* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Part 8
Condensed matter physics wikipedia , lookup
Transformation optics wikipedia , lookup
Colloidal crystal wikipedia , lookup
Tunable metamaterial wikipedia , lookup
Self-assembled monolayer wikipedia , lookup
Sol–gel process wikipedia , lookup
Silicon photonics wikipedia , lookup
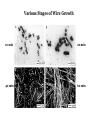
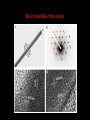
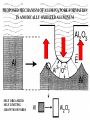
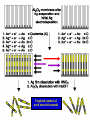
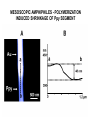
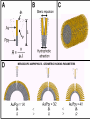
TOPOTACTIC NANOCHEMISTRY APPROACH TO SILVER SELENIDE NANOWIRES • Silver selenide Ag2Se • Silver ion superionic conductor • Photoconductor • Thermoelectric - large Seebeck coefficient • Thermochromic 133°C alpha-beta phase transition • Therefore interesting to synthesize nanowires of silver selenide • Idea is to synthesize c-Se nanowires and topotactically convert them with Ag+ to c-Ag2Se nanowires with shape retention - similar for ZnSe, Be2Se3 Unique Features of Selenium • Intrinsic Optical Chirality • Highest Photoconductivity (s = 8 x 104 S/cm for t-Se) • Piezoelectric and Nonlinear Optical (NLO) Properties • Thermoelectric Properties • Useful Catalytic Properties (Halogenation, Oxidation) • Reactivities to Form Other Se Chain Trigonal Selenium (t-Se) Functional Materials such as ZnSe, CdSe and Ag2Se Growth of c-Se Nanowires from a-Se Seeds 100 oC 100 oC a-Se R.T. a-Se t-Se (t-Se) t-Se a-Se Various Stages of Se Wire Growth Nanowires of t-Se with f~30 nm XRD Absorption Spectra of t-Se Nanowires ~30 nm wires ~10 nm wires Photoresponse of t-Se Nanowire Synthesis of Silver Nanowires AgNO3 + HO(CH2)2OH PtCl2 ( CH2 CH )n O N (PVP) PVP:Ag=1:1 160-180 oC Mechanism: Chemistry versus Art PtCl2 AgNO3 (CH2OH)2 PVP Pt seeds PVP ? Growth Various Stages of Wire Growth 10 min 20 min 40 min 60 min Silver Nanowires with f~40 nm XRD Bi-Crystalline Structure TOPOTACTIC TRANSFORMATION OF ORIENTED c-Se NWS TO ORIENTED C-Ag2Se NWS 3Se(s) + Ag+(aq) + 3H2O 2Ag2Se(s) + Ag2SeO3 (aq) + 6H+(aq) 0.71 + AgNO3 0.49 t-Se 0.44 0.49 (f<30 nm) + AgNO3 (f>40 nm) 0.44 (tetragonal Ag2Se) 0.78 0.70 (orthorhombic Ag2Se) PXRD MONITORING OF TOPOTACTIC CONVERISON OF c-Se NWs TO c-Ag2Se NWs • Rapid solution-solid phase reaction • Complete in less than 2 hours • Samples washed with hot water to remove Ag2SeO3 by product 3Se(s) + Ag+(aq) + 3H2O • Time evolution of PXRD shows c-Se converts to cAg2Se + 2Ag2Se(s) + Ag2SeO3 (aq) + 6H (aq) Tetragonal a-Ag2Se (f~30 nm) EDX Orthorhombic a-Ag2Se (f>40 nm) FILMS - FORM? • Supported - substrate type and effect of interface • Free standing - synthetic strategy • Epitaxial - lattice matching - tolerance • Superlattice - artificial • Patterned - chemical or physical lithography FILMS - WHEN IS A FILM THICK OR THIN? • Monolayer - atomic, molecular thickness • Multilayer - compositional superlattice - scale periodicity • Bulk properties - scale - thickness greater than l(e,h) • Quantum size effect - 2D confinement - free electron behavior in third dimension - quantum wells THIN FILMS ARE VITAL IN MODERN TECHNOLOGY • Protective coatings • Optical coatings, electrochromic windows • Filters, mirrors, lenses • Microelectronic devices • Optoelectronic devices • Photonic devices THIN FILMS ARE VITAL IN MODERN TECHNOLOGY • Electrode surfaces • Photoelectric devices, photovoltaics, solar cells • Xerography, photography • Electrophoretic and electrochromic ink, displays • Catalyst surfaces • Information storage, magnetic, magneto-resistant, magneto-optical, optical memories FILM PROPERTIES - ELECTRICAL, OPTICAL, MAGNETIC, MECHANICAL, ADSORPTION, PERMEABILTY, CHEMICAL • Thickness and surface : volume ratio • Structure - surface vs bulk, surface reconstruction, roughness • Hydrophobicity, hydrophilicy • Composition • Texture, single crystal, microcrystalline, orientation • Form, supported or unsupported, nature of substrate METHODS OF SYNTHESIZING THIN FILMS • ELECTROCHEMICAL, PHYSICAL, CHEMICAL • Cathodic deposition, anodic deposition, electroless deposition • Laser ablation • Cathode sputtering, vacuum evaporation • Thermal oxidation, nitridation METHODS OF SYNTHESIZING THIN FILMS • ELECTROCHEMICAL, PHYSICAL, CHEMICAL • Liquid phase epitaxy • Self-assembly, surface anchoring • Discharge techniques, RF, microwave • Chemical vapor deposition CVD, metal organic chemical vapour deposition MOCVD • Molecular beam epitaxy, supersonic cluster beams, aerosol deposition ANODIC OXIDATIVE DEPOSITION OF FILMS • Deposition of oxide films, such as alumina, titania • Deposition of conducting polymer films by oxidative polymerization of monomer, such as thiophene, pyrrole, aniline • Oxide films formed from metallic electrode in aqueous salts or acids ANODIC OXIDATION OF Al IN OXALIC OR PHOSPHORIC ACID TO FORM ALUMINUM OXIDE • Pt|H3PO4, H2O|Al • Al Al3+ + 3e- • PO43- +2e- PO33- + O2- anode cathode • Overall electrochemistry: potential control of oxide thickness • Oxide anions diffuse through growing layer of aluminum oxide • 2Al3+ + 3O2- g-Al2O3 (annealing) a-Al2O3 ANODIC OXIDATION OF PATTERNED Al DISC TO MAKE PERIODIC NANOPOROUS Al2O3 MEMBRANE Aqueous HgCl2 dissolves Al to give Hg and Al(H2O)63+ and H3PO4 dissolves Al2O3 barrier layer to give Al(H2O)63+ - yields open channel membrane 2Al + 3PO43- Al2O3 + 3PO332Al + 3C2O42- Al2O3 + 6CO + 3O2- ANODIC OXIDATION OF LITHOGRAPHIC PATTERNED Al TO PERIODIC NANOPOROUS Al2O3 ANODIC OXIDATION OF LITHOGRAPHIC PATTERNED Al TO PERIODIC NANOPOROUS Al2O3 40V 60V 80V PROPOSED MECHANISM OF ALUMINA PORE FORMATION IN ANODICALLY OXIDIZED ALUMINUM SELF ORGANIZED SELF LIMITING GROWTH OF PORES Templated synthesis of metal barcoded nanorods MESOSCOPIC AMPHIPHILES MESOSCOPIC AMPHIPHILES CURRENT CONTROL OF LENGTH OF POLYMER AND METAL SEGMENTS MESOSCOPIC AMPHIPHILES - POLYMERIZATION INDUCED SHRINKAGE OF Ppy SEGMENT MESOSCOPIC AMPHIPHILES - GEOMETRIC PACKING PARAMETERS ANODIC OXIDATION OF Si TO FORM POROUS Si: THROWING SOME LIGHT ON SILICON • Typical electrochemical cell to prepare PS by anodic oxidation of heavily doped p+-type Si • PS comprised of interconnected nc-Si with H/O/F surface passivation • nc-Si right size for QSEs and red light emission observed during anodic oxidation LIGHT WORK BY THE SILICON SAMURAI: WHERE IT ALL BEGAN AND WHERE IT IS ALL GOING FROM CANHAM’S 1990 DISCOVERY OF PL AND EL ANODICALLY OXIDIZED p-DOPED Si WAFERS, TO NEW LIGHT EMITTING SILICON NANOSTRUCTURES, TO SILICON OPTOELECTRONICS, TO PHOTONIC COMPUTING ELECTRONIC BAND STRUCTURE OF DIAMOND SILICON LATTICE • • • • • • band structure of Si computed using density functional theory with local density and pseudo-potential approximation diamond lattice, sp3 bonded Si sites VB maximum at k = 0, the G point in the Brillouin zone, CB minimum at distinct k value indirect band gap character, very weakly emissive behavior absorption-emission phonon assisted photon-electron-phonon three particle collision very low probability, thus band gap emission efficiency low, 10-5% SEMICONDUCTOR BAND STRUCTURE: CHALLENGE, EVOKING LIGHT EMISSION FROM Si • EMA Rexciton ~ 0.529e/mo where e = dielectric constant, reduced mass of exciton mo = memh/(me + mh) • Note exciton size within the bulk material defines the size regime below which significant QSEs on band structure are expected to occur, clearly < 5 nm to make Si work REGULAR OR RANDOM NANNSCALE CHANNELS IN ANODICALLY OXIDIZED SILICON WAFERS • Anodized forms of p+type Si wafer • Showing formation of random (left) and regular (right) patterns of pores • Lithographic pretexturing directs periodic pore formation