* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Absolute vs. Relative Dating of Rocks
Survey
Document related concepts
Transcript
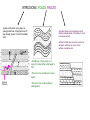
Absolute vs. Relative Dating of Rocks -As time passes, the rock cycle creates and destroys rocks. This process leaves behind layers of different types of rocks, which scientists can determine the age of in two different ways. Relative Dating - Determining the age of a rock based on its position in ground compared to other rocks. Absolute Dating - Determining the age of a rock based on its half-life (radioactive decay) Relative Dating -This process uses the relative position of a rock layer to determine its age. YOUNGEST ROCK AT THE TOP -The law of superposition - In any undisturbed layer of rock, the layers at the bottom will be the oldest, and the layers on top will be the youngest. ***IF THE ROCK LAYERS ARE UNDISTURBED, THE DEEPER YOU DIG, THE OLDER THE ROCKS*** Similar to counting the rings on a tree, the inner rings are older, the outer rings are newer. OLDEST ROCK AT THE BOTTOM INTRUSIONS, FOLDS, FAULTS -Igneous intrusions occur when an underground flow of magma forces its way through layers of rock that already exist. -At times, these rocks can bend so much that they actually break. This break or crack is known as a fault. -Similar to folds, they can occur is some or all layers, and they can occur on the surface or underground. -Sometimes, a force is put on a layer of rocks and the rocks begin to fold. -This can occur in all layers or some layers. Igneous Intrusion -This can occur on the surface or underground. Absolute Dating -To age rocks using this method, YOU DO NOT NEED TO KNOW WHERE THE ROCK CAME FROM. Scientists use a method called radioactive decay to determine the absolute age of a rock. Rocks contain thousands of particles called isotopes that decay (fall apart) over time. As they decay, they turn from one isotope to another. For example: Isotope - A variation of a chemical element that has an unusual number of neutrons. For example, Carbon normally has 6 neutrons. Carbon isotopes could contain 7, 8, 9, etc. These isotopes break down over KNOWN AMOUNTS OF TIME, and this break down can be measured by seeing how much product is made. Red isotope Blue isotope (product of red isotope decay) Half-life The time it takes for half of the isotopes in a sample to decay If the half-life of the red isotopes is 1 million years, how many years does it take for all but one of the red isotopes to decay (turn blue)? LETS PRACTICE RELATIVE DATING BY ANSWERING A FEW QUESTIONS... 1. Which layer in the picture will hold the oldest rocks? 2. Which layer in the picture will hold the youngest rocks? 3. The law of ___________________ states that layer G is made of younger rock than layer B. 4. Do you think the igneous intrusion occurred before or after the formation of layer A? What about layer G? 5. If layer H was created 5 million years ago, and layer B was formed 4 million years ago. Hypothesize an approximate date as to when the fold in layers D,C,I, and H could have occurred. 6. Name a type of rock that could have formed within the igneous intrusion (letter F). LETS PRACTICE SOME ABSOLUTE DATING........ The four pictures below are of a rock that is going through radioactive decay of a Uranium (U) isotope. Over time, the Uranium decays and slowly turns into Lead (Pb). Drag these 4 pictures in order, with the youngest rock on the left, and the oldest on the right. Each arrow above represents 1.1 billion years. What is the half-life of this sample of Uranium? So, how is determining the age of rocks useful to humans?