* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Graphing Periodic Trends – Ana Julia Silva
Survey
Document related concepts
Transcript
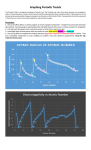
Graphing Periodic Trends The Periodic Table is arranged according to Periodic Law. The Periodic Law states that when elements are arranged in order of increasing atomic number, their physical and chemical properties show a periodic pattern. These patterns can be discovered by examining the changes in properties of elements on the Periodic Table. The properties that will be examined in this lesson are: atomic size, electronegativity, and ionization energy. Procedure 1. Use excel (office 365) or a similar program to create 3 graphs (scatterplot – straight link connections between data points). Give the graph an appropriate title and label the axes (the x-axis is ‘atomic number’ for all graphs). 2. Cut and paste the graphs onto a word document so that their x-axes are in line. 3. Label/high-light the data points with the symbol of each alkali metal, halogen and noble gas. 4. Use your graphs to complete the analysis questions (type your response in a different colour!). below. Post your completed assignment to your edublog (2 students may post identical assignments) using the tag: mstilsnerchem11coop Atomic number vs Atomic Radius 300 Atomic Radius (pm) 250 K 200 Na Li 150 100 Cl 50 F He Br Kr Ar Ne 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 Atomic Number Atomic Number vs Ionization Energy Ionization Energy (kj/mol) 2500 He Ne 2000 F Ar 1500 Kr Cl Br 1000 500 Li Na K 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 Atomic Number Atomic Number vs Electronegativity 4.5 Electronegativity (Pauling Scale) 4 F 3.5 Cl 3 Br 2.5 2 1.5 1 Li Na K 0.5 0 He Ne Ar Kr 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 Atomic Number ANALYSIS QUESTIONS: use the periodic table below to summarize the following trends. 1. Based on your graphs, what is the trend in atomic radius across a period? Down a family? The alkali metals all have very big atomic radiuses, while the noble gases and halogens have much smaller atoms. Based on my graph, atoms of the same families tend to have similar sized atoms. However, atom radius appear to get smaller across a period rather than have similar sizes. 2. Based on your graphs, what is the trend in ionization energy across a period? Down a family? Once again, atoms of the same families have similar ionization energies. For instance, the noble gases all have very high ionization energies, while the alkali metals have very low ionization energies. Across the first two periods, the gaps in ionization energies between elements is very noticeable, however once we add the transition metals in the third period we can see that they all have similar ionization energies. 3. Based on your graphs, what is the trend in electronegativity across a period? Down a family? All families display very similar electronegativity, for example, all noble gases have an electronegativity of zero, while the halogens are the most electronegative out of all other families on the periodic table. The Alkali metals also range around the same number, showing that the families all have similar electronegativity. Electronegativity seems to increase across a period, going up from the alkali metals which all range at around one to the halogens with the highest values, until reaching the noble gases and crashing down to zero. 4a) What is happening to the number of protons and the number of energy levels as you move across the periodic table from left to right? How and why does this affect atomic radius. As you move across the periodic table, the number of protons increases by one. This affects the atomic radius because the highest number of protons you have, the smaller the atom is going to be due to the increase of attractive forces which pull the electrons closer to the nucleus. Therefore, the elements on the right side of the table such as the halogens and noble gases all have very small atoms while the elements on the left such as the alkali metals have much bigger radiuses. b) What happens to the number of energy levels as you move down a column on the periodic table. How and why does this effect ionization energy? As you move down on the periodic table, the number of energy levels increases by one. This affects the ionization energy because the more energy levels the atom has, the further away the valence electrons are from the nucleus, and for atoms with bigger radiuses and less protons such as the alkali metals, the distance makes their ionization energy very low. c) What happens to the effective nuclear charge as you move across a period on the periodic table? How does this affect ionization energy and electronegativity? As you move across a period, the effective nuclear charge increases by one, which in turn increases both the ionization energy and the electronegativity as can be seen on the graphs above. 5a) Which group contains elements which are easiest to ionize? Explain why this is the case. The alkali metals are the easiest elements to ionize because they have the biggest atoms and the lowest ionization energy. It is very easy for other elements to rip the valence electron off an alkali metal. b) Explain why the third ionization energy of Ca would be much higher than the 1st and 2nd ionization energy Ca The reason why the third ionization energy of calcium is much higher than the first and second is because once the first two electrons are removed, the remaining ones are held much closer and stronger to the nucleus due to the fact that the overall charge of the atom will be positive, making the attractive forces stronger, and there will be less repulsive force. 6. Which element would have the highest electronegativity in each set below? Explain why this is. a) Ca, Be or Mg b) B, Li, or F Fluorine would have the highest electronegativity because it has the smallest atom and it has the highest effective nuclear charge which would attract other electrons much stronger than Lithium or Boron would. Beryllium would have the highest electronegativity because it has the smallest atom, therefore its attractive forces range further than that of calcium or magnesium. 7. Write (or type) the electron configuration of each atom (high-light the valence electrons) and it’s corresponding ion below each sketch (atomic radii are given in angstroms (1 x 10-10 M). 1s2, 2s2, 2p3 1s2, 2s2, 2p6 1s2, 2s2, 2p6, 3s1 1s2, 2s2, 2p6 1s2, 2s2, 2p4 1s2, 2s2, 2p6 1s2, 2s2, 2p6, 3s2 1s2, 2s2, 2p6 1s2, 2s2, 2p5 1s2, 2s2, 2p6 1s2, 2s2, 2p6, 3s2, 3p1 1s2, 2s2, 2p6 9. Over the blank periodic table provided, write or type the number of valence electrons and the expected ion charge for the transition metal block and for the families to the left and right of the transitions metals (the alkali metals have been done as an example). Note: Carbon and boron do not normally form ions and are thus blanked-out # valence e: Ion charge: 1 2 2 2 2 2 2 2 2 2 2 2 X X +1 +2 +3 +4 +2 +2 +2 +2 +2 +2 +2 +2 X X 5 6 7 8 -3 -2 -1 0