* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download General Steps in Viral Replication Cycles
West Nile fever wikipedia , lookup
Ebola virus disease wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Marburg virus disease wikipedia , lookup
Hepatitis B wikipedia , lookup
Orthohantavirus wikipedia , lookup
Henipavirus wikipedia , lookup
Influenza A virus wikipedia , lookup
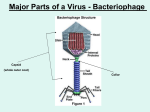
College of Dentistry Third stage Asst.Proff. Dina M.R. Alkhafaf Oral Virology. Viruses are the smallest infectious agents (ranging from about 20 to 300 nm in diameter) and contain only one kind of nucleic acid (RNA or DNA) as their genome. The nucleic acid is encased in a protein shell, which may be surrounded by a lipid-containing membrane. The entire infectious unit is termed a virion. Viruses are parasites at the genetic level, replicating only in living cells and are inert in the extracellular environment. The viral nucleic acid contains information necessary to cause the infected host cell to synthesize virus-specific macromolecules required for the production of viral progeny. During the replicative cycle, numerous copies of viral nucleic acid and coat proteins are produced. The coat proteins assemble together to form the capsid, which encases and stabilizes the viral nucleic acid against the extracellular environment and facilitates the attachment and penetration by the virus upon contact with new susceptible cells. The virus infection may have little or no effect on the host cell or may result in cell damage or death. The spectrum of viruses is rich in diversity. Viruses vary greatly in structure, genome organization and expression, and strategies of replication and transmission. The host range for a given virus may be broad or extremely limited. Viruses are known to infect unicellular organisms, such as mycoplasmas, bacteria, and algae, and all higher plants and animals. . Much informati on on virus–host relationships has been obtained from studies on bacteriophages, the viruses that attack bacteria. Properties of individual viruses TERMS AND DEFINITIONS IN VIROLOGY Indicated viral components are described below: Capsid: The protein shell, or coat, that encloses the nucleic acid genome. Capsomeres: Morphologic units seen in the electron microscope on the surface of icosahedral virus particles. Capsomeres represent clusters of polypeptides, but the morphologic units do not necessarily correspond to the chemically defined structural units. Defective virus: A virus particle that is functionally deficient in some aspect of replication. Envelope: A lipid-containing membrane that surrounds some virus particles. It is acquired during viral maturation a budding process through a cellular membrane (see Figure by 29-3). Virus-encoded glycoproteins are exposed on the surface of the envelope. These projections are called peplomers. Nucleocapsid: The protein–nucleic acid complex representing the packaged form of the viral genome. The term is commonly used in cases in which the nucleocapsid is a substructure of a more complex virus particle. Structural units: The basic protein building blocks of the coat. They are usually a collection of more than one nonidentical protein subunit. The structural unit is often referred to as a protomer. Subunit: A single folded viral polypeptide chain. Virion: The complete virus particle. In some instances (eg, papillomaviruses, picornaviruses), the virion is identical with the nucleocapsid. In more complex virions (herpesviruses, orthomyxoviruses), this includes the nucleocapsid plus a surrounding envelope. This structure, the virion, serves to transfer the viral nucleic acid from one cell to another Prions Prions are infectious particles composed solely of protein with no detectable nucleic acid. They are highly resistant to inactivation by heat, formaldehyde, and ultraviolet light that inactivate viruses. The infectious prion protein is misfolded and able to change the conformation of the native prion protein which is encoded by a single cellular gene. Prion diseases, called “transmissible spongiform encephalopathies,” include scrapie in sheep, mad cow disease in cattle, and kuru and CreutzfeldtJakob disease in humans. EVOLUTIONARY ORIGIN OF VIRUSES The origin of viruses is not known. There are profound differences among the DNA viruses, the RNA viruses, and viruses that use both DNA and RNA as their genetic material during different stages of their life cycle. It is possible that different types of agents are of different origins. Two theories of viral origin can be summarized as follows: 1. Viruses may be derived from DNA or RNA nucleic acid components of host cells that became able to replicate autonomously and evolve independently. They resemble genes that have acquired the capacity to exist independently of the cell. Some viral sequences are related to portions of cellular genes encoding protein functional domains. It seems likely that at least some viruses evolved in this fashion. 2. Viruses may be degenerate forms of intracellular parasites. There is no evidence that viruses evolved from bacteria, although other obligately intracellular organisms (eg, rickettsiae and chlamydiae) presumably did so. However, poxviruses are so large and complex that they might represent evolutionary products of some cellular ancestor A. Cubic Symmetry All cubic symmetry observed with animal viruses is of the icosahedral pattern, the most efficient arrangement for subunits in a closed shell. The icosahedron has 20 faces (each an equilateral triangle), 12 vertices, and fivefold, threefold, and twofold axes of rotational symmetry. The vertex units have five neighbors (pentavalent), and all others have six (hexavalent). There are 60 identical subunits on the surface of an icosahedron. To build a particle size adequate to encapsidate viral genomes, viral shells are often composed of multiples of 60 structural units. Larger capsid structures are formed in some cases to accommodate the size of the viral genome with the association of additional protein subunits. Most viruses that have icosahedral symmetry do not have an icosahedral shape rather, the physical appearance B. Helical Symmetry In cases of helical symmetry, protein subunits are bound in a periodic way to the viral nucleic acid, winding it into a helix. The filamentous viral nucleic acid–protein complex (nucleocapsid) is then coiled inside a lipid-containing envelope. Thus, as is not the case with icosahedral structures, there is a regular, periodic interaction between capsid protein and nucleic acid in viruses with helical symmetry. It is not possible for “empty” helical particles to form. All known examples of animal viruses with helical symmetry contain RNA genomes and, with the exception of rhabdoviruses, have flexible nucleocapsids that are wound into a ball inside envelopes . C. Complex Structures Some virus particles do not exhibit simple cubic or helical symmetry but are more complicated in structure. For example, poxviruses are brick shaped, with ridges on the external surface and a core and lateral bodies inside CLASSIFICATION OF VIRUSES Basis of Classification The following properties have been used as a basis for the classification of viruses. The amount of information available in each category is not the same for all viruses. Genome sequencing is now often performed early in virus identification, and comparisons with databases provide detailed information on the viral classification, predicted protein composition, and taxonomic relatedness to other viruses. 1. Virion morphology, including size, shape, type of symmetry, presence or absence of peplomers, and presence or absence of membranes. 2. Virus genome properties, including type of nucleic acid (DNA or RNA), size of the genome, strandedness (single or double), whether linear or circular, sense (positive, negative, ambisense), segments (number, size), nucleotide sequence, percent GC content, and presence of special features (repetitive elements, isomerization, 5′-terminal cap, 5′terminal covalently linked protein, 3′-terminal poly(A) tract). 3. Genome organization and replication, including gene order, number and position of open reading frames, strategy of replication (patterns of transcription, translation), and cellular sites (accumulation of proteins, virion assembly, virion release). 4. Virus protein properties, including number, size, amino acid sequence, modifications (glycosylation, phosphorylation, myristoylation), and functional activities of structural and nonstructural proteins (transcriptase, reverse transcriptase, neuraminidase, fusion activities). 5. Antigenic properties, particularly reactions to various antisera. 6. Physicochemical properties of the virion, including molecular mass, buoyant density, pH stability, thermal stability, and susceptibility to physical and chemical agents, especially solubilizing agents and detergents. 7. Biologic properties, including natural host range, mode of transmission, vector relationships, pathogenicity, tissue tropisms, and pathology. General Steps in Viral Replication Cycles A variety of different viral strategies have evolved for accomplishing multiplication in parasitized host cells. Although the details vary from group to group, the general outline of the replication cycles is similar. The growth cycles of a doublestranded DNA virus and a positive-sense, single-stranded RNA virus are diagrammed in Figure 29-5. Details are included in the following chapters devoted to specific virus groups. A. Attachment, Penetration, and Uncoating The first step in viral infection is attachment, interaction of a virion with a specific receptor site on the surface of a cell. Erally glycoproteins. In some cases, the virus binds protein sequences (eg, picornaviruses) and in others oligosaccharides (eg, orthomyxoviruses and paramyxoviruses). The presence or absence of receptors plays an important determining role in cell tropism and viral pathogenesis. Not all cells in a susceptible host will express the necessary receptors; for example, poliovirus is able to attach only to cells in the central nervous system and intestinal tract of primates. Each susceptible cell may contain up to 100,000 receptor sites for a given virus. After binding, the virus particle is taken up inside the cell. This step is referred to as penetration or engulfment. In some systems, this is accomplished by receptor-mediated endocytosis, with uptake of the ingested virus particles within endosomes. There are also examples of direct penetration of virus particles across the plasma membrane. In other cases, there is fusion of the virion envelope with the plasma membrane of the cell. Those systems involve the interaction of a viral fusion protein with a second cellular receptor or coreceptor. Uncoating occurs concomitantly with or shortly after penetration. Uncoating is the physical separation of the viral nucleic acid from the outer structural components of the virion so that it can function. The genome may be released as free nucleic acid (picornaviruses) or as a nucleocapsid (reoviruses). The nucleocapsids usually contain polymerases. Uncoating may require acidic pH in the endosome. The infectivity of the parental virus is lost at the uncoating stage. Viruses are the only infectious agents for which dissolution of the infecting agent is an obligatory step in the replicative B. Expression of Viral Genomes and Synthesis of Viral Components The synthetic phase of the viral replicative cycle ensues after uncoating of the viral genome. The essential theme in viral replication is that specific mRNAs must be transcribed from the viral nucleic acid for successful expression and duplication of genetic information. After this is accomplished, viruses use cell components to translate the mRNA. Various classes of viruses use different pathways to synthesize the mRNAs depending on the structure of the viral nucleic acid. Table 29-2 summarizes various pathways of transcription (but not necessarily those of replication) of the nucleic acids of different classes of viruses. Some viruses (eg, rhabdoviruses) carry RNA polymerases to synthesize mRNAs. RNA viruses of this type are called negative-strand (negativesense) viruses because their single-strand RNA genome is complementary to mRNA, which is conventionally designated positive strand (positive sense). The negative-strand viruses must supply their own RNA polymerase because eukaryotic cells lack enzymes able to synthesize mRNA off an RNA template. In the course of viral replication, all of the virus-specified macromolecules are synthesized in a highly organized sequence. In some viral infections, notably those involving double-stranded DNA-containing viruses, early viral proteins are synthesized soon after infection and late proteins are made only late in infection after viral DNA synthesis begins. Early genes may or may not be shut off when late products are made. In contrast, most, if not all, of the genetic information of RNA-containing viruses is expressed at the same time. In addition to these temporal controls, quantitative controls also exist because not all viral proteins are made in the same amounts. Viral microRNAs or virus-specific proteins may regulate the extent of transcription of the genome or the translation of viral mRNA. C. Morphogenesis and Release Newly synthesized viral genomes and capsid polypeptides assemble together to form progeny viruses. Whereas icosahedral capsids can condense in the absence of nucleic acid, nucleocapsids of viruses with helical symmetry cannot form without viral RNA. In general, nonenveloped viruses accumulate in infected cells, and the cells eventually lyse and release the virus particles.