking effects of
the stapedius contractions on auditory-nerve fiber responses can be completely explained by the
mechanistic model which predicts the unmasking based only on the growth-rate of neuronal
masking and the stapedius-produced linear attenuation of sounds reaching the inner ear. (4) The
average growth-rate of neuronal masking was 2 dB/dB for the maskers and signals studied. (5)
Maximum growth-rate of masking and maximum stapedius-unmasking occurs in auditory-nerve
fibers whose characteristic frequencies are near the test-signal frequency.
8.2 Masking Patterns of Auditory Responses
Discussion in this section will focus on two topics: (1) the relationship of the masking by
low-frequency noise of high-CF auditory-nerve fiber responses as reported here to other physio-
_
__II I I
___I
__1
_I
_
I
____
_II
_ _ _I
_
- 210 -
Figure 8.1
Schematic integration of the main-line results.
(A) Description of the signal-flow pathways. The acoustic input to the tympanic membrane is shown to the left as a high-frequency tone in the background of low-frequency
noise. For simplicity only the 6 kHz tone is shown. The middle ear is a linear processor
of acoustic input with and without contractions of the stapedius muscle. "H(f,s)" is
middle-ear transfer function, which, besides being a function of acoustic frequency ("f'),
is also a function of the status of the stapedius ("s"). The stapedius is shown here to act
in a "open-loop" manner with respect to middle-ear transmission. The CM response provides a measure of the input to the cochlea. The cochlea is a non-linear processor of
acoustic input. In particular, the cochlear output for the high-frequency tone will generally be masked by the low-frequency noise.
(B) A schematized "typical" example of the stapedius-induced attenuation of sound
reaching the inner ear in the frequency regions of the tone (AS) and the noise ('N),
with
realistic numerical values.
(C) A schematized masking function for the auditory-nerve responses to the highfrequency tone and explanation of the stapedius unmasking. The key element for a 20 dB
attenuation of noise to result in a 32 dB unmasking is the growth-rate (GR) of masking,
which has an average value of 2 dB/dB for most auditory-nerve fibers that respond to
6 kHz tones. For fibers whose CFs are near 6 kHz, the GR, and consequently the
unmasking, is likely to be larger. The quantitative model that explains the stapedius
unmasking is shown in the box. Note that of all neuronal attributes only the growth-rate
of masking is needed for the model.
__
_
_________
-211-
©
:,-
CI)u
LU
°ow
3-
nc
Ž
=
.r- Q
w
_ S
a)
-c
s ~~
~~~:
_-5
co
'
z
5
A
V)
_a1)
c
JL
U,
Z 'o
e±Q
,J
_
N
.
co
'a0
<3 ·
.11
-
4,i
nca
=9aa
§0-
f )-
o:o/,.,'~.
<
.
C
x
a)
- Q)
aII
z
I
v
x
z o
11
II
04
11
I
CdJ O
o
CD
Ai
O
Cj
(la!nO u! ploqseaJql
'
O
J sp)
ploqsaJqL euoi
m
n
I
n
IL
-4
cn o
-CO
.. .
c
,O
I0-
IN
C
LL
0
w
1
~~-
k
c;L
·
i
I
aD
I
C\
(8p) punoS o uoisenualu
peonpul-snpadelS
a)
a)
o0 '0 O
Z
0)
c
L
LL
(
0
~b 3
A
a)
CD
N
I
F-
C.
a)
0*
g
___
_
I__I
_·I
III_·_LIY·_L·^I__IY-l·l-
- ---.-
141C-
-11111_
-----·
·-·--
1(1-·11-_-·1_--^
--.---(-----
-·-
-
II
- 212 -
logical studies on masking by noise o tones of auditory-nerve fiber responses to tones, and (2)
the relationship of the physiological studies with psychophysical studies on masking.*
Systematic measurements of masking by low-frequency noise of auditory-nerve fiber
responses to high-frequency tones are not available in the literature. Previous studies on physiological masking can, however, be discussed in two categories for the purpose of comparing with
the present results: (1) masking by noise or tones which are distantly lower (by more than 2
octaves) in frequency than the test tone, and (2) masking by broadband noise or by noise or tones
which are close in frequency to the test tone. There is evidence indicating that there might be
different mechanisms dominating each of these categories of masking, as will be seen later in the
discussion. A step that can be useful towards identifying the mechanisms involved in these two
categories of masking is the identification of the features that are specific to the first category of
masking. This issue will be pursued by first comparing the features extracted from the systematic
measurements of this study with the data (relatively small in sample size) of the first category of
masking in the literature, and then comparing this with the features of the second category of
masking, which has received more coverage in the literature.
Kiang and Moxon (1974) showed an example of the masking of a high-CF auditory-nerve
fiber's tuning responses by a continuous narrow-band low-frequency noise at four noise levels in
a cat. Their data are similar to a typical measurement in the present study in every comparable
aspect. Namely, at each noise level the masking was strongest near the fiber CF and the growthrate of masking at the tip of the tuning curve was 2 dB/dB. They also showed in another example
that when the masking noise was either narrow-band centered at the fiber CF or was broadband,
the "selective" masking of the tip disappeared and the tuning curve was more uniformly elevated.
Abbas (1978) measured the effect of a second tone on auditory-nerve fiber rate-level responses to
a primary tone in the cat. He noted that with the frequency of the second tone "well below the
* Due to the lack of consistent definition in the literature, the words "masking" and "suppression" are often used by
different authors with partial or complete overlap in meaning. In this study and for the purpose of this discussion, physiological "masking" refers to the reduction in responsiveness of an auditory-nerve fiber to one sound by the simultaneous presence of another.
I__I
_
_
- 213 -
fiber CF", maximum masking in the form of shift of the rate-level function occurred when the
frequency of the primary tone was near the fiber CF. From his example showing masking by a
400 Hz tone of rate-level responses to a CF tone in a fiber with CF=3.4 kHz, the growth-rate of
masking can be calculated as 1.6 dB/dB with a mid-rate criterion. Geisler and Sinex (1980)
showed measurements from cat auditory-nerve fibers of the masking of tonal rate-level responses
by noises of different bandwidth. With noise low-pass filtered at 500 Hz, masking of rate-level
responses of a high-CF (9 kHz) fiber was strongest at CF, with a growth-rate of masking of
1.5 dB/dB (measured at mid-rate). In general, the growth-rate of masking also seemed to be largest when the test-tone frequency was at the fiber CF. With a 10 kHz broadband noise masking
the rate responses of a fiber with CF of 1.5 kHz, however, there was no consistent difference in
the masking pattern of rate-level functions measured at the fiber CF versus at 0.5, 1.0 or 2.0 kHz.
A more recent study by Delgutte (1986) on "two-tone rate suppression" using a constant-rate criterion (60% of the dynamic range) indicated that with a 1 kHz masking tone, the growth-rate of
the masking of cat auditory-nerve fiber rate responses to CF tones increases with fiber CF, reaching a value of approximately 2 dB/dB for fibers with CFs in the 6-8 kHz region (while for fibers
with CFs around 2 kHz, the GR was about 1 dB/dB). In addition, a feature common to all of
these studies is that when the masker and signal are widely separated in frequency, the threshold
level for the masker to be effective is rather high, i.e., the "masker to signal" sound-level ratio
has to be rather high to produce masking. This is also consistent with the results of the present
study.
Data from the second category of masking will now be compared with results of the
present study for similarities and differences in masking patterns. Costalupes et al. (1984) studied
the effect of continuous broadband background noise on the BF-tone 'rate-level responses of cat
auditory-nerve fibers. They reported the following features that are shared by the present study:
(1) Noise can produce an elevation of the background discharge rate, a reduction of the plateau
rate, and often a change in the slope of the rate-level function. (2) Such effects are less prominent
in low-SR fibers than in high-SR fibers. (3) The background noise causes a shift of the tonal
- 214 -
rate-level function towards higher tone levels. The primary difference in the masking pattern,
however, occurs at this stage: the mean rate of the shift of the level function, or the mean
growth-rate of masking, observed in their study was only 0.61 dB/dB, much smaller than the
2 dB/dB observed in the present study. Moreover, their growth-rate of masking was independent
of fiber BF (best frequency, the "equivalent" of CF in the suprathreshold region). They proposed
that such masking was a result of the "suppression of the response to the BF test tone by energy
present in the noise at surrounding frequencies", which is a notion closely related to the idea of
"critical band" as an explanation for the mechanism dominating the second category of masking.
Clearly, this cannot be the case with the first category of masking where the spectral regions of
the signal and of the masker do not overlap. Also using broadband noise, Young and Barta
(1986) measured the masking of BF-tone D functions (similar to the
function in the present
study) in cat auditory-nerve fibers. The mean growth-rate of masking at D=l can be measured
from their data as about 1 dB/dB, consistent with Costalupes et al.'s result (GR measured from D
functions is expected to be larger than that measured from average-rate responses, see Chapter
VI) and again distinctly smaller than the mean growth-rate of masking obtained in the present
study. Another difference in the masking pattern can be seen from data reported by Javel et al.
(1978) on "two-tone suppression" in cat auditory-nerve fibers. When the masker (or "suppressor')
and the signal are close in frequency, significant masking can be produced with a small "masker
to signal" sound-level ratio.
In summary, of the masking features in the present study, the following seem to be
specific to the first category of masking (masking by low-frequency noise or tones distantly lower
in frequency than the test tone): (1) The average value of the growth-rate of masking is higher
than 1 dB/dB for test tones near fiber CF. (2) For a given masking noise, maximum masking, and
probably maximum growth-rate of masking as well, occur in fibers whose CFs are near the testtone frequency. (3) A high "masker to signal" sound-level ratio is required for masking. The
features that seem to be common to both categories of masking are: (A) The shift of tonal ratelevel functions towards higher tone levels. (B) The masker-produced elevation of background
o'
- 215 -
discharge rate, suppression of plateau rate, and change in the slope of rate-level functions. (C)
The difference between low- and high-SR fibers in their susceptibility to the effects of (B).
The above comparisons are made for the situation when the signal and masker are present
simultaneously. A recent study comparing the effects of simultaneous masking with those of
"non-simultaneous masking",* however, provided some interesting clue for the physiological
mechanisms of masking by low-frequency sounds of the responses to high-frequency sounds.
Delgutte (1988) studied simultaneous and non-simultaneous "masking" by a 1 kHz "masker" of
the "near CF" tone responses of cat auditory-nerve fibers, in an attempt to identify the relative
contributions of two possible mechanisms of masking. One is called "excitatory masking" or
"spread of excitation" which presumably occurs in either simultaneous or non-simultaneous
"masking", and the other is called "suppressive masking" or simply "suppression" which presumably only occurs in simultaneous masking. When measured with rate-level functions, "excitatory
masking" is evidenced by an increase in the background discharge rate by the "masker", and
"suppressive masking" results in a shift of the level function towards higher tone levels. Most
masking patterns reported in the literature, however, show a combination of the two. Results
showed that masking was predominantly "excitatory" when the "near CF' test-tone signals were
close to the "masker" in frequency, and was predominantly "suppressive" when the "near CF"
test-tone signals were far above the "masker" in frequency. This distinction between the two
kinds of masking was clearer when the "masker" level was higher. The growth-rate of masking
of high-frequency tones was about 1 dB/dB with non-simultaneous "masking" and about 2 dB/dB
with simultaneous masking. It was suggested by Delgutte that fast growth of the "suppressive
masking" is primarily responsible for the 2 dB/dB GR of masking. Results of the present study
are consistent with this interpretation in that the masking by low-frequency noise of the responses
to high-frequency tones is largely suppressive, i.e., the primary effect of the masking noise on
rate-level function is a shift of the function towards higher tone levels.
* The conventional definition of masking implicitly refers to the situation when the signal and masker are simultaneous
present. Therefore for the non-simultaneous situation, the effect of one sound on the response to another is only termed
"masking" with quotation marks.
- 216 -
The psychophysical studies on masking to be discussed here include measurements from
both cat and human. As was shown in Fig. 2.2, Borg and Zakrisson (1974) measured from two
human subjects masking functions at 6 kHz in the presence and absence of stapedius contractions, using continuous narrow-band low-frequency masking noise (300 Hz bandwidth centered at
500 Hz). The features regarding the growth-rate of masking in the absence of stapedius contractions from both subjects are similar to those observed from cat auditory-nerve fibers, namely, that
the GR increased with noise intensity and that the mean of the average GR (above 5 dB of masking) was approximately 2 dB/dB.* Their measurements also show that the mean growth-rate of
masking at 3, 4 and 8 kHz was approximately 1.9, 2 and 2.1 dB/dB, respectively. There seems to
be a difference, however, in that the masking noise threshold in the human experiment at 6 and
8 kHz is about 15 dB higher than the average masking threshold observed in single auditorynerve fiber measurements at these frequencies from the cat (e.g., Fig. 6.14). Three factors may
contribute to this difference: (1) a difference in the calibration of acoustic systems (their indication of noise intensity was calibrated with a 6 cc coupler while in the present study noise intensity was directly measured at the tympanic membrane); (2) the difference in tonal audibility threshold between the human and cat, assuming that the masking threshold is related the hearing threshold (cat hearing threshold at 6 and 8 kHz is lower than that of the human and is more comparable to human threshold at 3 and 4 kHz (Sivian & White, 1933; Miller et al., 1963); in fact, the
masking threshold at 3 and 4 kHz in the above mentioned human experiment is much closer than
that at 6 and 8 kHz to the masking threshold at 6 and 8 kHz measured in the present study in the
cat); (3) masking of a psychophysical response might not occur till the responses of most or all
relevant auditory-nerve fibers are masked, and there is a spread of masking thresholds among the
auditory-nerve fibers.**
With broadband masking noise or noise bands surrounding the frequency of the test tone
* This is not the GR measured in the noise region corresponding to the stapedius attenuation, which had a value of
2.56 dB/dB.
** Even for fibers of similar CFs in the sadne animal, there can be a spread of masking thresholds, if not the growthrate of masking, with the spontaneous rate of the fibers.
- 217 -
(analogous to the "second category of masking"), Hawkins and Stevens (1950) and Watson
(1963) showed for human subjects and behaving cats, respectively, that the growth-rate of masking has a value of 1 dB/dB. On the other hand, studies by Wegel and Lane (1924) and by Egan
and Hake (1950) in human subjects using low-frequency tone or narrow-band noise maskers
reveal that while the growth-rate of masking has a value of 1 dB/dB when the masker and test
tone are close in frequency, when the masker frequency is held fixed, the GR of masking
increases with the test-tone frequency. This is consistent with the physiological data obtained by
Delgutte (1986) as was discussed earlier.
To summarize, comparisons of physiological and psychophysical studies on masking suggest the following: (1) The relevant features specific to the first category of physiological masking are also specific to the corresponding psychophysical masking. (2) The monaural psychophysical masking patterns can be largely accounted for by masking mechanisms at the cochlear stage
of the auditory periphery. (3) Masking in cat and human are similar at least with respect to the
growth-rate of masking for both the first and second categories of masking.
8.3 Effects of Stapedius-Muscle Contractions
In this section, the stapedius-induced attenuation of sound reaching the inner ear will be
discussed first, followed by a discussion of the effects of stapedius contractions on information
processing at more central stages of the auditory system.
8.3.1 Linearity of the middle ear with stapedius contractions
From both the measurements of the effects of stapedius contractions on single-tone level
functions of CM and auditory-nerve fiber responses and the test of the hypothesis for the stapedius unmasking effects, it is concluded that the stapedius-induced attenuation of acoustic
transmission through the middle ear does not depend on sound level. This result is consistent
with and extends considerably the results of previous physiological studies on the issue (Wever &
- 218 -
Bray, 1942; Nedzelnitsky, 1979). By combining the result of the present study with that of
Nedzelnitsky (1979), this conclusion can be maintained for sound levels from the physiological
threshold (see "Methods" chapter) to up to 140 dB SPL. Some of the psychophysical studies on
this issue (see Section 2.2.2 for details) have been reviewed by Rabinowitz (1981), with appropriate comments on the possible sources of the apparent discrepancy with physiological studies,
namely, that factors other than middle-ear transmission affect psychophysical judgments and that
the level of -stapedius contractions was not sufficiently documented in these psychophysical studies.
One significant aspect of the determination of middle-ear linearity with stapedius contractions is that it makes possible predictions of the effects of stapedius contractions on auditory
responses in general. The successful prediction of the stapedius-unmasking effects in this study
provides one such example. Another aspect of the significance is that any effect of stapedius contractions on information processing at more central stages, such as the unmasking effect, can, if
desired, be simulated by a linear filtering of the sound reaching the tympanic membrane. Possible
applications of such filtering in the design of hearing-aid devices will be discussed later.
It is observed that for a given stapedius contraction there is no significant difference
between the shifts of auditory-nerve-fiber rate-level or d-level functions and the shift of CM-level
functions. This observation provides another support for the notion that the olivocochlear
efferents were not electrically stimulated by the shocks to the stapedius region, since the effects
of the olivocochlear efferents on the CM responses are very different (opposite in direction, e.g.,
Gifford & Guinan, 1983, 1987) from their effects on auditory-nerve fiber responses. This observation is also relevant to the issue of possible "natural" interactions between the stapedius and the
olivocochlear system. While the existence of such interactions has no effect on the claims of the
present study for the stapedius effects on auditory-nerve fiber responses, it can nevertheless be an
interest issue in its own stand. Specifically, one may ask the question whether the contraction of
the stapedius (rather than its attenuation of sound) sends a signal to the brain to affect the activity
of the olivocochlear efferents (i.e., to affect the coordination of the two systems by the brain).
- 219 -
While it is not known whether there exist mechano-sensory pathways from the stapedius to the
brain, the observations in this research indicate an absence of effect mediated by such pathways
on the brain control of the olivocochlear efferents.
8.3.2 Stapedius unmasking
For comparable stapedius-produced attenuation of the masking noise (20 dB at around
0.5 kHz), the maximum stapedius-unmasking effect observed in single auditory-nerve fibers of
the cat (40 dB at both 6 and 8 kHz) is the same as the average effect observed psychophysically
in the human (39 dB at 6 kHz, two subjects; and 40 dB at 8 kHz, one subject), but is less than
the maximum effect (47 dB at 6 kHz, one subject) observed psychophysically (Borg and Zakrisson, 1974). Other than possible individual variabilities, any of the following can account for the
difference in the maximum effect (assuming that this difference is "real" or "systematic", even
though it is small). (1) There can be a difference in the stapedius-produced high-frequency
attenuation between man and cat. While the amount of stapedius-induced attenuation of sound in
the high-frequency region was not given in Borg and Zakrisson's report, it can be predicted using
the quantitative model tested in the present study as about 4 dB, based on the data shown in
Fig. 2.2 (for the subject shown in Fig. 2.2A). The stapedius-produced high-frequency attenuation
in the present study, on the other hand, had a typical value of 10 dB (e.g., see Fig. 7.12). (2)
Their result was obtained with a masking noise of 115 dB SPL, approximately 20 dB higher in
level than the noise used in the present study, and the growth-rate of masking in the absence of
stapedius contraction is an increasing function of noise intensity, as shown by masking functions
obtained from both auditory-nerve fiber measurements and psychophysical measurements
(Figs. 6.14 and 2.2). (3) In performing the psychoacoustic task of detecting high-frequency tones
in low-frequency noise at high sound levels, the central nervous system may well use information
from all "relevant" auditory-nerve fibers instead of just from one.* Here "relevant" fibers can be
* Among other reasons, the number of primary auditory neurons innervating each inner hair cell of the cochlea has been
reported to be in the range of 7-20 for the human and cat (Spoendlin, 1972; Liberman, 1980; Nadol, 1983).
- 220 -
either fibers with similar CFs tuned for the tone frequency or fibers with very different CFs or
both. The way information from these fibers is used can be either by selecting the maximum
unmasking effect or by some combination. On the other hand, one has no means of telling how
"complete" an experimental sampling of single auditory-nerve fibers is in connection with a particular psychoacoustic task. In other words, one possibility is that there are experimentally unsampled fibers in which stapedius-unmasking effect was greater (more will be said on this in the next
paragraph). Another possibility is that even though the experimental sampling was complete, the
stapedius-unmasking effect observed in any individual fiber is still smaller than that achievable
psychoacoustically, since as long as the discharge patterns in these fibers are not perfectly correlated, individual information from these fibers can always be combined in ways that yield larger
combined information. Taking the (hypothetical) case of the brain using information from fibers
with similar CFs as an example, since, as was shown in Chapter VI, masking patterns in two
high-CF auditory-nerve fibers with similar tuning properties can be very similar (while their
discharge patterns remain presumably uncorrelated), the stapedius-unmasking effect in such two
fibers will also be very similar (based on the results of Chapter VII). Therefore even if the brain
only uses information from these two fibers, it can combine the information in such a way that
the combined
value will be 1.4 times or 3 dB larger than that of either of the fiber (in the limit-
ing case of the brain functioning as an optimal processor for the detection task). The small
difference between the maximum stapedius unmasking observed psychophysically and that
observed physiologically is also consistent with the conclusion reached by Young and Barta
(1986) that rate information from a small number of auditory-nerve fibers seems sufficient to
account for the observed level of behavioral performance. In summary, it can be concluded that
the result of this study at the stage of auditory-nerve fibers does provide neuronal mechanisms
that are adequate for explaining the stapedius-unmasking effect observed psychophysically.
The data obtained in the present study also provide a basis for exploring how large a stapedius unmasking can be in the response of a single auditory-nerve fiber. As shown in Chapters
VI and VII, the maximum growth-rate of masking observed is in excess of 3 dB/dB,* and the
* This is for a large noise range (25 dB or more), as is necessary for the discussion here. For small noise ranges, GR
as large as 3.8 dB/dB was observed.
- 221 -
largest stapedius-induced attenuation of sound in the noise frequency region is 30 dB, with the
corresponding high-frequency attenuation of about 15 dB. Although due to technical reasons,
direct data of stapedius unmasking in single-fiber responses with all the above conditions combined were not obtained, since the test of the hypothesis that predicts stapedius unmasking based
on GR and stapedius-induced attenuation of sound gave positive result, however, the maximum
stapedius unmasking in the responses of single fibers can be prediced as 3x30-15 = 75 dB.
Although the stapedius contractions in this study were elicited by electric stimulation of
the muscle, the attenuation of sound required to produce an unmasking effect of 30-40 dB in
auditory-nerve fibers is comparable in magnitude to that producible by monaural acoustic stimulation in human stapedius acoustic reflex (e.g., Borg & Zakrisson, 1974) and in human vocalization
process (Borg & Zakrisson, 1975a; see Section 2.2.2). Moreover, since in natural circumstances
the acoustic reflex is mostly binaural and since binaural acoustic stimulation produces stronger
stapedius contraction than monaural (M6ller, 1962; Guinan & McCue, 1987), an even larger
stapedius-unmasking effect can be expected from binaurally activated acoustic reflexes.
On one hand, electric stimulation of the stapedius muscle as used in this study can be
considered as a simulation of "stapedius activation with signals of internal origin", such as vocalization, in that the activation of the muscle is independent of the level of the external sound. On
the other hand, for the result of this "open-loop" study to be quantitatively applicable in predicting the stapedius unmasking in the case of acoustic reflex (the "closed-loop" situation), one needs
to know the steady-state "closed-loop transfer function" or "regulation factor" involved in acoustic reflex, i.e., the fraction of an eliciting sound attenuated by the reflex. Borg (1968) and Borg
and Zakrisson (1974) reported for human subjects that above the acoustic reflex threshold, for
1 dB increase in a 0.5 kHz eliciting tone, 0.7 dB is attenuated by the middle-ear acoustic reflex,
i.e., the regulation factor is 0.7 dB/dB. Borg also reported that at 1.45 kHz the maximum regulation factor observed was only 0.38 dB/dB and that the regulation factor was even smaller at
higher frequencies, presumably due to the weaker stapedius-attenuation of sound at higher frequencies. Rabinowitz (1977), using a different method, estimated a regulation factor of 0.5 dB/dB
- 222 -
in the low-frequency region for human subjects. For the cat, a regulation factor of 0.43 dB/dB at
1 kHz can be calculated from data shown by Simmons (1963), and the regulation factor at
0.5 kHz is likely to be larger (inferring from Borg's data on the frequency dependence of the
regulation factor). If one accepts a regulation factor of 0.7 dB/dB at 0.5 kHz by stapedius contractions and if the stapedius-attenuation of sound at high frequency is negligible (as with
moderate stapedius contractions), then in the closed-loop situation of the acoustic reflex, the
growth-rate of the masking of a high-frequency tone by the low-frequency noise should change
from 2 dB/dB, as in the absence of stapedius contraction, to 0.6 dB/dB (a 1 dB increase in the
tympanic noise level results in a 0.3 dB increase of cochlear excitation). An examination of the
measurement by Borg and Zakrisson (Fig. 2.2) shows the validity of this prediction: the average
growth-rate of masking with stapedius reflex for the two subjects is approximately 0.62 dB/dB.
The conclusion is that the working hypothesis on stapedius unmasking proposed and tested in this
study can quantitatively explain stapedius-unmasking effect not only in single auditory-nerve
fibers, but also in psychophysical measurements in both open- and closed-loop conditions with
respect to the interaction between transmission change and stapedius contractions.
In order to assess what conclusions can be drawn from the results of this study on the
significance of stapedius contractions for acoustic communication in noise for both cat and man,
one may ask how relevant are the choices of the masker and signal frequencies in this study to
the natural acoustic environments surrounding cat and man. It is known that most environmental
noise (such as related to bad weather) and many kinds of industrial and traffic noise are predominantly of low frequency (Kryter, 1970; White, 1975; Harris, 1979). For example, Fletcher (1953)
reported that average street noise is predominantly of low frequency, with its spectral level falling
at a rate faster than 30 dB/decade towards high frequency. Similar measurements have been
reported by Ono et al. (1983) for subway and railway noise, with maximum spectral levels in the
region of 0.1-0.5 kHz. Also pointed out by Fletcher (1953) is that the maximum spectral level of
human speech is in the region of 0.25-0.5 kHz; and while this is not the frequency region important for the information content of speech, acoustic components in this region (and in the entire
- 223 -
low-frequency region) can, at high intensities, mask the high-frequency components of speech,
which are very important for the intelligibility of speech. Stevens et al. (1946) and Miller (1947)
measured masking of speech intelligibility by non-speech sounds and found that low-frequency
tones and noise have masking power much greater than high-frequency ones. In particular,
"intense sine waves produce the most masking when their frequency is about 300 cps, whereas
for weaker sine waves the most effective frequency is about 500 cps". Miller (1947) found that
among all narrow-band noises he used in the frequency region of 135 to 4000 Hz, noise in the
region of 350-700 Hz masked the speech signals most. In discussing the application of measurements from cat to the understanding of human hearing, Kiang and Moxon (1974) suggested that
since the audible frequency range for cat is about one octave higher than that for the human, the
mapping of cat cochlea to human cochlea can, to a first order approximation, be accomplished by
lowering the CF by one octave (among other things, the lowest hearing threshold for cat is at
about 8 kHz while that for the human is at about 4 kHz (Sivian & White, 1933; Miller et al.,
1963)). With such an assumption, 6 and 8 kHz sounds to cat would be analogous to 3 and 4 kHz
sounds to the human, which are the frequency region important for speech communication (Licklider & Miller, 1951). Data of Borg and Zakrisson (1974) are also consistent with such an
assumption in that the growth-rate of human psychophysical masking function at 3 and 4 kHz is
approximately 2 dB/dB as well and that the human masking threshold at 3 and 4 kHz is much
closer than that at 6 and 8 kHz to the cat masking threshold at 6 and 8 kHz, as was discussed in
the previous section. Concerning the cat, the frequency region of 6-8 kHz is well within the
acoustic spectrum of its own vocalization as well as some of its primary prey's (such as mice and
rats) (Busnel, 1963). The answer to the question at the beginning of this paragraph is therefore
that the choices of the noise and tone frequencies in this study do provide reasonable simulation
of important maskers and signals in the real acoustic world. This also seems to be an appropriate
place to conclude that the results of this study, by providing neuronal evidence of unmasking
mechanisms, supports the notions that contractions of the stapedius muscle can (1) for the cat, be
of important survival value for it to hear sounds important to it (such as squeaks of a mouse) in
- 224 -
noisy circumstances (such as bad weatidr), and (2) for the human, in addition to survival value*
from an evolutionary point of view, significantly improve speech communication both in noisy
environment and at high speech levels (Stevens & Davis, 1938; Lid6n et al., 1964; Borg &
Zakrisson, 1973; Mahoney et al., 1979; Dorman et al., 1986, 1987; for more background information, see Section 2.3.2).
8.4 Clinical Implications
Having obtained a clearer understanding of the functional benefits that the stapedius muscle can bring to a normal-hearing person or animal and of the physiological mechanisms involved
as a result of this study, one can take a step further to probe the possibility of applying the
results of this study to improve pathological hearing. Specifically, two kinds of hearing problems
will be considered: "normal" hearing in a quiet background and at moderate sound levels but
lacking a functioning stapedius, and with functioning stapedius but having high-frequency hearing
loss.
It has been reported from clinical studies that for otosclerosis patients after a stapedectomy which removed the stapedius tendon, although the hearing in quiet background was greatly
improved as measured by pure-tone and speech audiograms, speech discrimination in noisy backgrounds and at high intensities was mostly worse than before the operation and was worse than
that of ears with comparable hearing thresholds and functioning stapedius muscle (Lid6n et al.,
1964; Girgis, 1966; McCandless & Goering, 1974). McCandless and coworkers (1974, 1979)
showed that in such patients the deterioration of speech discrimination scores for speech at high
sound intensities had the same pattern (as a function of sound intensity) as that measured in
patients with stapedius paralysis.* Furthermore, such patients frequently suffer from intolerance to
noise, particularly low-frequency noise such as traffic noise. On the other hand, with the stapedius tendon (and part of the stapes) preserved in the surgery so that the stapedius muscle could
* Their results also agree with that of Borg and Zakrisson (1973), see Section 2.3.2.
- 225 -
influence the impedance of the prosthesis,* the speech discrimination score of the patients in
low-frequency background noise was significantly better than that in the patients with the tendon
removed, while there was no difference in the post-surgery improvement of hearing in quiet
between these two groups of patients (Lid6n et al., 1964).
While the hearing problems associated with the absence of a functioning stapedius system
is not typically treated with hearing-aid devices, the second kind of hearing problem to be considered here, the problem of sensori-neural hearing loss, is frequently dealt with through the use
of hearing aids. A common feature of this kind of hearing problem is a loss of high-frequency
hearing and a decrease in the capability for speech discrimination (Sataloff, 1966; Meyerhoff,
1984). This is also probably the most wide-spread hearing problem,** and it is typically progressive, irreversible, and with little effective medical treatment The modem development of
hearing-aid devices for sensori-neural hearing loss can be viewed as having progressed through
three stages. As a conventional hearing aid was essentially an audio-amplifier, the first major
improvement was the introduction of an automatic gain control, so that very loud sounds would
not reach the inner ear and cause discomfort and further hearing loss. The problem with a simple
automatic gain control is that it reduces both undesirable sounds such as environmental noise and
desirable sounds such as speech. Since environmental noise is predominantly of low-frequency
and since people with sensori-neural hearing loss mostly have impaired hearing for highfrequency sounds, the second improvement was the introduction of a high-pass filter in addition
to the uniform gain control. While the addition of a high-pass filter was reported to be effective
* Two such techniques were described in some detail by Girgis (1966). One is to fracture the stapes crura just below
the stapes neck, remove the whole stapes footplate, and insert the stapes head (with the tendon attached) into a polyethylene tube. The other end of the tube is inserted into the oval window whose membrane is replaced by a piece of
vein graft. The other technique is to drill a hole through the footplate and fit a teflon piston attached to the incus. Instead of completely removing the portion of the stapes above the footplate as in more conventional operations, however,
only the anterior crus of the stapes is removed and the posterior crus is cut at its lower end to form a false joint so that
when the stapedius muscle contracts this lower end of the posterior crus serves as a fulcrum. With both techniques the
incudo-stapedial joint is left intact. It has been reported (Rasmy, 1986) that with the above procedures of stapedectomy
operation the stapedius reflex is generally preserved, as measured with changes in acoustic impedance at the tympanic
membrane in response to intense sound. The change of impedance presumably results from a change of the friction
coefficient between the "piston head" of the prosthesis and the surrounding structures, because when the stapedius contracts a force "normal" to the direction of prosthesis vibration can be exerted by the stapedius tendon on the prosthesis.
** It can be caused by genetic reasons, inner-ear infections, oto-toxic drugs, traitnra, and most commonly, presbycusis
(aging) (Sataloff, 1966; Meyerhoff, 1984).
- 226 -
in suppressing some evironmental noises, a fixed high-pass filtering created new problems of
sound distortion and the user's difficulty in deciding when to use the filter (Ono et al., 1983). A
third major step forward was made rather recently in the category of "automatic signal processing" when the low-frequency attenuation of the overall frequency transfer characteristic was made
to change automatically with the power content of the sound in the low-frequency region (Ono
et al., 1981, 1983; Kates, 1986). The low- and high-frequency attenuations are approximately
constant within the respective frequency band with low-frequency attenuation always being larger,
and the transition frequency band is fixed. As the low-frequency power content of the sound
increases, the low-frequency attenuation increases, with little change in the high-frequency
attenuation. Results of a clinical test showed that with this type of hearing aid, there was on the
average an improvement of over 15% in speech discrimination score in traffic noise for 50 of a
total sample of 53 subjects with sensori-neural hearing loss, including both moderate (<50 dB)
and severe (>50 dB) loss, with flat, sloping or abruptly falling audiogram patterns (Ono et al.,
1983). Moreover, the improvement in speech discrimination score increased with noise level.
Such a signal-processing scheme apparently resembles that offered by the stapedius muscle and
can be viewed as an extension and augmentation of the stapedius function to improve impaired
hearing (although interestingly the designers of this type of hearing aids were apparently not
aware of the frequency characteristics of the attenuation of middle-ear transmission produced by
stapedius contractions or did not think of making use of such knowledge, as judged from their
publications). While the improvement has been significant, this hearing aid is probably not
optimal in the sense that the parameters of the automatic high-pass filtering have been largely
chosen subjectively and somewhat arbitrarily by the designers. This is where knowledge of stapedius unmasking might possibly be applied and make a contribution. Specifically, the following
are suggested as possible measures to minimize sound distortion and increase efficiency in combating noise: (1) the location and width of the transition frequency band be made to match those
of the stapedius attenuation of transmission; (2) the threshold sound level for applying the filtering be made to match, at least approximately, the threshold of noise masking; (3) the growth-rate
- 227 -
of the masking function (e.g., the masking of speech by low-frequency noise) with and without
contractions of the stapedius muscle (e.g., below and above the threshold of the acoustic reflex)
be measured in subjects with sensori-neural hearing loss (either for individual fitting or for populations with distinguishable types of the loss) and incorporated into the determination of the
growth of the low-frequency attenuation with noise level in the design of the hearing aid. While
there have been few data reported for people with sensori-neural hearing loss on the growth-rate
of masking by low-frequency sounds of the responses to high-frequency sounds, one piece of evidence (Gagn6, 1983) suggests that (A) the GR in the case of sensori-neural hearing loss is
smaller than the GR in the "normal" case, and (B) with a 1/3 octave bandwidth noise masker
centered at 1 kHz, the GR of masking of a 2.75 kHz tone had a value of 1 dB/dB. Two inferences based on these results and the working hypothesis of stapedius unmasking tested in the
present study are: (A) an attenuation of acoustic transmission in the low-frequency region larger
than that in the high-frequency region should guarantee an unmasking effect because the GR of
masking reported for the case of sensori-neural hearing loss is not less than one; and (B) people
with sensori-neural hearing loss need external augmentation of the stapedius function to combat
masking by high-intensity low-frequency noise, both because of their high-frequency loss and
because of the smaller growth-rate of masking.
In summary, besides agreeing with the notion that the stapedius tendon is worth saving in
stapes surgery even at the cost of increased operational complexity (Lid6n, et al., 1964; Girgis,
1966; Rasmy, 1986), it is proposed, based on the results of the present study on the mechanisms
and advantages of the stapedius function, that hearing-aid devices with gain-control mechanisms
simulating that of the stapedius muscle be used for both people without functioning stapedius
(such as resulting from stapedectomy or stapedius paralysis) and for people with sensori-neural
hearing loss.
8.5 Comparison of Stapedius Effects with Olivocochlear Effects
--___----------_-_III------
--_L_
_1
I_
___
1__1
·_I
- 228 -
Before ending this chapter, it may be of interest to make a comparison of the effects of
stapedius contractions on auditory-nerve responses with the effects of another efferent system
from the brain to the auditory periphery, the olivocochlear efferents. The olivocochlear efferents
run from the olivery complex to the hair cells in the cochlea, and can be activated by moderate
sound stimulation ("signals of external origin") as well as inputs from higher centers in the central nervous system ("signals of internal origin") (Fex, 1962; Desmedt, 1975; Liberman & Brown,
1986). It has been well documented that electric stimulation of the olivocochlear efferents can
have a significant effect on the responses of auditory-nerve fibers (Wiederhold & Kiang, 1970;
Gifford & Guinan, 1983; Winslow & Sachs, 1987; Guinan & Gifford, 1988a & b). With respect
to auditory-nerve fiber responses to single-tone stimulation, the effects of electric stimulation of
the olivocochlear efferents resemble the effects of stapedius contractions in that the direction of
both effects is towards a reduction in the effective acoustic stimulus. The specific features of
olivocochlear efferent effects are, however, quite different from those of stapedius contractions in
the following aspects: (1) With rate-level function measurements, the effect of a given stimulation
to the olivocochlear efferents varies in general with the level of the test tone, and frequently
results in a change in the slope and a lowering of the plateau rate of the level function (Guinan &
Gifford, 1988a). (2) With tuning-curve measurements, the effect of stimulating the olivocochlear
efferents is a maximum elevation of the tuning curve near the tip region (Kiang et al., 1970;
Guinan & Gifford, 1988b).* (3) The overall effect of stimulating the olivocochlear efferents is
like a band-reject filter with maximum attenuation of responses to sound in the high-frequency
region of 4-10 kHz (Wiederhold, 1970; Guinan & Gifford, 1988a & b). As a result of the complicated dependence of olivocochlear effects on sound level and frequency, it can be expected
that in the presence of masking noise, the effects of stimulating the olivocochlear efferents on
Qualitatively, the effect of stimulating the olivocochlear efferents bears some resemblance to the effect of lowfrequency masking noise on auditory-nerve fiber responses to single tones, especially at moderate noise levels where the
noise does not by itself excite the fiber. One major difference, however, seems to be in the selective effect on fibers of
different SRs in that while low-SR fibers seem to be more resistant to changes in the plateau rate and slope of rate-level
functions in the case of noise masking, it is the opposite with stimulation of the olivocochlear efferents (Guinan &
Gifford, 1988a).
*
- 229 -
auditory-nerve fiber responses to tones would be very different from and more complicated than
the effects of stapedius contractions.
Winslow and Sachs (1987) studied in cats the effects of electric stimulation of a subset of
olivocochlear efferents, the crossed olivocochlear bundle (COCB), on the CF-tone rate-level
responses of auditory-nerve fibers in the background of broadband noise. Their results indicate
that COCB stimulation usually produced a shift of the rate-level function towards higher tone levels which was in addition to the shift produced by the masking noise. However, stimulation of
the COCB helped to restore the range of differential rate response which had been decreased by
the masking noise. The additional shift of the level function produced by COCB stimulation
decreased with increasing noise level; however, unlike the case of stapedius contractions, the shift
was never negative. It was also reported that COCB stimulation increased the threshold for noise
masking and two-tone rate suppression. The authors suggested that in the case of two-tone
suppression, the amount of the elevation in suppressor threshold produced by a COCB stimulation equals the amount of the shift of the suppressor-tone rate-level function produced by the
COCB stimulation. This, however, is unlikely to be of general validity because it fails to consider
the effect of the COCB stimulation on the test tone. A general statement that COCB stimulation
increased the threshold for two-tone suppression also seems to be at odds with the result of
Bonfils et al. (1986, 1987), which indicates that two-tone suppression in the cochlear compound
action potential (CAP) responses of the guinea pig was stronger with intact COCB than with
severed COCB. A more recent study (Dolan & Nuttall, 1988) on the effect of COCB stimulation
on the masking of high-frequency CAP level functions by broadband noise in the guinea pig
shows that the effect of COCB stimulation on the masked CAP magnitude can be either an
enhancement or a further reduction, depending on the tone and noise level.
There have also been some behavioral studies on the function of olivocochlear efferents.
Dewson (1968) trained monkeys to discriminate two vowel sounds in background masking noise.
After the COCB was severed, the noise level had to be reduced to maintain a constant discrimination score (while the discrimination score in quiet background remained largely unchanged).
_
_
_·
1__1_1___1____1_1____
-YIIII
-
-------
-·I----·II
L--I-----(PII
-
··-
-··-
---1--_
-
-- -----
- 230 -
This study, therefore,suggests that the COCB aids the discrimination of signals in noise. Another
study by Borg (1971) in behaving rabbits suggests that the function of the olivocochlear efferents
and of the stapedius muscle can be "complementary" in that when the COCB was severed,
activation of the middle-ear acoustic reflex by high-frequency tones started at lower elicitor levels
and for a given eliciting tone level, the stapedius muscle contracted harder to regulate the sound
reaching the inner ear.
To summarize, while the overall picture of the effect of olivocochlear efferents on masking of auditory-nerve fiber responses is still unclear, it seems to be different and more complicated than the effect of stapedius contractions. Current evidence indicates, however, that an
unmasking effect of the olivocochlear efferents is possible and that one functional aspects of this
efferent system may be to act as a complementary means for the central nervous system to combat masking in frequency and sound intensity regions different from those covered by the stapedius action.
- 231 -
Appendix
This appendix is intended to point out a distinction maintained in this thesis between the
stapedius-induced attenuation of sound reaching the inner ear and the stapedius-induced attenuation of acoustic transmission through the middle ear. When the stapedius muscle contracts, the
impedance of the stapes changes and consequently the input acoustic impedance at the tympanic
membrane changes. Since the sound source has a finite output impedance, when the impedance of
the load (the ear) changes, the pressure drop across the load, i.e., the sound pressure at the tympanic membrane, changes. The stapedius effect measured from the CM or auditory-nerve
responses using a constant-response (constant middle-ear output) criterion, as was the case in this
study, is the stapedius-induced attenuation of sound reaching the inner ear. This measure includes
the effects of both the attenuation of acoustic transmission through the middle ear and the change
in the input sound pressure to the middle ear.
The difference between the attenuation of sound reaching the inner ear and attenuation of
middle-ear transmission does not change the results of this study as long as a consistent measure,
such as the constant-response measure, of the attenuation is used, because what counts in the
measurement and prediction of stapedius unmasking is the effective attenuation of sound reaching
the inner ear. Furthermore, it should be made clear that conclusions drawn from measurements of
the stapedius-induced attenuation of sound can also be applied to the stapedius-induced attenuation of transmission. For a given stapedius contraction, the change in sound pressure at the tympanic membrane at a given frequency is a constant. Therefore, the stapedius-induced attenuation
of sound and attenuation of transmission only differ by a constant, and the conclusion concerning
the linearity of middle ear with stapedius contractions is the same regardless of which measure of
the stapedius effect is used. The direction of the stapedius-induced change in sound pressure at
the tympanic membrane in the frequency region explored in this study is an increase in the magnitude of sound pressure. This is because the corresponding change in the input impedance at the
tympanic membrane is an increase in magnitude. As a result, the stapedius-induced attenuation of
- 232 -
sound reaching the inner ear provides an under-estimate of the corresponding attenuation of
transmission. With the sound source used in this study, the increase in sound pressure at the tympanic membrane with maximum stapedius contractions was smaller than 2 dB in magnitude in the
low-frequency region and approximately zero in the high-frequency region. When the stapediusinduced low-frequency attenuation of sound was 15 dB (a typical level used in this study), the
increase in sound pressure at the tympanic membrane was typically 1 dB. Therefore, the
stapedius-induced attenuation of sound measured in this study is a close approximation of the
attenuation of transmission in the low-frequency region and an even closer approximation in the
high-frequency region.
It should also be pointed out that a difference between the stapedius-induced attenuation
of sound and attenuation of transmission exists in all cases involving stapedius contractions,
including the case with open-field acoustic stimulation. The extent of the difference depends on
the sound source used and acoustic frequency (With the sound source used in this study, the
maximum difference was 6 dB between 1 and 2 kHz). While this difference has been largely
ignored in the literature (with a few exceptions, such as Pang & Peake (1985)), the ignorance of
this difference. may lead to errors in or even invalidation of the conclusion of a study. One possible example of the contamination of a conclusion due to negligence of this difference is the
report by Morgan et al. (1978), which was mentioned in Section 2.2.2. When they concluded that
stapedius contractions had no effect on threshold detection of low-frequency tones by human subjects, they probably neglected the fact that when the stapedius contracted, the real sound pressure
at the tympanic membrane, for the same voltage input to the sound source, was increased from
that without stapedius contraction. In other words, even if the auditory threshold in terms of the
voltage input to the sound source remained the same with and without contractions of the stapedius, the threshold in terms of the sound pressure at the tympanic membrane had increased
with contractions of the stapedius muscle.*
* Other reasons such as the poor level resolution involved (the voltage input to the sound source was changed in 4 dB
steps in the determination of auditory thresholds both with and without stapedius contractions) and sizable variabilities
of the measurement (on the same order of magnitude as the level step size) might also have contaminated their conclusion.
- 233 -
Literature Cited
Abbas, P.J. (1978) Effects of stimulus frequency on two-tone suppression: A comparison of physiological and psychophysical results. J. Acoust. Soc. Am., 63(6): 1878-1886.
Anderson, S.D. (1976) The intratympanic muscles. In: Hinchcliff, R. & Harrison, D. (Eds.),
Scientific Foundationsof Otolaryngology, Chicago: Year Book, pp. 257-280.
Brkrsy, G. von (1960) Experiments in Hearing, New York: McGraw-Hill.
Bonfils, P., Remond, M.C. & Pujol, R. (1986) Efferents tracts and cochlear frequency selectivity.
Hearing Res., 24: 277-283.
Bonfils, P. & Puel, J.L. (1987) Functional properties of the crossed part of the medial olivocochlear bundle. Hearing Res., 28: 125-130.
Borg, E. (1968) A quantitative study of the effect of the acoustic stapedius reflex on sound
transmission through the middle ear of man. Acta Oto-Laryng., 66: 461-472.
Borg, E. (1971) Efferent inhibition of afferent acoustic activity in the unanesthetized rabbit. Exp.
Neurol., 31: 301-312.
Borg, E. (1972) Regulation of middle ear sound transmission in the nonanesthetized rabbit. Acta
Physiol. Scand., 86: 175-191.
Borg, E., Counter, S.A. & Rosier, G. (1984) Theories of middle-ear muscle function. In: Silman,
S. (Ed.), The Acoustic Reflex -- Basic principles and clinical applications, Academic Press, pp.
63-99.
Borg, E. & M6ller, A.R. (1967) Effect of ethylalcohol and pentobarbital sodium on the acoustic
middle ear reflex in man. Acta Oto-Laryng., 64: 415-426.
Borg, E. & M6ller, A.R. (1975) Effect of central depressants on the acoustic middle ear reflex in
rabbit. Acta Physiol. Scand., 94: 327-338.
Borg, E. & Zakrisson, J. (1973) Stapedius reflex and speech features.
J. Acoust. Soc. Am.,
54(2): 525-527.
Borg, E. & Zakrisson, J. (1974) Stapedius reflex and monaural masking. Acta Otolaryng., 78:
155-161.
Borg, E. & Zakrisson, J. (1975a) The activity of the stapedius muscle in man during vocalization.
Acta Otolaryng., 79: 325-333.
Borg, E. & Zakrisson, J. (1975b) The stapedius muscle and speech perception. Symp. Zool. Soc.
Lond., 37: 51-68.
Busnel, R.G. (Ed.) Acoustic Behaviour of Animals, Elsevier, 1963.
Carmel, P.W. & Starr, A. (1963) Acoustic and nonacoustic factors modifying middle-ear muscle
activity in waking cats. J. Neurophysiol., 26: 598-616.
- Z3
-
Colburn, H.S. (1981) Intensity perception: relation of intensity discrimination to auditory-nerve
firing patterns. Internal memorandum, Res. Lab. Electron., M.I.T., Cambridge, MA.
Costalupes, J.A. (1983) Broadband masking noise and behavioral pure tone thresholds in cats. J.
Acoust. Soc. Am., 74(3): 758-764.
Costalupes, J.A. (1985) Representation of tones in noise in the responses of auditory nerve fibers
in the cats: I. Comparisons with detection thresholds. J. Neurosci., 5(12): 3261-3269.
Costalupes, J.A., Young, E.D. & Gibson, D.J. (1984) Effects of continuous noise backgrounds on
rate response of auditory nerve fibers in cat. J. Neurophysiol., 51(6): 1326-1344.
Dallos, P. (1984) Peripheral mechanisms of hearing. In: Darian-Smith, I. (Vol. Ed.), Handbook of
Physiology (Section I, Vol. III, Part 2), Bethesda, MD: Am. Physiol. Soc., pp. 595-637.
Delgutte, B. (1986) Two-tone rate suppression in auditory-nerve fibers: variations with suppressor
level and frequency. Presented at the Ninth Midwinter Research Meeting of Association for
Research in Otolaryngology, Clearwater, Fl.
Delgutte, B. (1987) Peripheral auditory processing of speech information: implications from a
physiological study of intensity discrimination. In: Schouten, M.E.H. (Ed.), Psychophysics of
Speech Perception, Martinus & Nijhoff, Dordrecht, The Netherlands, pp. 333-353.
Delgutte, B. (1988) Physiological mechanisms of masking. To appear in: Duifhuis, D. & Horst,
W. (Eds.), Basic Issues in Hearing, Academic Press. (In press).
Desmedt, J.E. (1975) Physiological studies of the efferent recurrent auditory system. In: Keidel,
W.D. & Neff, W.D. (Eds.), Handbook of Sensory Physiology, V5/2, Springer, Berlin, pp. 219-
246.
Dewson, J.H. (1968) Efferent olivocochlear bundle: some relationships to stimulus discrimination
in noise. J. Neurophysiol., 31: 122-130.
Djupesland, G. (1964) Middle ear muscle reflexes elicited by acoustic and nonacoustic stimulation. Acta Oto-Laryng. Suppl., 188: 287-292.
Djupesland, G. (1967) Contractions of the tympanic muscles in man. Thesis, Oslo: Universitetsforlaget.
Dolan, D. & Nuttall, A.L. (1988) Masked cochlear whole-nerve response intensity functions
altered by electric stimulation of the crossed olivocochlear bundle. J. Acoust. Soc. Am., 83(3):
1081-1086.
Dorman, M., Cedar, I., Hannley, M., Leek, M. & Lindholm, J.M. (1986) Influence of the acoustic reflex on vowel recognition. J. Speech & Hearing Res., 29: 420-424.
Dorman, M.F., Lindholm, J.M., Hannley, M.T. & Leek, M.R. (1987) Vowel intelligibility in the
absence of the acoustic reflex: performance-intensity characteristics. J. Acoust. Soc. Am., 81(2):
562-564.
Durlach, N.I. (1968) A decision model for psychophysics. Internal memorandum, Dept. Elec.
Eng. & Res. Lab. Electron., M.I.T., Cambridge, MA.
11111111
- 235 -
,
Egan, J.P. & Hake, H.W. (1950) On the masking pattern of a simple auditory stimulus. J.
Acoust. Soc. Am., 22(5): 622-630.
Evans, E.F. & Palmer, A.R. (1980) Relationship between dynamic range of cochlear nerve fibers
and their spontaneous activity. Exp. Brain Res., 40: 115-118.
Fex, J. (1962) Auditory activity in centrifugal and centrapetal cochlear fibers in cat. Acta Physiol.
Scand., 55, Suppl. 189: 5-86.
Fletcher, H. (1953) Speech and Hearing in Communication. Van Nostrand, Princeton, NJ.
Fletcher, J.L. (1962) Reflex response of middle-ear muscles: protection of the ear from noise.
Sound, 1: 17.
Gacek, R.R. & Rasmussen, G.L. (1961) Fiber analysis of the statoacoustic nerve of guinea pig,
cat and monkey. Anat. Rec., 139: 455-463.
Gagn6, J.P. (1983) Excess masking among listeners with high-frequency sensorineural hearing
loss. Ph.D. Dissertation, Central Inst. for the Deaf, Washington Univ., St. Louis, MO.
Geisler, C.D. & Sinex, D.G. (1980) Responses of primary auditory fibers to combined noise and
tonal stimuli. Hearing Res., 3: 317-334.
Gifford, M.L. & Guinan, J.J., Jr. (1983) Effects of crossed-olivocochlear-bundle stimulation on
cat auditory nerve fiber responses to tones. J. Acoust. Soc. Am., 74(1): 115-123.
Gifford, M.L. & Guinan, J.J., Jr. (1987) Effects of electrical stimulation of medial olivocochlear
neurons on ipsilateral and contralateral cochlear responses. Hearing Res., 29: 179-194.
Girgis, I.H. (1966) Preservation of the stapedius tendon in stapes surgery. J. Larygol. Otol., 80:
733-742.
Goldstein, M.H., Jr. & Kiang, N.Y.S. (1958) Synchrony of neural activity in electric response
evoked by transient acoustic stimuli. J. Acoust. Soc. Am., 30: 107-114.
Green, D.M. & Swets, J.A. (1966) Signal Detection Theory and Psychophysics. John Wiley &
Sons, New York.
Green, D.M. & Wier, C.C. (1984) Auditory perception. In: Darian-Smith, I. (Vol. Ed.) Handbook
of Physiology (Section I, Vol. III, Part 2), Bethesda, MD: Am. Physiol. Soc., pp. 557-594.
Guinan, J.J., Jr. & Gifford, M.L. (1988a) Effects of electrical stimulation of efferent olivocochlear neurons on cat auditory-nerve fibers. I. Rate-level functions. Hearing Res., in press.
Guinan, J.J., Jr. & Gifford, M.L. (1988b) Effects of electrical stimulation of efferent olivocochlear neurons on cat auditory-nerve fibers. III. Tuning curves and threshold at CF. Hearing
Res., in press.
Guinan, J.J., Jr. & McCue, M.P. (1987) Asymmetries in the acoustic reflexes of the cat stapedius
muscle. Hearing Res., 26: 1-10.
Guinan, J.J., Jr. & Peake, W.T. (1967) Middle-ear characteristics of anesthetized cats. J. Acoust.
Soc. Am., 41(5): 1237-1261.
___111_
- 236 -
Harris, C.M. (Ed.) Handbook of Noise Control. McGraw-Hill, New York, 1979.
Hawkins, J.E., Jr. & Stevens, S.S. (1950) The masking of pure tones and of speech by white
noise. J. Acoust. Soc. Am., 22; 6-13.
Hilding, D.A. (1960) The intratympanic muscle reflex as a protective mechanism against loud
impulsive noise. Ann. Oto. Rhin. Laryng., 69: 51-60.
Humes, L.E. (1978) The effect of middle ear muscle contraction on auditory and overload thresholds. Audiology, 17: 360-367.
Irvine, D.R.F., Clarey, J.C., Morton, R.E. & Newman, R.G. (1983) Masking by internally generated noise and protection by middle ear muscle activity. Hearing Res., 10: 371-374.
Irvine, D.R.F. & Wester, K.G. (1974) Middle ear muscle effects on cochlear responses to boneconducted sound. Acta Physiol. Scand., 91: 482-496.
Javel, E., Geisler, C.D. & Ravindran, A. (1978) Two-tone suppression in auditory nerve of the
cat: Rate-intensity and temporal analyses. J. Acoust. Soc. Am., 63(4): 1093-1104.
Johnson, D.H. (1980) The relationship between spike rate and synchrony in responses of
auditory-nerve fibers to single tones. J. Acoust. Am. Soc., 68(4): 1115-1122.
Johnson, D.H. & Kiang, N.Y.S. (1976) Analysis of discharges recorded simultaneously from
pairs of auditory-nerve fibers. Biophys. J., 16: 719-734.
Joseph, M.P., Guinan, J.J., Jr., Fullerton, B.C., Norris, B.E. & Kiang, N.Y.S. (1985) Number and
distribution of stapedius motoneurons in cats. J. Comp. Neurol., 232: 43-54.
Kates, J.M. (1986) Signal processing for hearing aids. Hearing Instruments, 37(2): 19-22.
Kiang, N.Y.S. (1975) Stimulus representation in the discharge patterns of auditory neurons. In:
Tower, D.B. (Ed. in Chief), The Nervous System, vol.3, Eagles, E.L. (Vol. Ed.), Human Communication and Its Disorders, New York: Raven, pp. 81-96.
Kiang, N.Y.S. (1984) Peripheral neural processing of auditory information. In: Darian-Smith, I.
(Vol. Ed.), Handbook of Physiology (Section I, Vol. III, Part 2), Bethesda, MD: Am. Physiol.
Soc., pp. 639-674.
Kiang, N.Y.S., Guinan, J.J., Jr., Liberman, M.C., Brown, M.C. & Eddington, D.K. (1987) Feedback control mechanisms of the auditory periphery: implications for cochlear implants. Presented
at "International Cochlear Implant Symposium 1987", Cologne, West Germany, Sept. 7-12, 1987.
Kiang, N.Y.S. & Moxon, E.C. (1974) Tails of tuning curves of auditory-nerve fibers. J. Acoust.
Soc. Am., 55(3): 620-630.
Kiang, N.Y.S., Moxon, E.C. & Levine, R.A. (1970) Auditory-nerve activity in cats with normal
and abnormal cochleas. In: Whostenholme, G.E.W. & Knight, J. (Eds.), SensorineuralHearing
Loss, Great Britain: J. & A. Churchill, pp. 241-273.
Kiang, N.Y.S., Watanabe, T., Thomas, E.C. & Clark, L.F. (1965) Discharge Patterns of Single
Fibers in the Cat's Auditory Nerve, Cambridge, MA: MIT Press.
- 237 -
Klockhoff, I.H. & Anderson, H. (1959) recording of the stapedius reflex elicited by cutaneous
stimulation. Acta Oto-Laryng., 50: 451-454.
Kobler, J.B., Vacher, S.R. & Guinan, J.J., Jr. (1987) The recruitment order of stapedius motoneurons in the acoustic reflex varies with sound laterality. Brain Res., 425: 372-375.
Kryter, K.D. (1970) The Effects of Noise on Man. Academic Press, New York.
Liberman, M.C. (1978) Auditory-nerve response from cats raised in a low-noise chamber. J.
Acoust. Soc. Am., 63(2): 442-455.
Liberman, M.C. (1980) Morphological differences among radial afferent fibers in the cat cochlea:
An electron-microscopic study of serial sections. Hearing Res., 3: 45-63.
Liberman, M.C. (1982) The cochlear frequency map for the cat: labelling auditory nerve fibers of
known frequency. J. Acoust. Soc. Am., 72: 1441-1449.
Liberman, M.C. & Brown, M.C. (1986) Physiology and anatomy of single olivocochlear neurons
in the cat. Hearing Res., 24: 17-36.
Liberman, M.C., Dodds, L.W. & Kiang, N.Y.S. (1984) Single-neuron labeling and chronic
cochlear pathology (I-IV). Hearing Res., 16: 33-90.
Licklider, J.C.R. & Miller, G.A. (1951) The perception of speech. In: Stevens, S.S. (Ed.), Handbook of Experimental Psychology, Wiley, New York.
Lidtn, G., Nordlund, B. & Hawkins, J.E., Jr. (1964) Significance of the stapedius reflex for the
understanding of speech. Acta Oto-Laryng., Suppl. 188: 275-279.
Loeb, M. & Riopelle, A.J. (1960) Influence of loud contralateral stimulation on the threshold and
perceived loudness of low-frequency tones. J. Acoust. Soc. Am., 32(5): 602-610.
Mahoney, T., Vernon, J. & Meikle, M. (1979) Function of the acoustic reflex in discrimination of
intense speech. Arch. Otolaryng., 105: 119-123.
Matthews, J.W. (1983) Modeling reverse middle ear transmission of acoustic distortion signals.
In: de Bore, E. & Viergever, M.A. (Eds.), Mechanics of Hearing, Delft Univ. Press, Delft, The
Netherlands, pp. 11-18.
McCandless, G.A. & Goering, D.M. (1974) Changes in loudness after stapedectomy. Arch. Otolaryngol., 100: 344-350.
McCandless, G.A. & Schumacher, M.H. (1979) Auditory dysfunction with facial paralysis. Arch.
Otolaryngol., 105: 271-274.
McCue, M.P. & Guinan, J.J., Jr. (1983) Functional segregation within the stapedius motoneuron
pool. Soc. Neurosci. Abstr., 9: 1085.
McMahon, A.T. (1984) Muscles, Reflexes, and Locomotion. Princeton University Press.
Meyerhoff, W.L. (Ed.) Diagnosis and Management of hearing Loss. Saunders, 1984.
Miller, G.A. (1947) The masking of speech. Psychol. Bull., 44: 105-129.
I-X
-C
--I -
- 238 -
Miller, J.D., Watson, C.S. & Covell, W.P. (1963) Deafening effects of noise on the cat. Acta
Oto-Laryngol. Suppl. 176.
M1ller, A.R. (1962) Acoustic reflex in man. J. Acoust. Soc. Am., 34(8): 1524-1534.
M6ller, A.R. (1965) An experimental study of the acoustic impedance of the middle ear and its
transmission properties. Acta Oto-Laryng., 60: 129-149.
Morgan, D.E. & Dirks, D.D. (1975) Influence of middle-ear muscle contraction on pure-tone
suprathreshold loudness judgement. J. Acoust. Soc. Am., 57(2): 411-420.
Morgan, D.E., Dirks, D.D. & Kamm, C. (1978) The influence of middle-ear muscle contraction
on auditory threshold for selected pure tones. J. Acoust. Soc. Am., 63(6): 1896-1903.
Nadol, J.B., Jr. (1983) Serial section reconstruction of the neural poles of hair cells in the human
organ of corti. I. Inner hair cells. Laryngoscope, 93: 599-614.
Nedzelnitsky, V. (1979) Effects of middle-ear muscle contraction on transmission of sound to the
inner ear (cat): some direct measurements. J. Acoust. Soc. Am., 65 Suppl (1): S10.
Ono, H., Kanzaki, J. & Mizoi, K. (1981) Hearing aid with noise-level-controlled selective
amplification. The Lancet, Dec. 5, 1981, 1286-1287.
Ono, H., Kanzaki, J. & Mizoi, K. (1983) Clinical results of hearing aid with noise-levelcontrolled selective amplification. Audiology, 22: 494-515.
Pang, X.D. & Peake, W.T. (1985) How do contractions of the stapedius muscle alter the acoustic
properties of the ear? In: Allen, J.B., et al. (Eds.), PeripheralAuditory Mechanisms, SpringerVerlag, pp. 36-43.
Pickles, J.O. (1979) Psychophysical frequency resolution in the cat as determined by simultaneous masking and its relation to auditory-nerve resolution. J. Acoust. Soc. Am., 66(6): 1725-1732.
Rabinowitz, W.M. (1977) Acoustic-reflex effects on the input admittance and transfer characteristics of the human middle-ear. Ph.D. thesis, Dept. of EECS, MIT, Cambridge, MA.
Rabinowitz, W.M. (1981) Acoustic-reflex effects on middle-ear performance. Presented at the
101th Meeting of Acoust. Soc. Am., 69 Suppl.: S44.
Rasmussen, A.T. (1940) Studies of the eighth cranial nerve of man. Laryngoscope, 50: 67-83.
Rasmy, E. (1986) Stapedius reflex after stapedectomy with preservation of the stapedius tendon.
J. Laryngol. Otol., 100: 521-527.
Relkin, E.M. & Pelli, D.G. (1987) Probe tone thresholds in the auditory nerve measured by twointerval forced-choice procedures. J. Acoust. Soc. Am., 82(5): 1679-1691.
Robles, L., Ruggero, M.A. & Rich, N.C. (1986) Basilar membrane mechanics at the base of the
chinchilla cochlea. I. Input-output functions, tuning curves, and response phase. J. Acoust. Soc.
Am., 80: 1364-1374.
Rose, C. (1985) Methods of frequency selectivity and synchronization measurement in single
auditory nerve fibers: application to the alligator lizard. Ph.D. thesis, Dept. of EECS, MIT,
_
__
- 239 -
Cambridge, MA.
Sachs, M.B. & Abbas, P.J. (1974) Rate versus level functions for auditory-nerve fibers in cats:
tone-burst stimuli. J. Acoust. Soc. Am., 56(6): 1835-1847.
Sachs, M.B. & Kiang, N.Y.S. (1968) Two-tone inhibition in auditory-nerve fibers. J. Acoust.
Soc. Am., 43(5): 1120-1128.
Sataloff, J. (1966) HearingLoss. Lippincott, Philadelphia.
Sesterhenn, G. & Breuninger, H. (1978) On the influence of middle ear muscle upon changes in
sound transmission. Arch. Otorhinolaryngol., 221: 47-60.
Siebert, W.M. (1965) Some implications of the stochastic behavior of primary auditory neurons.
Kybernetik, 2: 206-215.
Siebert, W.M. (1968) Stimulus transformations in the peripheral auditory system. In: Kollers,
P.A. & Eden, M. (Eds.), Recognizing Patterns, MIT press, pp. 104-133.
Simmons, F.B. (1959) Middle ear muscle activity at moderate sound levels. Ann. Oto. Rhin. Laryng., 68: 1126-1143.
Simmons, F.B. (1960) Middle ear muscle protection from the acoustic trauma of loud continuous
sound. Ann. Oto. Rhin. Laryng., 69(4): 1063-1161.
Simmons, F.B. (1963) Individual sound damage susceptibility: role of middle ear muscles. Ann.
Oto. Rhin. Laryng., 72(2): 528-547.
Simmons, F.B. (1964) Perceptual theories of middle ear muscle function. Ann. Oto. Rhin. Laryng., 73: 724-739.
Sivian, L.J. & White, S.D. (1933) On minimum audible sound fields. J. Acoust. Soc. Am., 4:
288-321.
Sokolovski, A. (1973) The protective action of the stapedius muscle in noise-induced hearing loss
in cats. Arch. fur Ohren-, Nasen- und Kehlkopfheilkunde, 203: 289-309.
Spoendlin, H. (1972) Innervation densities of the cochlea. Acta Otolaryng., 73: 235-248.
Stevens, S.S. & Davis, H. (1938) Hearing: Its Psychology and Physiology. Wiley, New York.
Stevens, S.S., Miller, J. & Truscott, I. (1946) The masking of speech by sine waves, square
waves, regular and modulated pulses. J. Acoust. Soc. Am., 18: 418-424.
Teich, M.C. & Khanna, S.M. (1985) Pulse-number distribution for the neural spike train in the
cat's auditory nerve. J. Acoust. Soc. Am., 77(3): 1110-1128.
Teig, E. (1973) Differential effect of graded contraction of middle ear muscles on the sound
transmission of the ear. Acta Physiol. Scand., 88: 382-391.
V6r, I.L., Brown, R.M. & Kiang, N.Y.S. (1975) Low-noise chambers for auditory research. J.
Acoust. Soc. Am., 58(2): 392-398.
____
_lii
_I_·II
__^^^_II_
240 -
Viemeister, N.F. (1983) Auditory intensity discrimination at high frequencies in the presence of
noise. Science, 221: 1206-1208.
Wang, B.S. (1979) The relation between the compound action potential and unit discharges of the
auditory nerve. Sc.D. thesis, Dept. of EECS, M.I.T., Cambridge, Mass.
Ward, W.D. (1961) Studies on aural reflex: I. contralateral remote masking as an indicator of
reflex activity. J. Acoust. Soc. Am., 33: 1034.
Ward, W.D. (1967) Further observations on contralateral remote masking and related phenomena.
J. Acoust. Soc. Am., 42: 593.
Warr, W.B. (1975) Olivocochlear and vestibular efferent neurons of the feline brain stem: their
location, morphology and number determined by retrograde axonal transport and acetylcholinesterase histochemistry. J. Comp. Neurol., 161: 159-181.
Watson, C.S. (1963) Masking of tones by noise for cat. J. Acoust. Soc. Am., 35: 167-172.
Wegel, R.L. & Lane, C.E. (1924) The auditory masking of one pure tone by another and its
probable relation to the dynamics of the inner ear. Physical Review, 23: 266-285.
Weiss, T.F., Goldmark, G.M., Altmann, D.W. & Brown, R.M. (1969): Automated system to control stimulus and measure response variables in experiments on the auditory system. Quarterly
Progress Report #95, Res. Lab. Electronics, MIT, Cambridge, MA, pp. 122-127.
Weiss, T.F. & Peake, W.T. (1972) Cochlear potential response at the round-window membrane of
the cat -- a reply to the comment of G.R. Price. J. Acoust. Soc. Am., 52(6): 1729-1734.
Wever, E.G. & Bray, C.W. (1942) The stapedius muscle in relation to sound conduction. J. Exp.
Psychol., 31: 35-43.
Wever, E.G. & Lawrence, M. (1954) Physiological Acoustics. Princeton University Press.
White, F.A. (1975) Our Aoustic Environment. Wiley, New York.
Wiederhold, M.L. (1970) Variations in the effects of electric stimulation of the crossed olivocochlear bundle on cat single auditory-nerve-fiber responses to tone bursts. J. Acoust. Soc. Am.,
48(4): 966-977.
Wiederhold, M.L. & Kiang, N.Y.S. (1970) Effects of electric stimulation of the crossed olivocochlear bundle on single auditory-nerve fibers in the cat. J. Acoust. Soc. Am., 48(4): 950-965.
Winslow, R.L. & Sachs, M.B. (1987) Effect of electrical stimulation of the crossed olivocochlear
bundle on auditory nerve response to tones in noise. J. Neurophysiol., 57(4): 1002-1021.
Young, E.D. & Barta, P.E. (1986) Rate responses of auditory nerve fibers to tones in noise near
masked threshold. J. Acoust. Soc. Am., 79(2): 426-442.
Zakrisson, J. (1975) The role of the stapedius reflex in poststimulatory auditory fatigue. Acta
Otolaryng., 79: 1-10.
Zakrisson, J. & Borg, E. (1974) Stapedius reflex and auditory fatigue. Audiology, 13: 231-235.
0o
1
_
_
- 241 -
Zakrisson,.J., Borg, E., Liden, G. & Nilsson, R. (1980) Stapedius reflex in industrial impact
noise: fatigability and role for temporary threshold shift (ITS). Scand. Audiol. Suppl., 12: 326334.
___ _____^
I1__·_III_1IIIIII·_1_11.-----··--_
L· I --
I-II
_..I
____