* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Synthesis of new nitric oxide donor derivatives
Cluster chemistry wikipedia , lookup
Jahn–Teller effect wikipedia , lookup
Bond valence method wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Hydroformylation wikipedia , lookup
Metal carbonyl wikipedia , lookup
Spin crossover wikipedia , lookup
Metalloprotein wikipedia , lookup
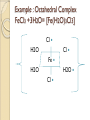
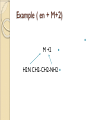
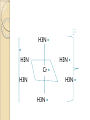
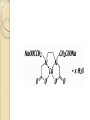
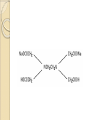
Complexation& Complexes used in Pharmacy 2016-2017 3rd Year Pharmacy Ligands Molecule or ion having a lone electron pair that can be used to form a bond to a metal ion (Lewis base). coordinate covalent bond: metal- ligand bond monodentate: one bond to metal ion two bond to metal ion bidentate: more than two polydentate: Example : Octahedral Complex FeCl3 +3H2O= [Fe(H2O)3Cl3] Cl H2O Cl Fe H2O H2O Cl Werner Theory 1- Two steps of valiancy are observed Primary ( ionizable )and Secondary non- ionizable valence. 2-Each metal exhibit a specific maximum numbers of secondary valence. 3-The primary valences are filled by anions, but secondary valence anions or neutral. 4- The ligands are arranged (Square planner, Tetrahedral, Octahedral ) The most stable complexes are found by cations of the transition series with anions. Why? The criteria for maximum stability of the metal in a complex involving ( + ) charge , a small cation radius and unoccupied . Properties of Ligands The ligands may be anions or neutral molecules . Neutral atoms arenot found in coordination agents. There are one factor that all ligands have in common that one non – bonded pairs of es , which is used to form a coordination covalent bond with metal. Table2: Complexation ligands Neutral ligands Anionic ligands NH3 H2O CN- NH2R HOR S2O3-2 NHR2 ROR F- NR3 R2C=O OH-1 - RHC=O Cl-1 Br-1 Bidentate ligands Ligands have two positions arranged, so that both can act simultaneously as donor sites in a complex Table 3:Some common bidentate Ligands Ethylene diamine En NH2CH2CH2NH2 Glycinate Gly NH2CH2COO- Oxalate - -ooccoo- Ethylen diamine tetraacetic acid EDTA - COO O OC N - N - O OC Ethylene diamine tetraacetic acid COO - Example ( en + M+2) M +2 H2N CH2-CH2-NH2 Importance of Chelation 1- Employ in pharmaceutical and reducing agent( Benedict reagent) for sugar test. 2- Drug Therapy ( various types of poisoning &Radioactive materials). 3- Titration with complexing agents in analytical methods. 4-Has been used to alter the physicochemical & biopharmaceutical properties of drugs 5-Chelation is used in solubility & stability of products. Bonding in Complexes There FOUR theories describe the bonding in complexation : 1-Valence bond theory 2-Crystal field theory 3-Ligand field theory 4-Molecular orbital theory( MOT) MOT now is used in common. Factors affecting the complexation The solution conditions that effect the size of the formation constant are: • Temperature, • Presence of competing ions, • pH, and • Ionic strength, stability …. EX: Cr+3 + 6CN-1 = Cr24 : Cr +3 d2 SP3 Cr( CN)6 4S2 3d4 4p0 4S0 3d3 4p0 -3 H3N H3N H3N Cr H3N H3N H3N C ompare between the following complexes Fe ( H2O)6 +3 Fe ( CN)6) -3 Answer [Fe ( H2O )6 ] +++ magnetic momentum = 5.9 B.M( Bohr magnaton ) 4s2 3d6 Fe+++= 4s0 3d5 (H2O)6 are on sp3d2 high spin complex Octahedral structure. [Fe( CN)6]-3 D.M= 2 B.M low spin complex 3d5 octahedral complex structure d2SP3 Why Ni(CN)4 is squar plannar while NiCl4 is tetrahedra When lighand CN is low spin , the d orbital be vacant & form dsp2 hybride is a squar plannar. In case of Cl high spin lighand , the d – orbital would only have SP3 and form tetrahedral . Pharmaceutical Products 1- Ca di Na edetate 2- Di Na edetate 3- penicillamine 4-Dicaperol 5- Iron injection ( imferone ) 1-Calciuum di sodium edetate Calcium Disodium Versenate (edetate calcium disodium injection, USP) is a sterile, injectable, chelating agent in concentrated solution for intravenous infusion or intramuscular injection. Each 5 ml ampoule contains 1000 mg of edetate calcium disodium (equivalent to 200 mg/ml) in water for injection Chelation therapy should not replace effective measures to eliminate or reduce further exposure to lead.( Plumblism) It may be used for posioing Cu, Ni , Cd, Zn, Cr , & Mn. It is no value in treatment for Hg,As & Au. 2-Disodium edetate. Endrate (Edetate Disodium Injection, USP) is a sterile, nonpyrogenic, concentrated solution of edetate disodium in water for injection which as a result of a pH adjustment with sodium hydroxide contains varying amounts of disodium and trisodium salts. After dilution, it is administered by intravenous infusion Uses. Endrate (Edetate Disodium Injection, USP) is indicated in selected patients for the emergency treatment of hypercalcemia and for the control of ventricular arrhythmias associated with digitalis toxicity 3- Penicillamine . The pharmaceutical form is D- penicillamine, as L-penicillamine is toxic (it inhibits the action of pyridoxine Uses of penicillamine In Wilson's disease, a rare genetic disorder of copper metabolism, penicillamine treatment relies on its binding to accumulated copper and elimination through urine. 4- Dimercaprol Dimercaprol (INN) or British anti- Lewisite (abbreviated BAL) Dimercaprol is itself toxic, with a narrow therapeutic range and a tendency to concentrate arsenic in some organs. Other drawbacks include the need to administer it by painful intramuscular injection.[7] Serious side effects include nephrotoxicity and hypertension 5-Imferon injection 1- Iron defiency ( anemia ) Pregnency or Children It give by Physician in applying equation to calculate the dose. 2-Iron replacement ( Blood loss). Iron+ Dextran=Complex Fe(OH)3 given as injection Fe+2 Fe SO4 given orally. Fe+2 Lactate orally