* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download crust - WordPress.com
Survey
Document related concepts
History of geomagnetism wikipedia , lookup
Schiehallion experiment wikipedia , lookup
Spherical Earth wikipedia , lookup
Post-glacial rebound wikipedia , lookup
Tectonic–climatic interaction wikipedia , lookup
Algoman orogeny wikipedia , lookup
History of Earth wikipedia , lookup
Provenance (geology) wikipedia , lookup
History of geology wikipedia , lookup
Plate tectonics wikipedia , lookup
Composition of Mars wikipedia , lookup
Age of the Earth wikipedia , lookup
Clastic rock wikipedia , lookup
Transcript
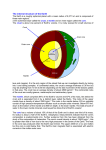
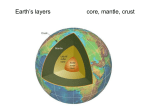
UNIT TWO: Structure and Composition of the Earth 2.1. Structure of the earth . We now know that Earth is comprised of layers. –The Crust. –The Mantle. –The Core. – Crust Continental Oceanic –Mantle Upper Lower –Core Outer – Liquid Inner – Solid 2.1.1. Crust, mantle and core The Crust The outermost “skin” of Earth with variable thickness. Thickest under mountain ranges (70 km – 40 miles). Thinnest under mid-ocean ridges (3 km – 2 miles). Covers 1% of the thickness of the earth The only portion of the earth that man has direct knowledge and accessibility. The crust is made up of silicate minerals, which is composed of silicon and oxygen. The components are made from lighter substances compared to mantle and core. Therefore, Crustal material contains lighter elements like Si, O, Al, Ca, K, Na, etc... Feldspars (Anorthite, Albite, and Orthoclase) are common minerals in the crust (CaAL2Si2O8, NaALSi3O8, and KALSi3O8). Generally Crustal Composition is that: 98.5% of the crust is comprised of just 8 elements. Oxygen is (by far!) the most abundant element in the crust. This reflects the importance of silicate (SiO2-based) minerals. As a large atom, oxygen occupies ~93% of crustal volume. Two Types of Crust Continental crust Underlies the continents. Covers 45% of the crust Avg. rock density about 2.7 g/cm3. Avg. thickness 35-40 km. Made from Sial (silica- alumina). Sial makes the rock granite Felsic composition. Avg. rock type = Granite Oceanic crust Underlies the ocean basins. Covers 55% of the crust Density about 3.0 g/cm3. Avg. thickness 7-10 km. Made from Sima (silica- magnesium). Sima makes the rock basalt Mafic composition Avg. rock type = Basalt/Gabbro The transition b/n crust and upper mantle is called Mohorovičić discontinuity or “Moho”. It is named after a Yugoslavian seismologist known as Andrija Mohorovicic. Who first identified it in 1909. Mantle A compositional layer between the crust and the core. It is made from ultramafic minerals (magnesium and ferrous) minerals particularly olivine. The mantle is made up of Si and O, like the crust, but it contains more Fe and Mg. Thus, Olivine (Fe2SiO4-Mg2SiO4) and pyroxene (MgSiO3-FeSiO3) are abundant in the mantle. The thickness of the mantle is about 2,885 km; the mantle is 82% of Earth’s volume. Below ~100-150 km, the rock is hot enough to flow. It convects: hot mantle rises, cold mantle sinks. Three subdivisions: upper, transitional, and lower. The upper mantle’s high temperatures of 2,800–3,200 °C can melt rocks. The semi-solid layer in the upper part of the mantle is called the asthenosphere. The asthenosphere is a solid that flows like a liquid. This physical property is called plasticity. Scientists believe that the lithosphere and the crust are able to move slowly over the top of the mantle and asthenosphere. Lower mantle (also called mesosphere): 660 km - 2900 km, strong ultramafic rock Based on composition and physical properties Mantle categorized in to two. Viz: Upper Mantle (lithosphere) Lithosphere provides plates that cause continental drift Upper mantle begins at the base of the crust Contains part of the lithosphere (or rigid sphere), the asthenosphere (or weak sphere, soft, easily deformable) Gradual increase in pressure and temperature Lower Mantle (asthenosphere) Asthenosphere = weak sphere – Near to molten rock – It is a source of energy for most of the tectonic processes. – Begins at 670 km discontinuity, ends at the core‐mantle – – boundary Gradual increase in pressure and temperature and seismic velocities Different from upper mantle due to an increase in temperature and in seismic velocities At the bottom of asthenosphere low velocity zone. P waves reduce velocity S waves cease to exist. Gutenberg discontinuity separates mantle from the core The Core A compositional layer found at the central position of the earth. What the core made of mostly is iron; it is high density and common in the solar system (one of the most abundant after H & He) An iron-rich sphere with a radius of 3,471 km. It accounts 17% of the earth’s volume. Mainly, it is composed of Nife (Nickel and Ferrous)(dense minerals) Little knowledge due to its remoteness Generates earth’s magnetism Extreme pressure due to compression Based on composition core categorized in to two. Viz: Outer core Liquid iron-nickel-sulfur Density – 10-12 g/cm3 The outer core is less dense than the inner core and, therefore, is located around the inner core. The temperatures range from 4,000 °C to 5,000 °C. The outer core is approximately 2,200 kilometers thick and is a combination of mostly molten Iron (Fe) and Nickel (Ni). Molten describes materials that change to liquid form when exposed to extreme amounts of thermal energy. Inner core Solid iron-nickel alloy Density – 13 g/cm3 Very extreme pressure The deepest layer in Earth is the inner core. It is located at the center of Earth because it contains the densest material of all of Earth’s layers. The inner core is solid and mostly composed of the element iron (Fe). It’s extremely hot temperature is estimated at 6,000 °C. This layer is approximately 1,250 km thick. 2.2. Composition of the earth 2.2.1. Rock forming minerals Meaning of rock forming minerals How many minerals do you think exist in the crust of the Earth? Geologists have discovered more than 3000 mineral species been in the Earth, but all of them are not of common occurrence. In fact more than 99% of rocks of the crust are made up from only 20 minerals and each rock being composed of two or three minerals or so. Many rocks contain only one mineral in abundance, and few others being present only in small accessory amounts. Such common minerals, which constitute the bulk of the crust of the Earth, are called Rock forming minerals. How do we classify minerals???? In most case, however minerals are classified by the dominant element they contain. For example, minerals that contain silicon-oxygen complexes are called silicates, those which contain sulfur ions, called sulfides, and those which contain carbonate are called carbonate minerals. Thus, rock- forming minerals may be broadly classified as silicate and non silicate minerals. Silicate minerals are those minerals whose dominant constituents are silicon with different proportion of oxygen and other elements. Most of rock forming minerals are composed largely of oxygen and silicon with Aluminium and at least one or more of the abundant eight elements. A. Silicates are the most abundant mineral groups, and because of their structure, the varied and the complexes of the mineral groups. These silicate minerals are again subdivided into groups on the base of the arrangement of the elements that are bounded together. 1. Feldspars are the most abundant rock forming minerals contain almost half of the Earth’s crust. It is found in more kinds of rocks than other minerals. They are major constituents of igneous rocks. It may present in various colours such as light, white, pink, gray etc. 2. Flow over the asthenosphere over geologic time. The lithosphere is also the zone of Mica Groups Mica: are again complex containing K, Mg, Fe and etc. hydrous silicates, They have sheet layers; thus, cleaves into their flexible layers. They occur in rocks in the form of thin, shiny, and flat plates. 3. Pyroxene Group: A complex group of silicate minerals containing different proportion of Ca, Ma, Fe, etc. It is known by dark colors, and hardness. It has many verities of minerals the commonest variety being Augite. It is found in many igneous and metamorphic rocks. It has poor luster. B. Non- silicate Minerals: are minerals without silica elements. They are classified on the base of their chemical composition as. a) Oxides: This group of minerals is composed of one or more metallic elements combined with oxygen, water, or hydroxyl (OH). The minerals in this mineral group show the greatest variations of physical properties. Some are hard, others soft. Some have metallic luster; some are clear and transparent. Some representative oxide minerals include the following ones. I. Magnetite: magnetite iron oxide (Fe3O4) Both its color and streaks are black Its hardness ranges from 5.5-6.5 Commonly occurs in igneous rocks in the form of small crystals. When in sufficient concentration, it is an important source of iron ore. II. Hematite: iron oxide (Fe2O3) Reddish-brown to black or gray in color; generally present in association to metamorphic rock and igneous rocks and chiefly used as source for extracting iron. III. Chromites: oxide of iron and chromium (Fe2Cr2O4) Occurs in ultra-basic igneous rocks Chief source of chromium used in the making of stainless steel IV. Bauxite: hydrated and aluminium oxide (Al2O.2H2O) Found as residual deposits and it is formed by the weathering of aluminium--bearing igneous rocks under humid, tropical conditions. b. Carbonate minerals: They are mainly found in sedimentary rocks. The carbonates group consist of minerals, which contain one or more metallic elements chemically associated with the compound CO4. Most carbonates are lightly coloured and transparent when relatively pure. All carbonates are soft and brittle. Some common carbonate minerals include: I. Calcite (calcium carbonate-CaCO3)-the most abundance mineral in the crust other than silicate mineral and is chief constituent of limestone. II. Dolomite (carbonate of ca and mg)-[CaMg(CO3)2]: Easily dissolved in water, but relatively resistant in dry climates. III. Sulphides: notable example, being (CaSO4.2H2O) hydrate sulphate of calcium. gypsum Most ores of important metals such as mercury (cinnabar-HgS) and lead (galena-PbS) are extracted from sulphides. 2.2.2. Physical properties of minerals Physical properties refer to the characteristics of a certain minerals that can be seen and measured. Specific gravity: the ratio of substances weight to the weight of an equal volume of pure water. Metals have high Specific gravity b/c atoms are closely arranged. But non-metals with loose atmospheric structure have smaller Specific gravity. 2. Cleavage: it is a line along which minerals break. Such a break forms smooth, flat surface is called cleavage plane. 1. 3. Fracture: minerals that do not cleave b/c their bonds are equally strong in all directions and are distributed uniformly throughout the crystal will breakdown or fracture. 4. Colour: show the external or partial expression of minerals. It is the weakest characteristics of minerals 5. Streak: a colour of mineral in its powdered form. Metallic minerals have a dark streak where as Non-metallic minerals yield a white powder. 6. Cluster: It describes how a mineral surface reflects light. Metals display a shiny metallic luster. Non metallic clusters are more poorly earthly, pearly, silky, and glassy. 7. Crystal form: Crystal form is the external expression of minerals that reflects the orderly internal arrangement of atoms. E.g. Quartz 8. Smell and taste: e.g. Sulphur → rotten egg Taste →rock salt 9. Hardness: refers to a measure of resistance from being scratched. E.g. diamond is the most hardest. Mohos scale of hardness The higher the value the hardest the element/ mineral. Besides each reference mineral is able to scratch any mineral with lower number. For instance, Talc will be scratched by any all known minerals. Conversely Diamond can scratch all known minerals. The end for unit two The Earth’s Layers Questions Label the layers of the earth. Read the questions below and circle the letter of the correct answer. 1. Which of the following is NOT one of the Earth’s layers? a. Inner core b. Crust c. Outer mantle d. Lower mantle 2. Starting with the outermost layer, what is the order of the Earth’s layers? a. Core, mantle, crust b. Crust, upper mantle, lower mantle, outer core, inner core c. Mantle, inner crust, core d. Crust, inner core, mantle 3. The core of the Earth is made up almost completely of which element? a. Iron b. Granite c. Sulfur d. Calcium 4. Which layer of the earth is the thickest? a. Mantle b. Core c. Crust d. All layers are the same thickness 5. Which of the earth’s layers is liquid? a. Lower crust b. Outer core c. Inner core d. mantle Read each statement below. If it is true, write a “T” in the blank; if it is false, write an “F”. ____1. The center of the Earth is made of molten rock. ____2. Most of the Earth’s heat is stored in the mantle. ____3. The outer core is liquid. ____4. Earthquakes occur when heat travels through the mantle and causes tectonic plates to shift. ____5. The thinnest parts of the Earth’s crust are its continents. ____6. Extreme pressure causes the inner core of the Earth to remain solid. ____7. The crust of the Earth is much cooler than its other layers. ____8. The Earth’s mantle is flexible and shifts under heavy loads. ____9. The core is where geologists look for oil, gas, and other resources. ___10. The crust is broken down into two parts: the upper and lower crust.