* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Heat and Energy of Ractions
Kinetic energy wikipedia , lookup
Energy Charter Treaty wikipedia , lookup
World energy consumption wikipedia , lookup
Energy storage wikipedia , lookup
International Energy Agency wikipedia , lookup
Regenerative brake wikipedia , lookup
Low-Income Home Energy Assistance Program wikipedia , lookup
Zero-energy building wikipedia , lookup
Energy efficiency in transport wikipedia , lookup
Low-carbon economy wikipedia , lookup
Energy returned on energy invested wikipedia , lookup
Alternative energy wikipedia , lookup
Negawatt power wikipedia , lookup
Energy harvesting wikipedia , lookup
Compressed air energy storage wikipedia , lookup
Energy policy of the European Union wikipedia , lookup
Cogeneration wikipedia , lookup
Environmental impact of electricity generation wikipedia , lookup
Energy in the United Kingdom wikipedia , lookup
Gibbs free energy wikipedia , lookup
Energy Independence and Security Act of 2007 wikipedia , lookup
Internal energy wikipedia , lookup
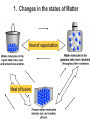
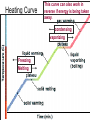
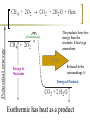
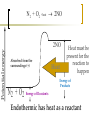
Heat and Energy of Ractions Chapter 10 Energy World of Chemistry Zumdahl Last revision Fall 2009 What is energy? The ability to cause a change. change in position or temperature 1st law of thermodynamics: Energy is neither created nor destroyed, only transformed from one type to another. The unit is the Energy transformations Joule (J) or Kgm2/s2 Kinetic: motion Potential: stored Work: applied forces to make motion Heat: really kinetic energy of molecules Temperature and Heat Temperature is a measure of the random motions of the particles of a substance. Hot water (90. oC) Cold water (10. oC) More motion in particles means higher temperatures Absolute Zero is the temperature at which all motion of particles stops. Scientists have yet to reach it. State of Matter- Matter can be solid, liquid, gas or plasma depending on how much kinetic energy (or motion) they have. Solid: Particles vibrate in place. It has a definite volume and a definite shape. Liquid: Particles roll around each other. It has a definite volume but no definite shape. Gas: Particles move around colliding with one another. It has no definite volume or shape. Plasma: Particles are so hot and excited that the electrons leave the nucleus and matter is ionized or charged Matter in Motion The Kinetic Molecular Theory states that atoms and molecules are always in motion. Remember, the temperature of a substance is the measure of its kinetic energy. That energy can only do one thing at a time: 1. Change the state of the substance (disrupt the intermolecular forces that hold it in a phase). 2. Increase the temperature (motion) of a substance. 1. Changes in the states of Matter Boiling Heat of vaporization Condensing Freezing Heat of fusion Melting Sublimating Depositing Heating Curve This curve can also work in reverse if energy is being taken away. condensing vaporizing Freezing Melting Energy in Reactions Exothermic Reactions – A reaction in which energy is released. Feels hot. Endothermic Reactions – A reaction which energy must be provided for it to continue. Absorbs energy and feels cold. Dissolution Reactions – When ionic compounds dissolve in water. Will this be endothermic or exothermic? (remember, breaking bonds requires energy) Endothermic What the heat? Heat is energy that transfers from one object to another because of a temperature difference between them. Heat, itself, cannot be detected by the senses or by instruments. Only the change can be detected. Heat always flows from a warmer object to a cooler object. Heat takes the perspective of the reaction so it is positive if it is absorbed and negative if it is released CH 4 + 2O 2 CO 2 + 2H 2 O + Heat The products have less energy than the reactants. It has to go somewhere Potential energy Activation Energy CH 4 + 2O 2 Heat Energy of Reactants Released to the surroundings (-) Energy of Products CO 2 + 2 H 2 O Exothermic has heat as a product N2 + O2 +heat 2NO Potential energy 2NO Absorbed from the surroundings (+) N2 + O2 Energy of Reactants Heat Heat must be present for the reaction to happen Energy of Products Endothermic has heat as a reactant Energy, potential energy, and Enthalpy. Energy (q) is the ability to do work or produce heat. Kinetic energy is the motion of the molecules and can be measured by taking it’s temperature. Potential energy is the energy stored in a substance because of its composition (like the types of bonds it formed). Enthalpy (H) is the heat released or absorbed in a chemical reaction. Heat Capacity and Specific Heat Heat Capacity (C) is the amount of heat energy a substance can absorb before the substance will increase its temperature by one degree Celsius. Specific heat (s) of a substance is the amount of heat required to raise the temperature of one gram of that substance by one degree Celsius. Calculating the Energy of a Reaction Any energy lost by the reaction must be gained by the surroundings. Energy must always be conserved. The sign on Q indicates if energy is leaving or entering the system. Try it yourself: What is the specific heat of a pure metal that has a mass of 2.8g and requires 10.1J of energy to raise the temperature from 21oC to 36 oC. Try this (Remember heat is conserved): -Qlost= Qgained What is the specific heat of the rock below if the rock begins at 100 oC and is placed in the beaker of water at 20.0oC with a specific heat capacity of 4.18J/g oC. They both end up at 23.5 oC.